 Found 311 hits with Last Name = 'scott' and Initial = 'wj'
Found 311 hits with Last Name = 'scott' and Initial = 'wj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215791
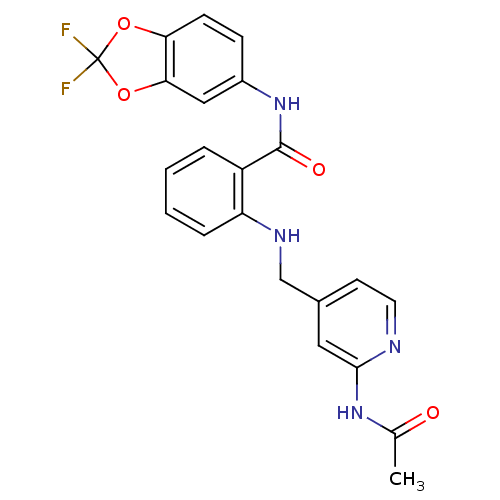
(2-((2-acetamidopyridin-4-yl)methylamino)-N-(2,2-di...)Show SMILES CC(=O)Nc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C22H18F2N4O4/c1-13(29)27-20-10-14(8-9-25-20)12-26-17-5-3-2-4-16(17)21(30)28-15-6-7-18-19(11-15)32-22(23,24)31-18/h2-11,26H,12H2,1H3,(H,28,30)(H,25,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139736

(US8895549, 11)Show SMILES COc1c(OC[C@H](O)CN(C)C)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:31,t:15| Show InChI InChI=1S/C22H26N6O4/c1-27(2)12-15(29)13-32-17-7-6-16-18(19(17)31-3)25-22(28-10-9-24-20(16)28)26-21(30)14-5-4-8-23-11-14/h4-8,11,15,29H,9-10,12-13H2,1-3H3,(H,25,26,30)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139746
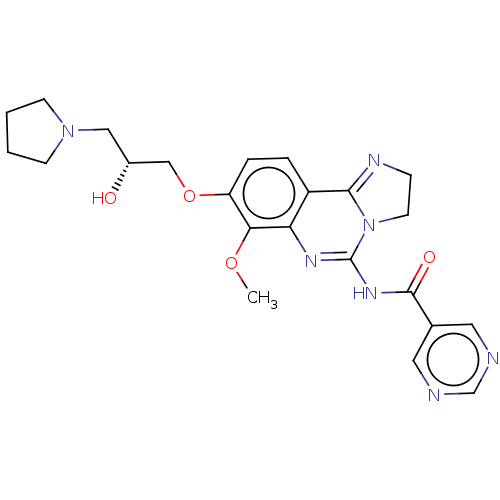
(US8895549, 24)Show SMILES COc1c(OC[C@H](O)CN2CCCC2)ccc2C3=NCCN3C(NC(=O)c3cncnc3)=Nc12 |r,c:34,t:18| Show InChI InChI=1S/C23H27N7O4/c1-33-20-18(34-13-16(31)12-29-7-2-3-8-29)5-4-17-19(20)27-23(30-9-6-26-21(17)30)28-22(32)15-10-24-14-25-11-15/h4-5,10-11,14,16,31H,2-3,6-9,12-13H2,1H3,(H,27,28,32)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50091927
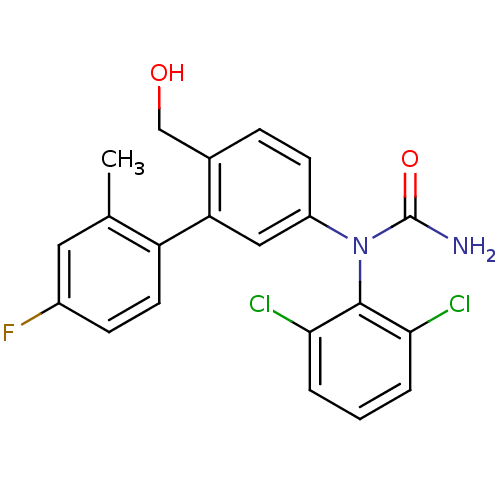
(1-(2,6-Dichloro-phenyl)-1-(4'-fluoro-6-hydroxymeth...)Show SMILES Cc1cc(F)ccc1-c1cc(ccc1CO)N(C(N)=O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C21H17Cl2FN2O2/c1-12-9-14(24)6-8-16(12)17-10-15(7-5-13(17)11-27)26(21(25)28)20-18(22)3-2-4-19(20)23/h2-10,27H,11H2,1H3,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 expressed in E. coli. |
Bioorg Med Chem Lett 10: 2047-50 (2000)
BindingDB Entry DOI: 10.7270/Q2FB526H |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215766
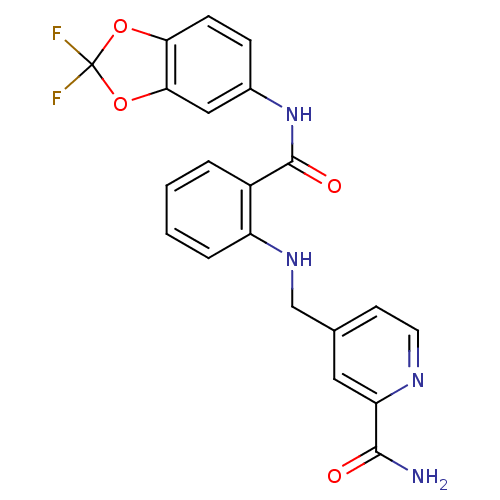
(4-((2-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)carba...)Show SMILES NC(=O)c1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C21H16F2N4O4/c22-21(23)30-17-6-5-13(10-18(17)31-21)27-20(29)14-3-1-2-4-15(14)26-11-12-7-8-25-16(9-12)19(24)28/h1-10,26H,11H2,(H2,24,28)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139750

