 Found 142 hits with Last Name = 'scott' and Initial = 'n'
Found 142 hits with Last Name = 'scott' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018759
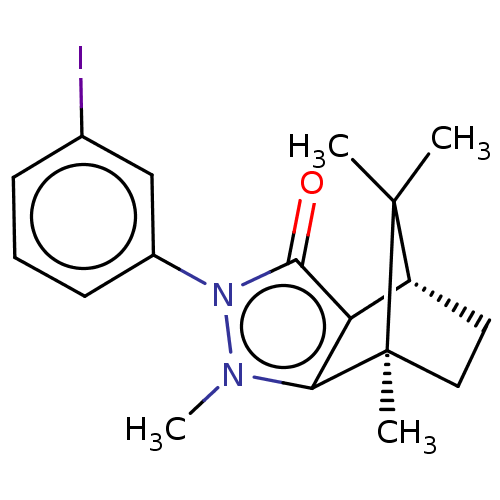
(CHEMBL3291350)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(I)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H21IN2O/c1-17(2)13-8-9-18(17,3)15-14(13)16(22)21(20(15)4)12-7-5-6-11(19)10-12/h5-7,10,13H,8-9H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018760
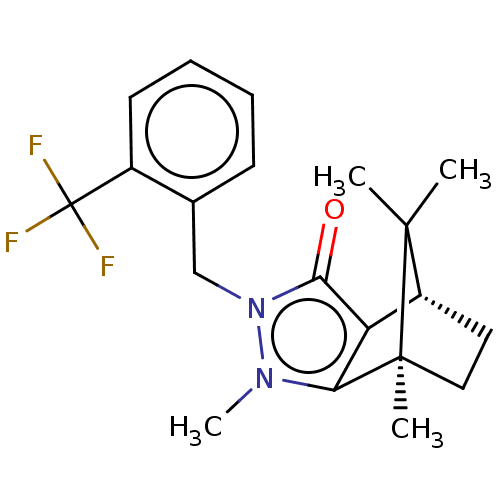
(CHEMBL3291357)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1C(F)(F)F)n3C)C2(C)C |r| Show InChI InChI=1S/C20H23F3N2O/c1-18(2)14-9-10-19(18,3)16-15(14)17(26)25(24(16)4)11-12-7-5-6-8-13(12)20(21,22)23/h5-8,14H,9-11H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018761
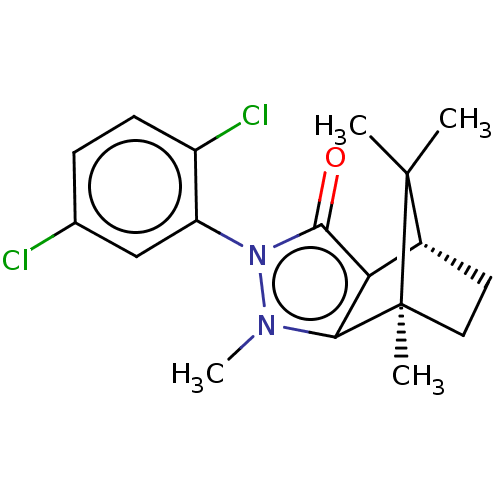
(CHEMBL3291348)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cc(Cl)ccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(16.87,-14.9,;16.87,-13.4,;18.16,-12.66,;18.16,-11.16,;16.87,-10.41,;16.45,-8.98,;15.58,-11.16,;15.58,-12.66,;14.16,-13.12,;13.7,-14.54,;13.28,-11.91,;11.73,-11.9,;10.97,-13.24,;9.43,-13.25,;8.66,-14.58,;8.65,-11.9,;9.43,-10.56,;10.97,-10.57,;11.75,-9.23,;14.16,-10.7,;13.68,-9.23,;17.73,-11.9,;19.22,-12.76,;19.22,-11.04,)| Show InChI InChI=1S/C18H20Cl2N2O/c1-17(2)11-7-8-18(17,3)15-14(11)16(23)22(21(15)4)13-9-10(19)5-6-12(13)20/h5-6,9,11H,7-8H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018793
(CHEMBL3291344)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H25FN2O/c1-23(2)17-13-14-24(23,3)21-20(17)22(28)27(19-12-8-7-11-18(19)25)26(21)15-16-9-5-4-6-10-16/h4-12,17H,13-15H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018761

(CHEMBL3291348)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cc(Cl)ccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(16.87,-14.9,;16.87,-13.4,;18.16,-12.66,;18.16,-11.16,;16.87,-10.41,;16.45,-8.98,;15.58,-11.16,;15.58,-12.66,;14.16,-13.12,;13.7,-14.54,;13.28,-11.91,;11.73,-11.9,;10.97,-13.24,;9.43,-13.25,;8.66,-14.58,;8.65,-11.9,;9.43,-10.56,;10.97,-10.57,;11.75,-9.23,;14.16,-10.7,;13.68,-9.23,;17.73,-11.9,;19.22,-12.76,;19.22,-11.04,)| Show InChI InChI=1S/C18H20Cl2N2O/c1-17(2)11-7-8-18(17,3)15-14(11)16(23)22(21(15)4)13-9-10(19)5-6-12(13)20/h5-6,9,11H,7-8H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018759