(US8895549, 9)Show SMILES COc1c(OC[C@H](O)CN2CCCCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:35,t:19| Show InChI InChI=1S/C25H30N6O4/c1-34-22-20(35-16-18(32)15-30-11-3-2-4-12-30)8-7-19-21(22)28-25(31-13-10-27-23(19)31)29-24(33)17-6-5-9-26-14-17/h5-9,14,18,32H,2-4,10-13,15-16H2,1H3,(H,28,29,33)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139741
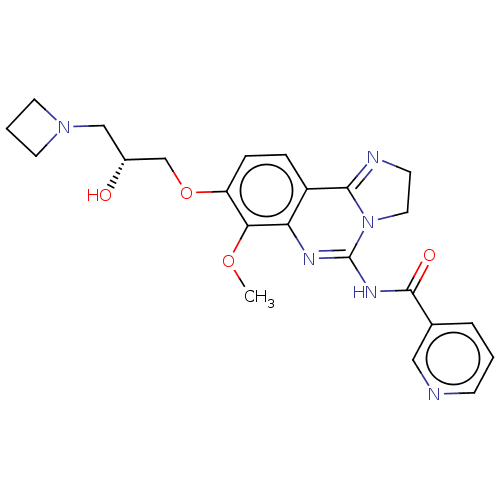
(US8895549, 7)Show SMILES COc1c(OC[C@H](O)CN2CCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:33,t:17| Show InChI InChI=1S/C23H26N6O4/c1-32-20-18(33-14-16(30)13-28-9-3-10-28)6-5-17-19(20)26-23(29-11-8-25-21(17)29)27-22(31)15-4-2-7-24-12-15/h2,4-7,12,16,30H,3,8-11,13-14H2,1H3,(H,26,27,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139743
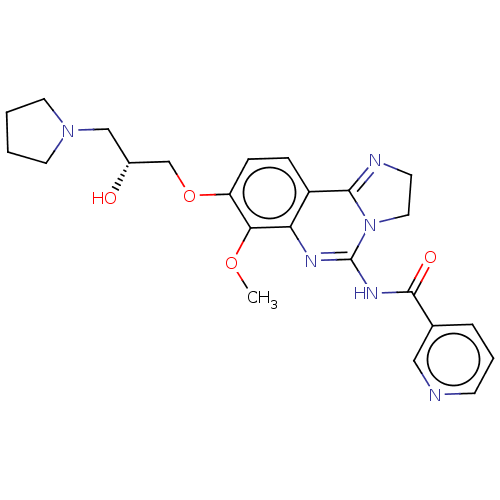
(US8895549, 8)Show SMILES COc1c(OC[C@H](O)CN2CCCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:34,t:18| Show InChI InChI=1S/C24H28N6O4/c1-33-21-19(34-15-17(31)14-29-10-2-3-11-29)7-6-18-20(21)27-24(30-12-9-26-22(18)30)28-23(32)16-5-4-8-25-13-16/h4-8,13,17,31H,2-3,9-12,14-15H2,1H3,(H,27,28,32)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139754
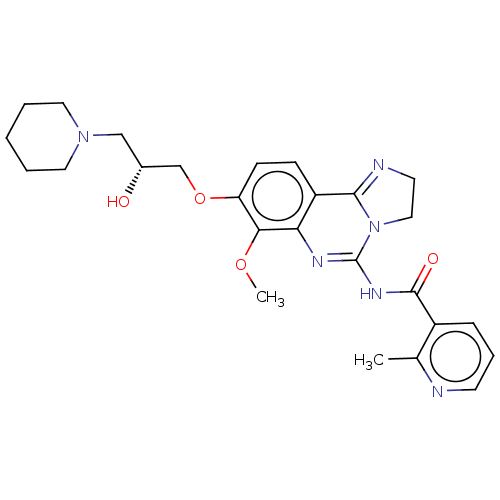
(US8895549, 19)Show SMILES COc1c(OC[C@H](O)CN2CCCCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3C)=Nc12 |r,c:36,t:19| Show InChI InChI=1S/C26H32N6O4/c1-17-19(7-6-10-27-17)25(34)30-26-29-22-20(24-28-11-14-32(24)26)8-9-21(23(22)35-2)36-16-18(33)15-31-12-4-3-5-13-31/h6-10,18,33H,3-5,11-16H2,1-2H3,(H,29,30,34)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139717

(US8895549, 2 | US9675616, 2 N-(8-{[(2R)-2-hydroxy-...)Show SMILES COc1c(OC[C@H](O)CN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:35,t:19| Show InChI InChI=1S/C24H28N6O5/c1-33-21-19(35-15-17(31)14-29-9-11-34-12-10-29)5-4-18-20(21)27-24(30-8-7-26-22(18)30)28-23(32)16-3-2-6-25-13-16/h2-6,13,17,31H,7-12,14-15H2,1H3,(H,27,28,32)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139763

(US8895549, 37)Show SMILES COc1c(OC[C@H](O)CN2CCCCC2)ccc2C3=NCCN3C(NC(=O)c3cncs3)=Nc12 |r,c:34,t:19| Show InChI InChI=1S/C23H28N6O4S/c1-32-20-17(33-13-15(30)12-28-8-3-2-4-9-28)6-5-16-19(20)26-23(29-10-7-25-21(16)29)27-22(31)18-11-24-14-34-18/h5-6,11,14-15,30H,2-4,7-10,12-13H2,1H3,(H,26,27,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50215761
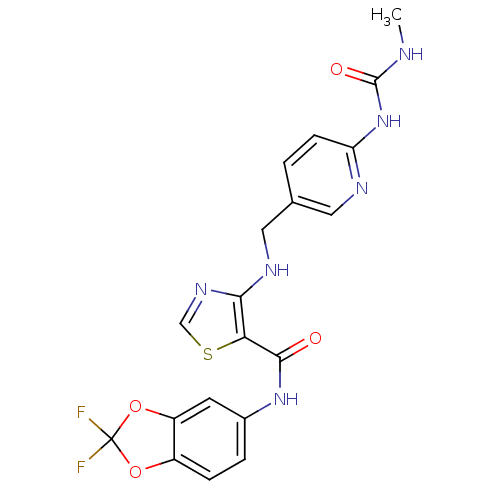
(1-(5-((5-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)ca...)Show SMILES CNC(=O)Nc1ccc(CNc2ncsc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)cn1 Show InChI InChI=1S/C19H16F2N6O4S/c1-22-18(29)27-14-5-2-10(7-23-14)8-24-16-15(32-9-25-16)17(28)26-11-3-4-12-13(6-11)31-19(20,21)30-12/h2-7,9,24H,8H2,1H3,(H,26,28)(H2,22,23,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215788
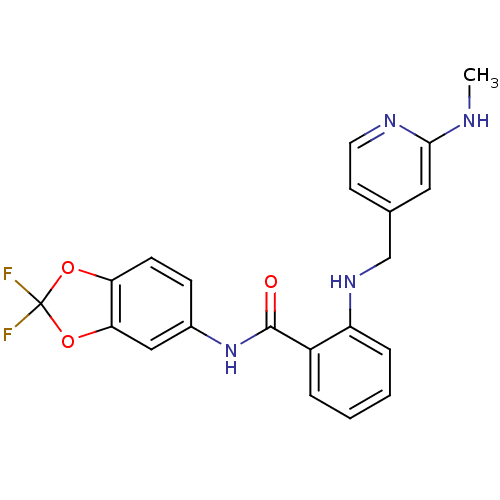
(CHEMBL399534 | N-(2,2-difluorobenzo[d][1,3]dioxol-...)Show SMILES CNc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C21H18F2N4O3/c1-24-19-10-13(8-9-25-19)12-26-16-5-3-2-4-15(16)20(28)27-14-6-7-17-18(11-14)30-21(22,23)29-17/h2-11,26H,12H2,1H3,(H,24,25)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215774
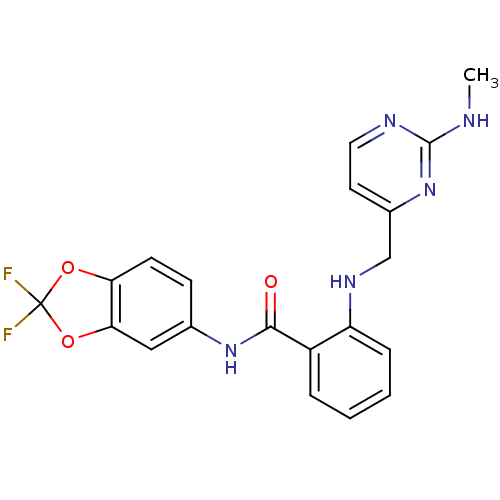
(CHEMBL246424 | N-(2,2-difluorobenzo[d][1,3]dioxol-...)Show SMILES CNc1nccc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)n1 Show InChI InChI=1S/C20H17F2N5O3/c1-23-19-24-9-8-13(27-19)11-25-15-5-3-2-4-14(15)18(28)26-12-6-7-16-17(10-12)30-20(21,22)29-16/h2-10,25H,11H2,1H3,(H,26,28)(H,23,24,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215793
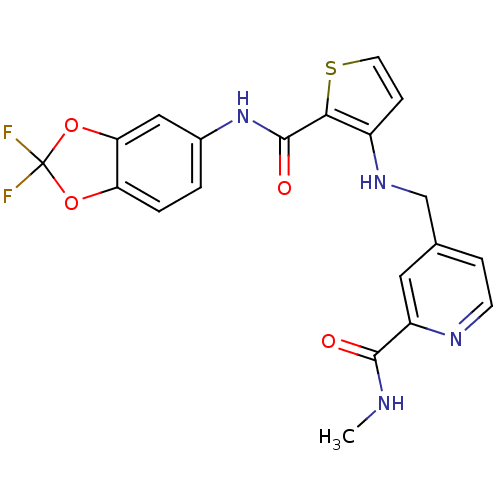
(4-((2-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)carba...)Show SMILES CNC(=O)c1cc(CNc2ccsc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C20H16F2N4O4S/c1-23-18(27)14-8-11(4-6-24-14)10-25-13-5-7-31-17(13)19(28)26-12-2-3-15-16(9-12)30-20(21,22)29-15/h2-9,25H,10H2,1H3,(H,23,27)(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139752