(CHEMBL3291350)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(I)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H21IN2O/c1-17(2)13-8-9-18(17,3)15-14(13)16(22)21(20(15)4)12-7-5-6-11(19)10-12/h5-7,10,13H,8-9H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018795
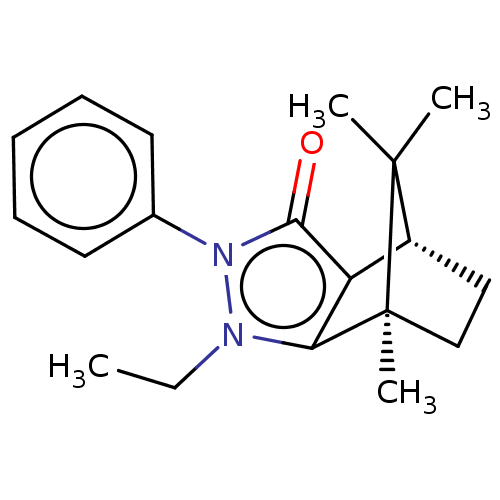
(CHEMBL3291339)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3CC)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-5-20-16-15(14-11-12-19(16,4)18(14,2)3)17(22)21(20)13-9-7-6-8-10-13/h6-10,14H,5,11-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018769
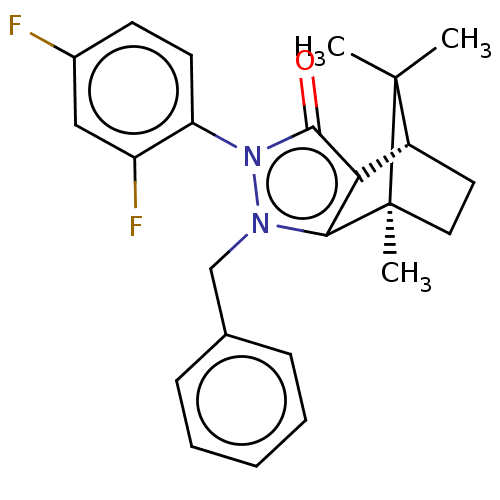
(CHEMBL3291346)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;9.91,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H24F2N2O/c1-23(2)17-11-12-24(23,3)21-20(17)22(29)28(19-10-9-16(25)13-18(19)26)27(21)14-15-7-5-4-6-8-15/h4-10,13,17H,11-12,14H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018797
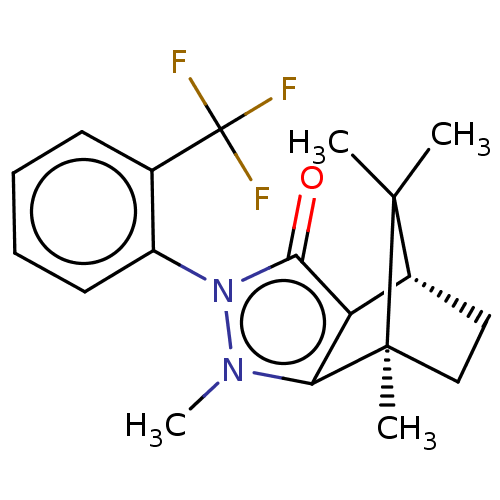
(CHEMBL3291345)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1C(F)(F)F)n3C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;13.37,-6.66,;14.46,-5.57,;11.88,-6.27,;12.97,-5.16,;16.96,-8.3,;16.48,-6.83,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C19H21F3N2O/c1-17(2)12-9-10-18(17,3)15-14(12)16(25)24(23(15)4)13-8-6-5-7-11(13)19(20,21)22/h5-8,12H,9-10H2,1-4H3/t12-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018760

(CHEMBL3291357)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1C(F)(F)F)n3C)C2(C)C |r| Show InChI InChI=1S/C20H23F3N2O/c1-18(2)14-9-10-19(18,3)16-15(14)17(26)25(24(16)4)11-12-7-5-6-8-13(12)20(21,22)23/h5-8,14H,9-11H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018794
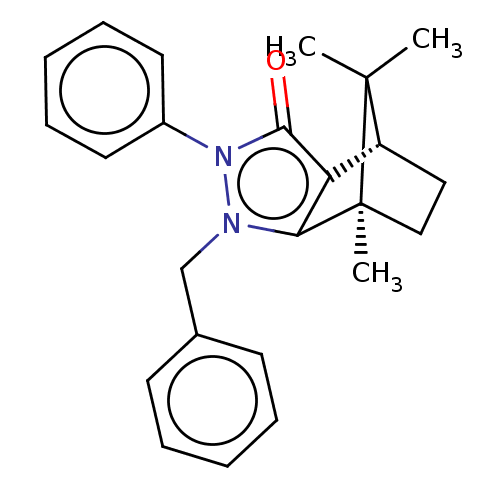
(CHEMBL3291340)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3Cc1ccccc1)C2(C)C |r| Show InChI InChI=1S/C24H26N2O/c1-23(2)19-14-15-24(23,3)21-20(19)22(27)26(18-12-8-5-9-13-18)25(21)16-17-10-6-4-7-11-17/h4-13,19H,14-16H2,1-3H3/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018793

(CHEMBL3291344)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H25FN2O/c1-23(2)17-13-14-24(23,3)21-20(17)22(28)27(19-12-8-7-11-18(19)25)26(21)15-16-9-5-4-6-10-16/h4-12,17H,13-15H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392984
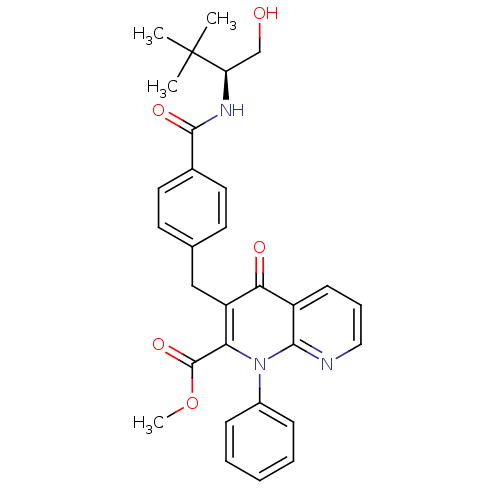
(CHEMBL2152384)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H](CO)C(C)(C)C)c(=O)c2cccnc2n1-c1ccccc1 |r| Show InChI InChI=1S/C30H31N3O5/c1-30(2,3)24(18-34)32-28(36)20-14-12-19(13-15-20)17-23-25(29(37)38-4)33(21-9-6-5-7-10-21)27-22(26(23)35)11-8-16-31-27/h5-16,24,34H,17-18H2,1-4H3,(H,32,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392983
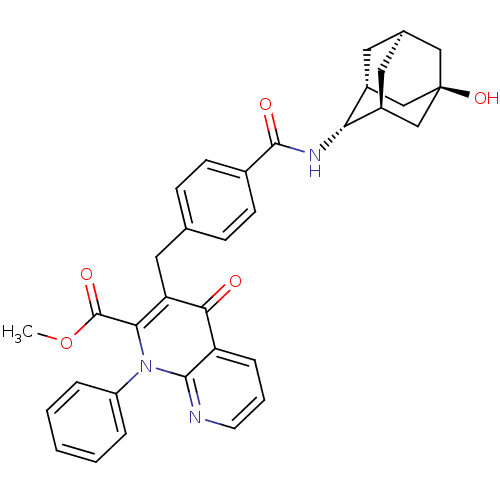
(CHEMBL2152383)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H]2[C@H]3C[C@H]4C[C@@H]2C[C@](O)(C4)C3)c(=O)c2cccnc2n1-c1ccccc1 |r,TLB:26:23:20:16.17.18,THB:18:17:22:20.19.25,18:19:16.17.26:22,15:16:22:20.19.25,24:23:20:16.17.18| Show InChI InChI=1S/C34H33N3O5/c1-42-33(40)29-27(30(38)26-8-5-13-35-31(26)37(29)25-6-3-2-4-7-25)16-20-9-11-22(12-10-20)32(39)36-28-23-14-21-15-24(28)19-34(41,17-21)18-23/h2-13,21,23-24,28,41H,14-19H2,1H3,(H,36,39)/t21-,23-,24+,28-,34- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018769

(CHEMBL3291346)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3Cc1ccccc1)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.96,-10.25,;20.96,-8.75,;19.67,-8,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.95,-10.71,;16.5,-12.13,;16.08,-9.5,;14.53,-9.5,;13.77,-10.83,;12.23,-10.84,;11.45,-9.49,;9.91,-9.49,;12.23,-8.16,;13.77,-8.16,;14.55,-6.83,;16.96,-8.29,;16.48,-6.82,;17.51,-5.68,;19.01,-6,;20.04,-4.85,;19.56,-3.38,;18.04,-3.07,;17.02,-4.22,;20.53,-9.49,;22.02,-10.35,;22.02,-8.63,)| Show InChI InChI=1S/C24H24F2N2O/c1-23(2)17-11-12-24(23,3)21-20(17)22(29)28(19-10-9-16(25)13-18(19)26)27(21)14-15-7-5-4-6-8-15/h4-10,13,17H,11-12,14H2,1-3H3/t17-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018797

(CHEMBL3291345)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1C(F)(F)F)n3C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;13.37,-6.66,;14.46,-5.57,;11.88,-6.27,;12.97,-5.16,;16.96,-8.3,;16.48,-6.83,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C19H21F3N2O/c1-17(2)12-9-10-18(17,3)15-14(12)16(25)24(23(15)4)13-8-6-5-7-11(13)19(20,21)22/h5-8,12H,9-10H2,1-4H3/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018798
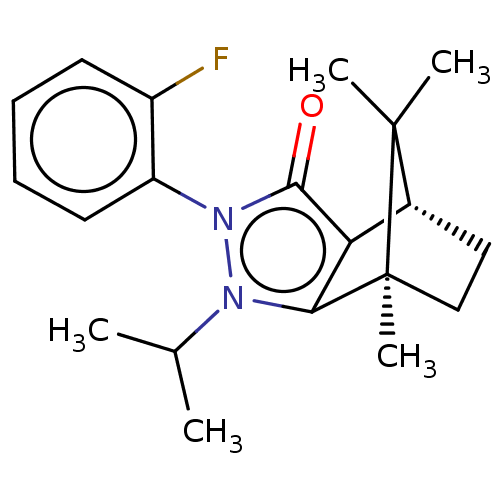
(CHEMBL3291342)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3C(C)C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;15.7,-5.48,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-12(2)22-17-16(13-10-11-20(17,5)19(13,3)4)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,12-13H,10-11H2,1-5H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018799
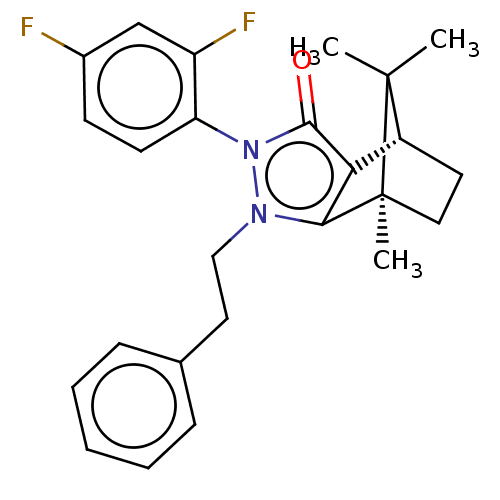
(CHEMBL3291347)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3CCc1ccccc1)C2(C)C |r,wU:4.4,1.0,(16.88,-14.91,;16.88,-13.41,;18.18,-12.67,;18.18,-11.17,;16.89,-10.42,;16.46,-8.98,;15.59,-11.17,;15.59,-12.67,;14.17,-13.14,;13.71,-14.55,;13.29,-11.92,;11.75,-11.92,;10.98,-13.26,;9.44,-13.26,;8.66,-11.91,;7.11,-11.91,;9.44,-10.58,;10.98,-10.58,;11.76,-9.24,;14.17,-10.71,;13.69,-9.24,;14.72,-8.09,;14.24,-6.63,;15.26,-5.48,;14.77,-4.02,;13.26,-3.71,;12.23,-4.88,;12.73,-6.33,;17.75,-11.92,;19.24,-12.77,;19.24,-11.05,)| Show InChI InChI=1S/C25H26F2N2O/c1-24(2)18-11-13-25(24,3)22-21(18)23(30)29(20-10-9-17(26)15-19(20)27)28(22)14-12-16-7-5-4-6-8-16/h4-10,15,18H,11-14H2,1-3H3/t18-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018802
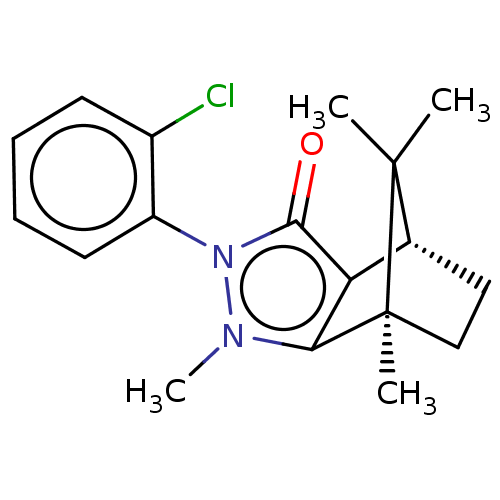
(CHEMBL3291354)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(22.13,-16.72,;22.13,-15.22,;23.42,-14.48,;23.42,-12.98,;22.13,-12.23,;21.71,-10.79,;20.84,-12.98,;20.84,-14.48,;19.42,-14.94,;18.96,-16.36,;18.54,-13.73,;16.99,-13.72,;16.23,-12.39,;14.69,-12.38,;13.91,-13.72,;14.69,-15.07,;16.23,-15.06,;17.01,-16.4,;19.42,-12.52,;18.94,-11.05,;22.99,-13.72,;24.49,-14.58,;24.49,-12.86,)| Show InChI InChI=1S/C18H21ClN2O/c1-17(2)11-9-10-18(17,3)15-14(11)16(22)21(20(15)4)13-8-6-5-7-12(13)19/h5-8,11H,9-10H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018803
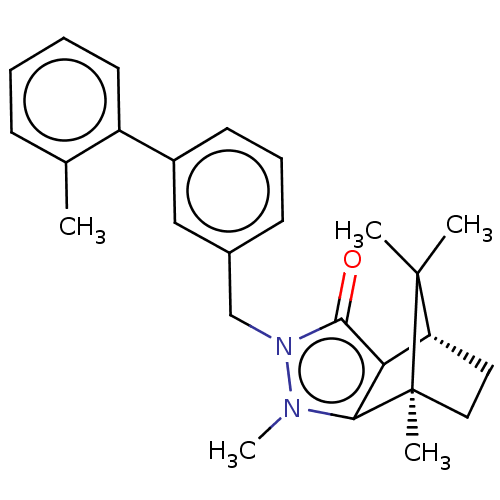
(CHEMBL3291358)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1cccc(c1)-c1ccccc1C)n3C)C2(C)C |r| Show InChI InChI=1S/C26H30N2O/c1-17-9-6-7-12-20(17)19-11-8-10-18(15-19)16-28-24(29)22-21-13-14-26(4,25(21,2)3)23(22)27(28)5/h6-12,15,21H,13-14,16H2,1-5H3/t21-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018794