(US8895549, 14)Show SMILES COc1c(OC[C@H](O)CN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3C)=Nc12 |r,c:36,t:19| Show InChI InChI=1S/C25H30N6O5/c1-16-18(4-3-7-26-16)24(33)29-25-28-21-19(23-27-8-9-31(23)25)5-6-20(22(21)34-2)36-15-17(32)14-30-10-12-35-13-11-30/h3-7,17,32H,8-15H2,1-2H3,(H,28,29,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139749

(US8895549, 40)Show SMILES COc1c(OC[C@H](O)CN2CCCC2)ccc2C3=NCCN3C(NC(=O)c3cnco3)=Nc12 |r,c:33,t:18| Show InChI InChI=1S/C22H26N6O5/c1-31-19-16(32-12-14(29)11-27-7-2-3-8-27)5-4-15-18(19)25-22(28-9-6-24-20(15)28)26-21(30)17-10-23-13-33-17/h4-5,10,13-14,29H,2-3,6-9,11-12H2,1H3,(H,25,26,30)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.09 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139747
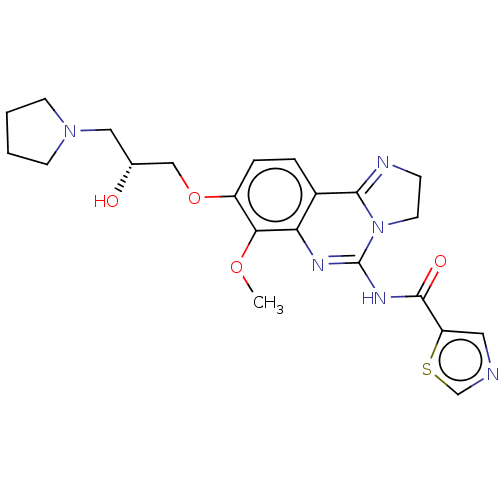
(US8895549, 36)Show SMILES COc1c(OC[C@H](O)CN2CCCC2)ccc2C3=NCCN3C(NC(=O)c3cncs3)=Nc12 |r,c:33,t:18| Show InChI InChI=1S/C22H26N6O4S/c1-31-19-16(32-12-14(29)11-27-7-2-3-8-27)5-4-15-18(19)25-22(28-9-6-24-20(15)28)26-21(30)17-10-23-13-33-17/h4-5,10,13-14,29H,2-3,6-9,11-12H2,1H3,(H,25,26,30)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139727
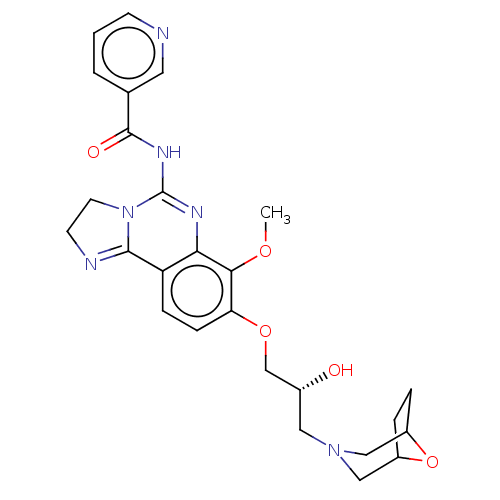
(US8895549, 5)Show SMILES COc1c(OC[C@H](O)CN2CC3CCC(C2)O3)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:38,t:22| Show InChI InChI=1S/C26H30N6O5/c1-35-23-21(36-15-17(33)12-31-13-18-4-5-19(14-31)37-18)7-6-20-22(23)29-26(32-10-9-28-24(20)32)30-25(34)16-3-2-8-27-11-16/h2-3,6-8,11,17-19,33H,4-5,9-10,12-15H2,1H3,(H,29,30,34)/t17-,18?,19?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139745