(CHEMBL3291340)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3Cc1ccccc1)C2(C)C |r| Show InChI InChI=1S/C24H26N2O/c1-23(2)19-14-15-24(23,3)21-20(19)22(27)26(18-12-8-5-9-13-18)25(21)16-17-10-6-4-7-11-17/h4-13,19H,14-16H2,1-3H3/t19-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392990
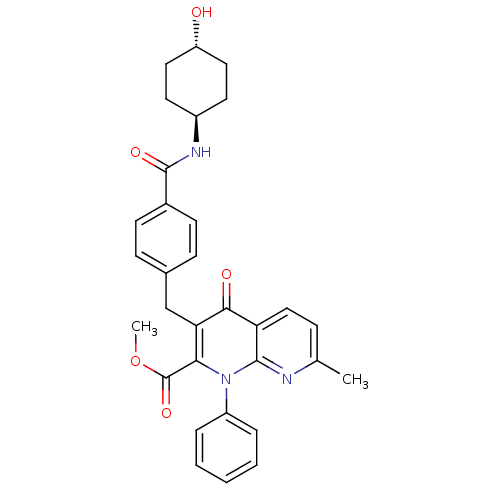
(CHEMBL2152390)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H]2CC[C@H](O)CC2)c(=O)c2ccc(C)nc2n1-c1ccccc1 |r,wU:16.16,wD:19.20,(1.04,-20.36,;-.3,-19.58,;-.29,-18.04,;1.04,-17.28,;-1.62,-17.27,;-1.62,-15.72,;-.28,-14.95,;-.29,-13.41,;1.04,-12.64,;1.04,-11.1,;-.3,-10.34,;-1.63,-11.12,;-1.62,-12.65,;-.3,-8.8,;1.03,-8.02,;-1.64,-8.03,;-1.64,-6.49,;-2.98,-5.73,;-2.98,-4.19,;-1.65,-3.42,;-1.65,-1.88,;-.31,-4.19,;-.31,-5.72,;-2.97,-14.93,;-2.97,-13.39,;-4.31,-15.71,;-5.64,-14.95,;-6.97,-15.72,;-6.97,-17.26,;-8.31,-18.03,;-5.64,-18.03,;-4.31,-17.27,;-2.97,-18.04,;-2.97,-19.57,;-4.31,-20.34,;-4.32,-21.88,;-2.98,-22.65,;-1.64,-21.88,;-1.65,-20.34,)| Show InChI InChI=1S/C31H31N3O5/c1-19-8-17-25-28(36)26(27(31(38)39-2)34(29(25)32-19)23-6-4-3-5-7-23)18-20-9-11-21(12-10-20)30(37)33-22-13-15-24(35)16-14-22/h3-12,17,22,24,35H,13-16,18H2,1-2H3,(H,33,37)/t22-,24- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018799

(CHEMBL3291347)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(F)cc1F)n3CCc1ccccc1)C2(C)C |r,wU:4.4,1.0,(16.88,-14.91,;16.88,-13.41,;18.18,-12.67,;18.18,-11.17,;16.89,-10.42,;16.46,-8.98,;15.59,-11.17,;15.59,-12.67,;14.17,-13.14,;13.71,-14.55,;13.29,-11.92,;11.75,-11.92,;10.98,-13.26,;9.44,-13.26,;8.66,-11.91,;7.11,-11.91,;9.44,-10.58,;10.98,-10.58,;11.76,-9.24,;14.17,-10.71,;13.69,-9.24,;14.72,-8.09,;14.24,-6.63,;15.26,-5.48,;14.77,-4.02,;13.26,-3.71,;12.23,-4.88,;12.73,-6.33,;17.75,-11.92,;19.24,-12.77,;19.24,-11.05,)| Show InChI InChI=1S/C25H26F2N2O/c1-24(2)18-11-13-25(24,3)22-21(18)23(30)29(20-10-9-17(26)15-19(20)27)28(22)14-12-16-7-5-4-6-8-16/h4-10,15,18H,11-14H2,1-3H3/t18-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018795