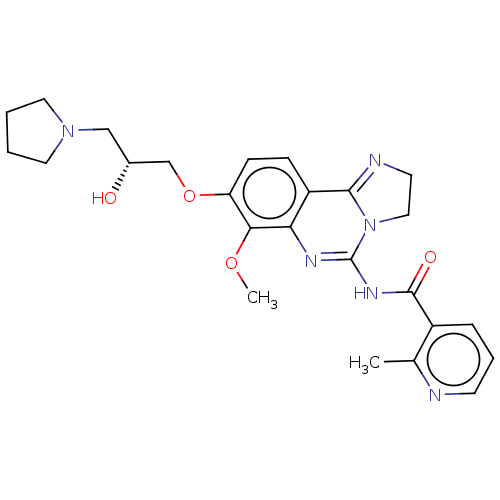
(US8895549, 18)Show SMILES COc1c(OC[C@H](O)CN2CCCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3C)=Nc12 |r,c:35,t:18| Show InChI InChI=1S/C25H30N6O4/c1-16-18(6-5-9-26-16)24(33)29-25-28-21-19(23-27-10-13-31(23)25)7-8-20(22(21)34-2)35-15-17(32)14-30-11-3-4-12-30/h5-9,17,32H,3-4,10-15H2,1-2H3,(H,28,29,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139729
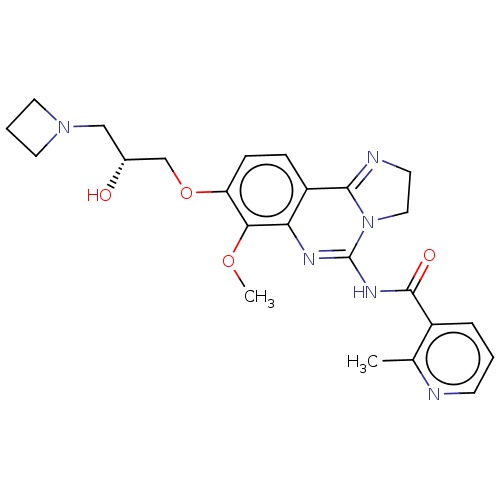
(US8895549, 16)Show SMILES COc1c(OC[C@H](O)CN2CCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3C)=Nc12 |r,c:34,t:17| Show InChI InChI=1S/C24H28N6O4/c1-15-17(5-3-8-25-15)23(32)28-24-27-20-18(22-26-9-12-30(22)24)6-7-19(21(20)33-2)34-14-16(31)13-29-10-4-11-29/h3,5-8,16,31H,4,9-14H2,1-2H3,(H,27,28,32)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215759
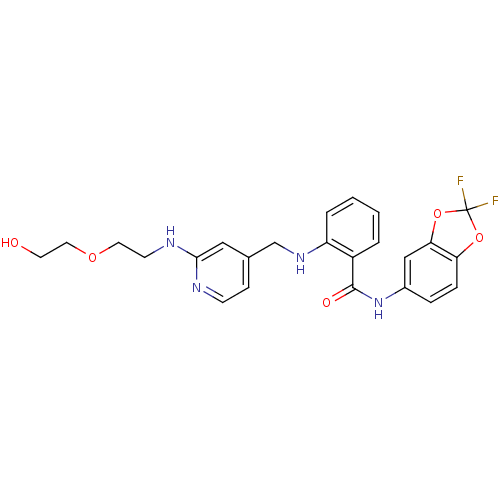
(CHEMBL247423 | N-(2,2-difluorobenzo[d][1,3]dioxol-...)Show SMILES OCCOCCNc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C24H24F2N4O5/c25-24(26)34-20-6-5-17(14-21(20)35-24)30-23(32)18-3-1-2-4-19(18)29-15-16-7-8-27-22(13-16)28-9-11-33-12-10-31/h1-8,13-14,29,31H,9-12,15H2,(H,27,28)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215758
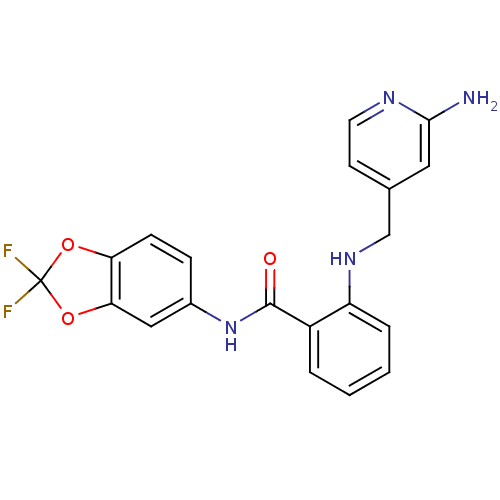
(2-((2-aminopyridin-4-yl)methylamino)-N-(2,2-difluo...)Show SMILES Nc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C20H16F2N4O3/c21-20(22)28-16-6-5-13(10-17(16)29-20)26-19(27)14-3-1-2-4-15(14)25-11-12-7-8-24-18(23)9-12/h1-10,25H,11H2,(H2,23,24)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215758