(CHEMBL3291339)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3CC)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-5-20-16-15(14-11-12-19(16,4)18(14,2)3)17(22)21(20)13-9-7-6-8-10-13/h6-10,14H,5,11-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018804
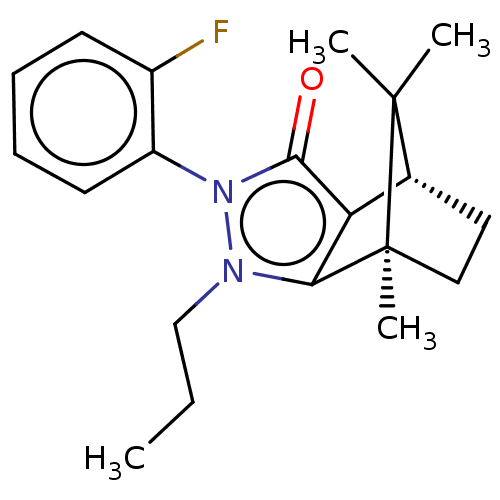
(CHEMBL3291341)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3CCC)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;17.04,-4.21,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-5-12-22-17-16(13-10-11-20(17,4)19(13,2)3)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,13H,5,10-12H2,1-4H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018803

(CHEMBL3291358)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1cccc(c1)-c1ccccc1C)n3C)C2(C)C |r| Show InChI InChI=1S/C26H30N2O/c1-17-9-6-7-12-20(17)19-11-8-10-18(15-19)16-28-24(29)22-21-13-14-26(4,25(21,2)3)23(22)27(28)5/h6-12,15,21H,13-14,16H2,1-5H3/t21-,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018798

(CHEMBL3291342)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3C(C)C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;15.7,-5.48,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-12(2)22-17-16(13-10-11-20(17,5)19(13,3)4)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,12-13H,10-11H2,1-5H3/t13-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018806

(CHEMBL3291343)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3CC=C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;17.04,-4.21,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H23FN2O/c1-5-12-22-17-16(13-10-11-20(17,4)19(13,2)3)18(24)23(22)15-9-7-6-8-14(15)21/h5-9,13H,1,10-12H2,2-4H3/t13-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018807
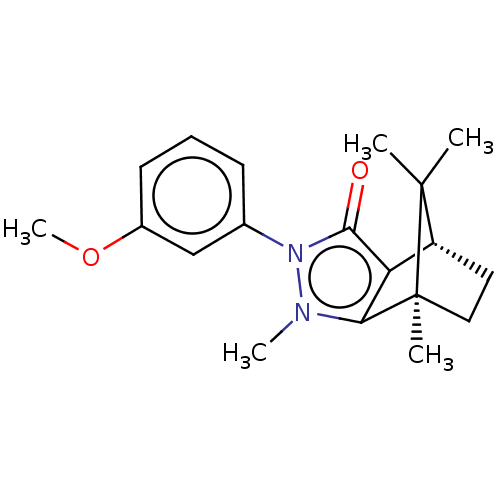
(CHEMBL3291355)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(OC)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O2/c1-18(2)14-9-10-19(18,3)16-15(14)17(22)21(20(16)4)12-7-6-8-13(11-12)23-5/h6-8,11,14H,9-10H2,1-5H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018808
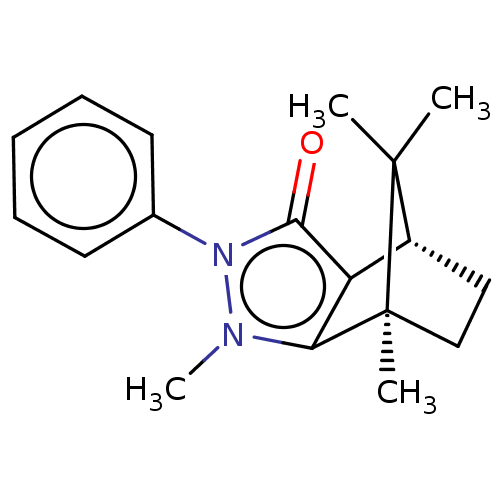
(CHEMBL3291337)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H22N2O/c1-17(2)13-10-11-18(17,3)15-14(13)16(21)20(19(15)4)12-8-6-5-7-9-12/h5-9,13H,10-11H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018809
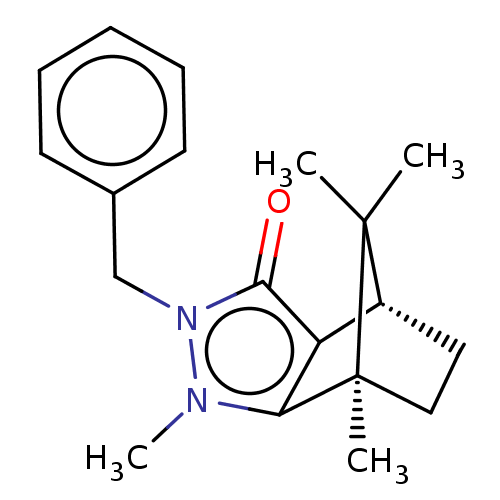
(CHEMBL3291356)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-18(2)14-10-11-19(18,3)16-15(14)17(22)21(20(16)4)12-13-8-6-5-7-9-13/h5-9,14H,10-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018806

(CHEMBL3291343)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3CC=C)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;17.04,-4.21,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H23FN2O/c1-5-12-22-17-16(13-10-11-20(17,4)19(13,2)3)18(24)23(22)15-9-7-6-8-14(15)21/h5-9,13H,1,10-12H2,2-4H3/t13-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018802

(CHEMBL3291354)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1Cl)n3C)C2(C)C |r,wU:4.4,1.0,(22.13,-16.72,;22.13,-15.22,;23.42,-14.48,;23.42,-12.98,;22.13,-12.23,;21.71,-10.79,;20.84,-12.98,;20.84,-14.48,;19.42,-14.94,;18.96,-16.36,;18.54,-13.73,;16.99,-13.72,;16.23,-12.39,;14.69,-12.38,;13.91,-13.72,;14.69,-15.07,;16.23,-15.06,;17.01,-16.4,;19.42,-12.52,;18.94,-11.05,;22.99,-13.72,;24.49,-14.58,;24.49,-12.86,)| Show InChI InChI=1S/C18H21ClN2O/c1-17(2)11-9-10-18(17,3)15-14(11)16(22)21(20(15)4)13-8-6-5-7-12(13)19/h5-8,11H,9-10H2,1-4H3/t11-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018807

(CHEMBL3291355)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1cccc(OC)c1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O2/c1-18(2)14-9-10-19(18,3)16-15(14)17(22)21(20(16)4)12-7-6-8-13(11-12)23-5/h6-8,11,14H,9-10H2,1-5H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392986
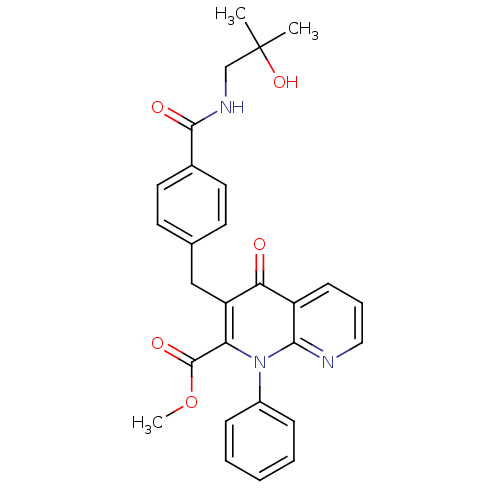
(CHEMBL2152386)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)NCC(C)(C)O)c(=O)c2cccnc2n1-c1ccccc1 Show InChI InChI=1S/C28H27N3O5/c1-28(2,35)17-30-26(33)19-13-11-18(12-14-19)16-22-23(27(34)36-3)31(20-8-5-4-6-9-20)25-21(24(22)32)10-7-15-29-25/h4-15,35H,16-17H2,1-3H3,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392982
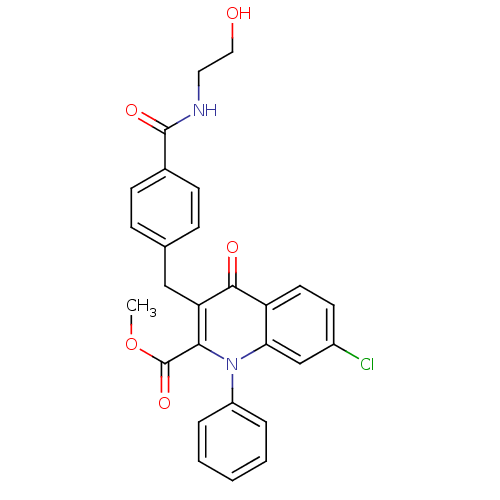
(CHEMBL2152382)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)NCCO)c(=O)c2ccc(Cl)cc2n1-c1ccccc1 Show InChI InChI=1S/C27H23ClN2O5/c1-35-27(34)24-22(15-17-7-9-18(10-8-17)26(33)29-13-14-31)25(32)21-12-11-19(28)16-23(21)30(24)20-5-3-2-4-6-20/h2-12,16,31H,13-15H2,1H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392989
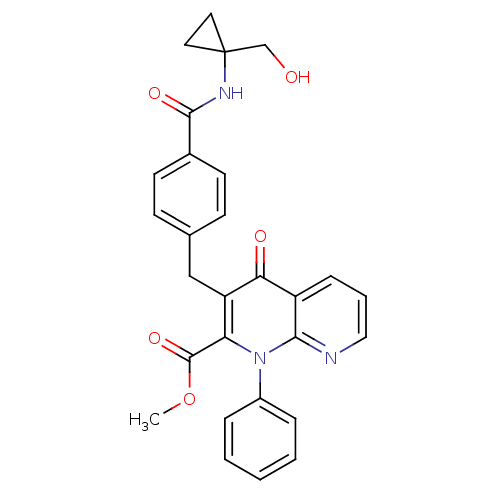
(CHEMBL2152389)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)NC2(CO)CC2)c(=O)c2cccnc2n1-c1ccccc1 Show InChI InChI=1S/C28H25N3O5/c1-36-27(35)23-22(16-18-9-11-19(12-10-18)26(34)30-28(17-32)13-14-28)24(33)21-8-5-15-29-25(21)31(23)20-6-3-2-4-7-20/h2-12,15,32H,13-14,16-17H2,1H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
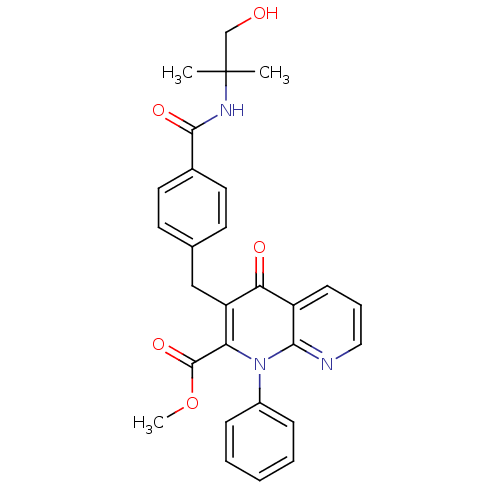
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392988
(CHEMBL2152388)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)NC(C)(C)CO)c(=O)c2cccnc2n1-c1ccccc1 Show InChI InChI=1S/C28H27N3O5/c1-28(2,17-32)30-26(34)19-13-11-18(12-14-19)16-22-23(27(35)36-3)31(20-8-5-4-6-9-20)25-21(24(22)33)10-7-15-29-25/h4-15,32H,16-17H2,1-3H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392985
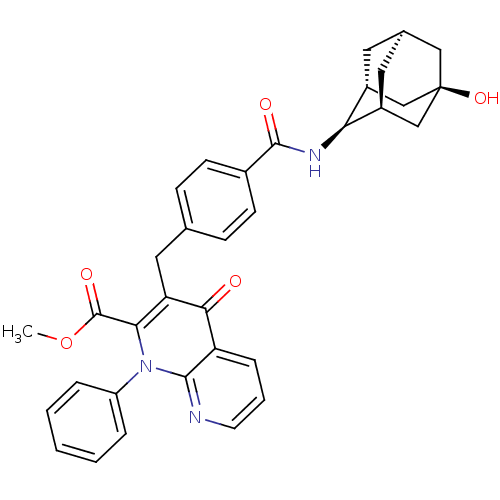
(CHEMBL2152385)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@@H]2[C@H]3C[C@H]4C[C@@H]2C[C@](O)(C4)C3)c(=O)c2cccnc2n1-c1ccccc1 |r,TLB:26:23:20:16.17.18,THB:18:17:22:20.19.25,18:19:16.17.26:22,15:16:22:20.19.25,24:23:20:16.17.18| Show InChI InChI=1S/C34H33N3O5/c1-42-33(40)29-27(30(38)26-8-5-13-35-31(26)37(29)25-6-3-2-4-7-25)16-20-9-11-22(12-10-20)32(39)36-28-23-14-21-15-24(28)19-34(41,17-21)18-23/h2-13,21,23-24,28,41H,14-19H2,1H3,(H,36,39)/t21-,23-,24+,28+,34- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392992
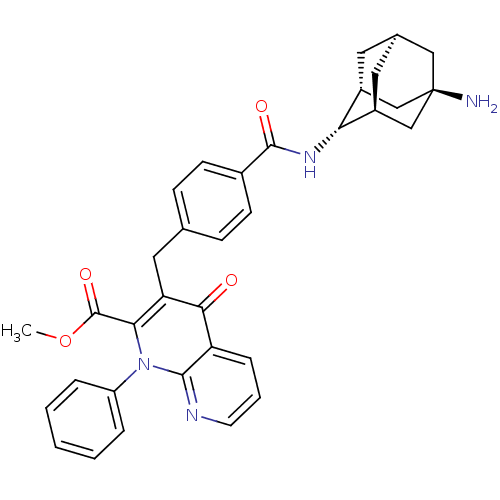
(CHEMBL2152392)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H]2[C@H]3C[C@H]4C[C@@H]2C[C@](N)(C4)C3)c(=O)c2cccnc2n1-c1ccccc1 |r,TLB:26:23:20:16.17.18,THB:18:17:22:20.19.25,18:19:16.17.26:22,15:16:22:20.19.25,24:23:20:16.17.18| Show InChI InChI=1S/C34H34N4O4/c1-42-33(41)29-27(30(39)26-8-5-13-36-31(26)38(29)25-6-3-2-4-7-25)16-20-9-11-22(12-10-20)32(40)37-28-23-14-21-15-24(28)19-34(35,17-21)18-23/h2-13,21,23-24,28H,14-19,35H2,1H3,(H,37,40)/t21-,23-,24+,28-,34- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392987
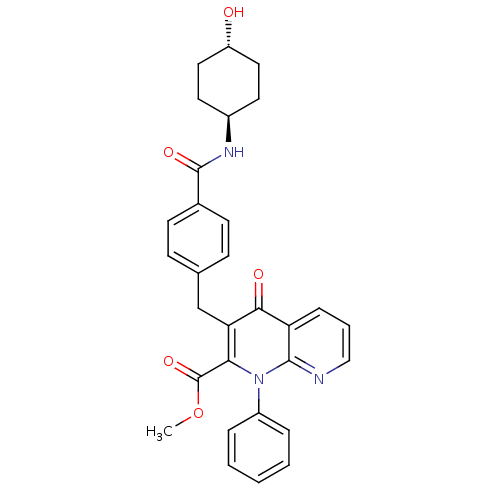
(CHEMBL2152387)Show SMILES COC(=O)c1c(Cc2ccc(cc2)C(=O)N[C@H]2CC[C@H](O)CC2)c(=O)c2cccnc2n1-c1ccccc1 |r,wU:16.16,wD:19.20,(13.6,-20.56,;12.27,-19.79,;12.27,-18.25,;13.61,-17.48,;10.94,-17.47,;10.94,-15.92,;12.28,-15.15,;12.27,-13.61,;13.61,-12.85,;13.61,-11.31,;12.28,-10.54,;10.94,-11.31,;10.95,-12.85,;12.28,-9,;13.61,-8.23,;10.94,-8.23,;10.94,-6.69,;9.61,-5.93,;9.61,-4.38,;10.95,-3.61,;10.95,-2.07,;12.28,-4.39,;12.28,-5.92,;9.59,-15.13,;9.59,-13.59,;8.25,-15.91,;6.92,-15.15,;5.59,-15.92,;5.59,-17.47,;6.92,-18.24,;8.25,-17.47,;9.59,-18.24,;9.59,-19.78,;8.25,-20.54,;8.24,-22.08,;9.58,-22.86,;10.92,-22.08,;10.91,-20.55,)| Show InChI InChI=1S/C30H29N3O5/c1-38-30(37)26-25(27(35)24-8-5-17-31-28(24)33(26)22-6-3-2-4-7-22)18-19-9-11-20(12-10-19)29(36)32-21-13-15-23(34)16-14-21/h2-12,17,21,23,34H,13-16,18H2,1H3,(H,32,36)/t21-,23- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018810
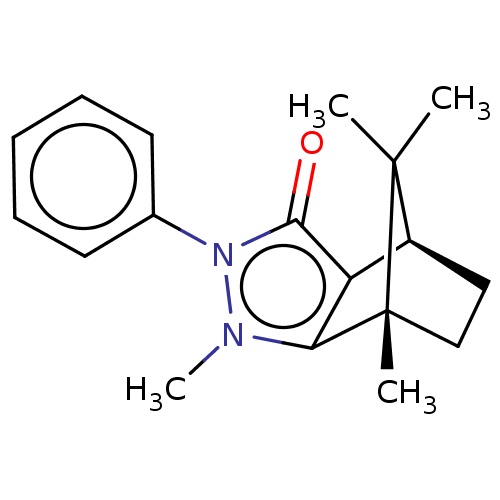
(CHEMBL3291338)Show SMILES [H][C@]12CC[C@](C)(c3c1c(=O)n(-c1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H22N2O/c1-17(2)13-10-11-18(17,3)15-14(13)16(21)20(19(15)4)12-8-6-5-7-9-12/h5-9,13H,10-11H2,1-4H3/t13-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018809