(2-((2-aminopyridin-4-yl)methylamino)-N-(2,2-difluo...)Show SMILES Nc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C20H16F2N4O3/c21-20(22)28-16-6-5-13(10-17(16)29-20)26-19(27)14-3-1-2-4-15(14)25-11-12-7-8-24-18(23)9-12/h1-10,25H,11H2,(H2,23,24)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
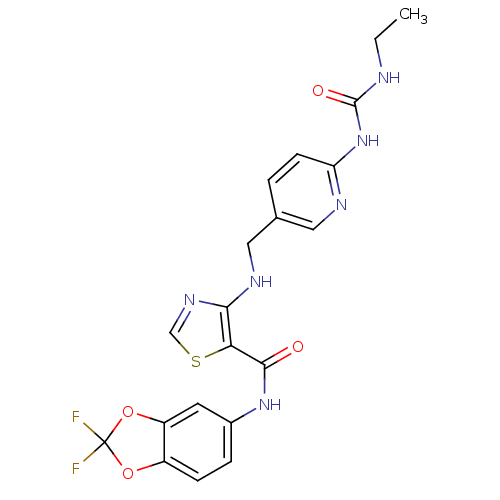
(Homo sapiens (Human)) | BDBM50215770
(1-(5-((5-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)ca...)Show SMILES CCNC(=O)Nc1ccc(CNc2ncsc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)cn1 Show InChI InChI=1S/C20H18F2N6O4S/c1-2-23-19(30)28-15-6-3-11(8-24-15)9-25-17-16(33-10-26-17)18(29)27-12-4-5-13-14(7-12)32-20(21,22)31-13/h3-8,10,25H,2,9H2,1H3,(H,27,29)(H2,23,24,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50114250
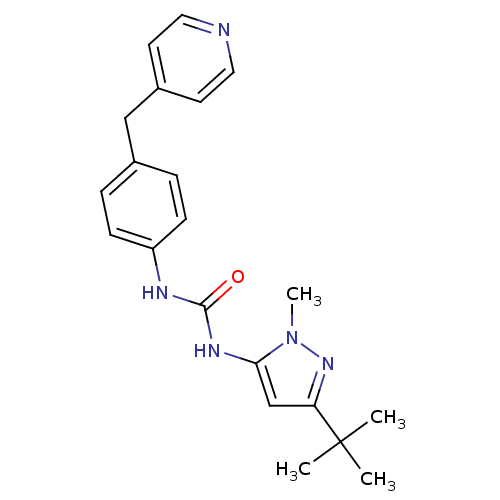
(1-(5-tert-Butyl-2-methyl-2H-pyrazol-3-yl)-3-(4-pyr...)Show SMILES Cn1nc(cc1NC(=O)Nc1ccc(Cc2ccncc2)cc1)C(C)(C)C Show InChI InChI=1S/C21H25N5O/c1-21(2,3)18-14-19(26(4)25-18)24-20(27)23-17-7-5-15(6-8-17)13-16-9-11-22-12-10-16/h5-12,14H,13H2,1-4H3,(H2,23,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human epidermal growth factor receptor-2 |
Bioorg Med Chem Lett 12: 1559-62 (2002)
BindingDB Entry DOI: 10.7270/Q2CV4H28 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139738
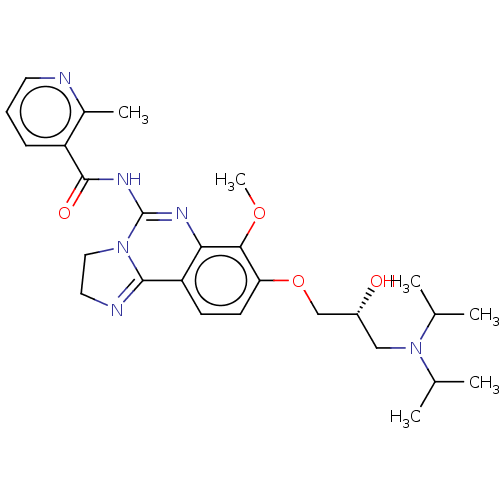
(US8895549, 20)Show SMILES COc1c(OC[C@H](O)CN(C(C)C)C(C)C)ccc2C3=NCCN3C(NC(=O)c3cccnc3C)=Nc12 |r,c:36,t:19| Show InChI InChI=1S/C27H36N6O4/c1-16(2)33(17(3)4)14-19(34)15-37-22-10-9-21-23(24(22)36-6)30-27(32-13-12-29-25(21)32)31-26(35)20-8-7-11-28-18(20)5/h7-11,16-17,19,34H,12-15H2,1-6H3,(H,30,31,35)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139731

(US8895549, 28)Show SMILES COc1c(OC[C@H](O)CN2CC3CCC(C2)O3)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |r,c:39,t:22| Show InChI InChI=1S/C25H30N8O5/c1-36-21-19(37-13-15(34)10-32-11-16-2-3-17(12-32)38-16)5-4-18-20(21)30-25(33-7-6-27-22(18)33)31-23(35)14-8-28-24(26)29-9-14/h4-5,8-9,15-17,34H,2-3,6-7,10-13H2,1H3,(H2,26,28,29)(H,30,31,35)/t15-,16?,17?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139737

(US8895549, 12)Show SMILES COc1c(OC[C@H](O)CN(C(C)C)C(C)C)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:35,t:19| Show InChI InChI=1S/C26H34N6O4/c1-16(2)32(17(3)4)14-19(33)15-36-21-9-8-20-22(23(21)35-5)29-26(31-12-11-28-24(20)31)30-25(34)18-7-6-10-27-13-18/h6-10,13,16-17,19,33H,11-12,14-15H2,1-5H3,(H,29,30,34)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139724

(US8895549, 4 | US9675616, 4 N-[8-({(2R)-3-[(2R,6S)...)Show SMILES COc1c(OC[C@H](O)CN2C[C@H](C)O[C@H](C)C2)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:37,t:21| Show InChI InChI=1S/C26H32N6O5/c1-16-12-31(13-17(2)37-16)14-19(33)15-36-21-7-6-20-22(23(21)35-3)29-26(32-10-9-28-24(20)32)30-25(34)18-5-4-8-27-11-18/h4-8,11,16-17,19,33H,9-10,12-15H2,1-3H3,(H,29,30,34)/t16-,17+,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.85 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215761

(1-(5-((5-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)ca...)Show SMILES CNC(=O)Nc1ccc(CNc2ncsc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)cn1 Show InChI InChI=1S/C19H16F2N6O4S/c1-22-18(29)27-14-5-2-10(7-23-14)8-24-16-15(32-9-25-16)17(28)26-11-3-4-12-13(6-11)31-19(20,21)30-12/h2-7,9,24H,8H2,1H3,(H,26,28)(H2,22,23,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215775

(1-(4-((2-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)ca...)Show SMILES CNC(=O)Nc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C22H19F2N5O4/c1-25-21(31)29-19-10-13(8-9-26-19)12-27-16-5-3-2-4-15(16)20(30)28-14-6-7-17-18(11-14)33-22(23,24)32-17/h2-11,27H,12H2,1H3,(H,28,30)(H2,25,26,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215773
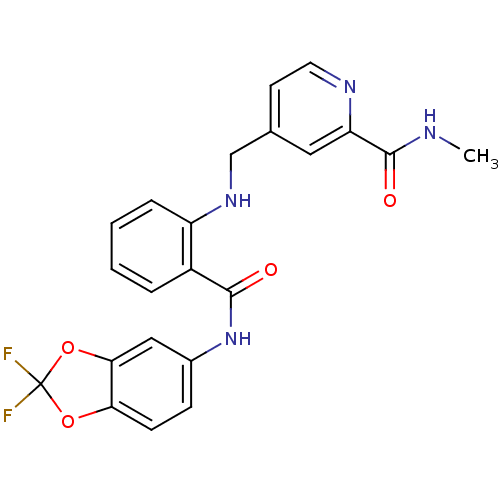
(4-((2-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)carba...)Show SMILES CNC(=O)c1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C22H18F2N4O4/c1-25-21(30)17-10-13(8-9-26-17)12-27-16-5-3-2-4-15(16)20(29)28-14-6-7-18-19(11-14)32-22(23,24)31-18/h2-11,27H,12H2,1H3,(H,25,30)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50215791

(2-((2-acetamidopyridin-4-yl)methylamino)-N-(2,2-di...)Show SMILES CC(=O)Nc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C22H18F2N4O4/c1-13(29)27-20-10-14(8-9-25-20)12-26-17-5-3-2-4-16(17)21(30)28-15-6-7-18-19(11-15)32-22(23,24)31-18/h2-11,26H,12H2,1H3,(H,28,30)(H,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of b-Raf |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139764

(US8895549, 39)Show SMILES COc1c(OC[C@H](O)CN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3sc(N)nc3C)=Nc12 |r,c:36,t:19| Show InChI InChI=1S/C23H29N7O5S/c1-13-19(36-22(24)26-13)21(32)28-23-27-17-15(20-25-5-6-30(20)23)3-4-16(18(17)33-2)35-12-14(31)11-29-7-9-34-10-8-29/h3-4,14,31H,5-12H2,1-2H3,(H2,24,26)(H,27,28,32)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139730