(CHEMBL3291356)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(Cc1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-18(2)14-10-11-19(18,3)16-15(14)17(22)21(20(16)4)12-13-8-6-5-7-9-13/h5-9,14H,10-12H2,1-4H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018811
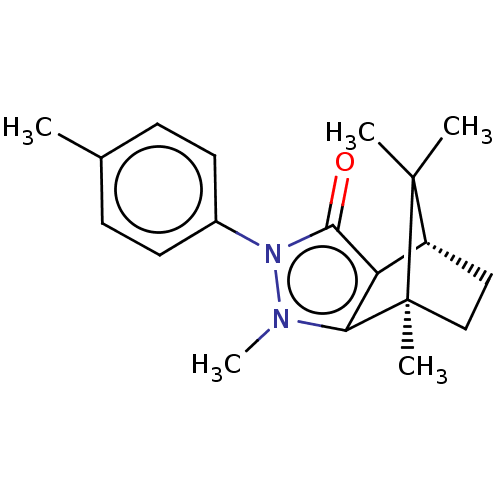
(CHEMBL3291351)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(C)cc1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-12-6-8-13(9-7-12)21-17(22)15-14-10-11-19(4,18(14,2)3)16(15)20(21)5/h6-9,14H,10-11H2,1-5H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50392991
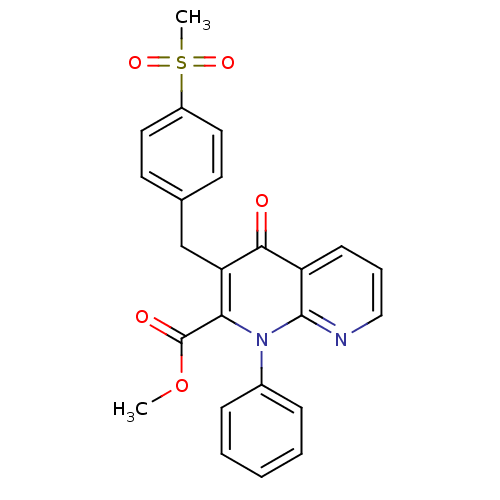
(CHEMBL2152391)Show SMILES COC(=O)c1c(Cc2ccc(cc2)S(C)(=O)=O)c(=O)c2cccnc2n1-c1ccccc1 Show InChI InChI=1S/C24H20N2O5S/c1-31-24(28)21-20(15-16-10-12-18(13-11-16)32(2,29)30)22(27)19-9-6-14-25-23(19)26(21)17-7-4-3-5-8-17/h3-14H,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 after 30 mins by TR-FRET assay |
ACS Med Chem Lett 3: 764-768 (2012)
Article DOI: 10.1021/ml300175c
BindingDB Entry DOI: 10.7270/Q22808P8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018811

(CHEMBL3291351)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccc(C)cc1)n3C)C2(C)C |r| Show InChI InChI=1S/C19H24N2O/c1-12-6-8-13(9-7-12)21-17(22)15-14-10-11-19(4,18(14,2)3)16(15)20(21)5/h6-9,14H,10-11H2,1-5H3/t14-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018804

(CHEMBL3291341)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1F)n3CCC)C2(C)C |r,wU:4.4,1.0,(19.67,-12.49,;19.67,-10.99,;20.97,-10.25,;20.97,-8.75,;19.67,-8.01,;19.25,-6.57,;18.38,-8.75,;18.38,-10.25,;16.96,-10.72,;16.5,-12.13,;16.09,-9.5,;14.54,-9.5,;13.78,-10.84,;12.24,-10.84,;11.46,-9.5,;12.23,-8.16,;13.78,-8.16,;14.55,-6.83,;16.96,-8.3,;16.48,-6.83,;17.51,-5.68,;17.04,-4.21,;20.54,-9.5,;22.03,-10.35,;22.03,-8.64,)| Show InChI InChI=1S/C20H25FN2O/c1-5-12-22-17-16(13-10-11-20(17,4)19(13,2)3)18(24)23(22)15-9-7-6-8-14(15)21/h6-9,13H,5,10-12H2,1-4H3/t13-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018810

(CHEMBL3291338)Show SMILES [H][C@]12CC[C@](C)(c3c1c(=O)n(-c1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H22N2O/c1-17(2)13-10-11-18(17,3)15-14(13)16(21)20(19(15)4)12-8-6-5-7-9-12/h5-9,13H,10-11H2,1-4H3/t13-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50018808

(CHEMBL3291337)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccc1)n3C)C2(C)C |r| Show InChI InChI=1S/C18H22N2O/c1-17(2)13-10-11-18(17,3)15-14(13)16(21)20(19(15)4)12-8-6-5-7-9-12/h5-9,13H,10-11H2,1-4H3/t13-,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11betaHSD1 assessed as reduction of cortisone to cortisol by ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50018812
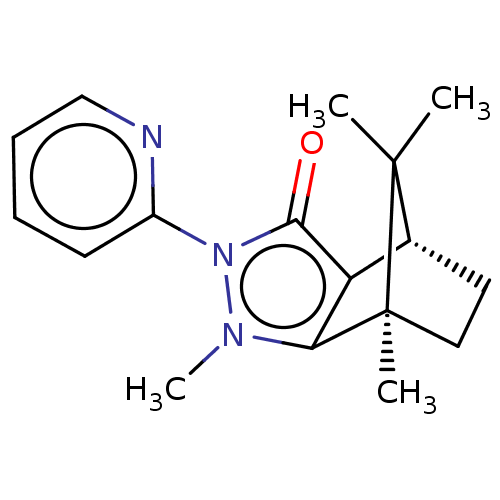
(CHEMBL3291353)Show SMILES [H][C@@]12CC[C@@](C)(c3c1c(=O)n(-c1ccccn1)n3C)C2(C)C |r| Show InChI InChI=1S/C17H21N3O/c1-16(2)11-8-9-17(16,3)14-13(11)15(21)20(19(14)4)12-7-5-6-10-18-12/h5-7,10-11H,8-9H2,1-4H3/t11-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 634 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of full-length human 11betaHSD1 expressed in HEK293 cells assessed as cortisol level by competitive ELISA |
Bioorg Med Chem Lett 24: 2707-11 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.049
BindingDB Entry DOI: 10.7270/Q2TM7CPF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data