(US8895549, 17)Show SMILES COc1c(OC[C@H](O)CN2C[C@H](C)O[C@H](C)C2)ccc2C3=NCCN3C(NC(=O)c3cccnc3C)=Nc12 |r,c:38,t:21| Show InChI InChI=1S/C27H34N6O5/c1-16-12-32(13-17(2)38-16)14-19(34)15-37-22-8-7-21-23(24(22)36-4)30-27(33-11-10-29-25(21)33)31-26(35)20-6-5-9-28-18(20)3/h5-9,16-17,19,34H,10-15H2,1-4H3,(H,30,31,35)/t16-,17+,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139735
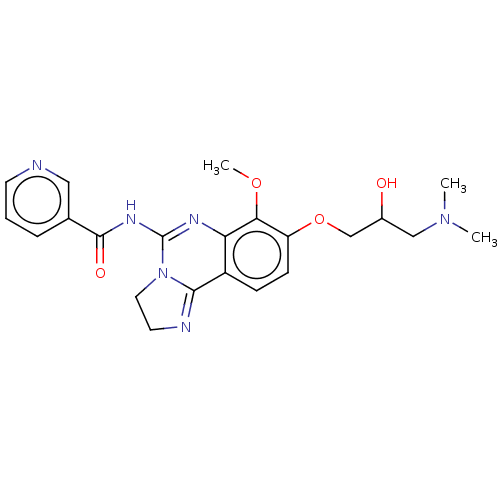
(US8895549, 10)Show SMILES COc1c(OCC(O)CN(C)C)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |c:31,t:15| Show InChI InChI=1S/C22H26N6O4/c1-27(2)12-15(29)13-32-17-7-6-16-18(19(17)31-3)25-22(28-10-9-24-20(16)28)26-21(30)14-5-4-8-23-11-14/h4-8,11,15,29H,9-10,12-13H2,1-3H3,(H,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139720
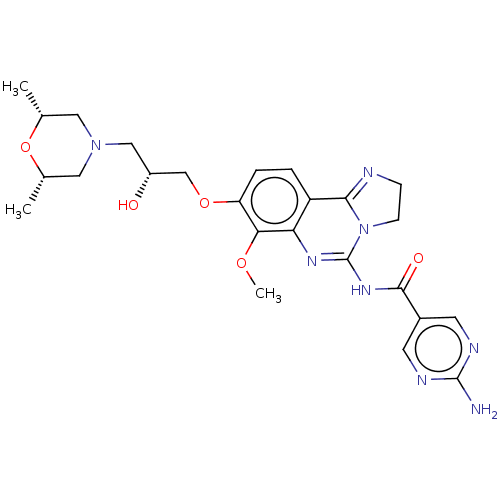
(US8895549, 27)Show SMILES COc1c(OC[C@H](O)CN2C[C@H](C)O[C@H](C)C2)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |r,c:38,t:21| Show InChI InChI=1S/C25H32N8O5/c1-14-10-32(11-15(2)38-14)12-17(34)13-37-19-5-4-18-20(21(19)36-3)30-25(33-7-6-27-22(18)33)31-23(35)16-8-28-24(26)29-9-16/h4-5,8-9,14-15,17,34H,6-7,10-13H2,1-3H3,(H2,26,28,29)(H,30,31,35)/t14-,15+,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139748

(US8895549, 38)Show SMILES COc1c(OC[C@H](O)CN2CCCC2)ccc2C3=NCCN3C(NC(=O)c3scnc3C)=Nc12 |r,c:34,t:18| Show InChI InChI=1S/C23H28N6O4S/c1-14-20(34-13-25-14)22(31)27-23-26-18-16(21-24-7-10-29(21)23)5-6-17(19(18)32-2)33-12-15(30)11-28-8-3-4-9-28/h5-6,13,15,30H,3-4,7-12H2,1-2H3,(H,26,27,31)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139740
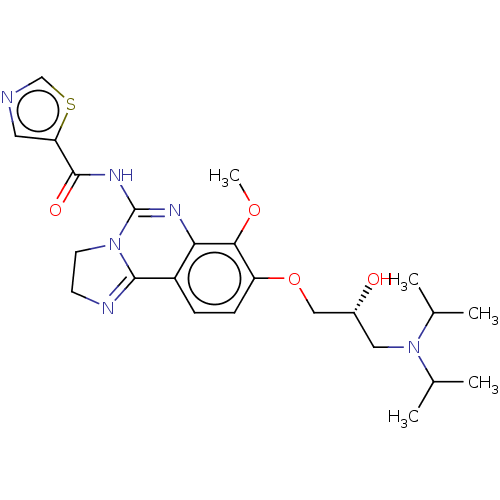
(US8895549, 41)Show SMILES COc1c(OC[C@H](O)CN(C(C)C)C(C)C)ccc2C3=NCCN3C(NC(=O)c3cncs3)=Nc12 |r,c:34,t:19| Show InChI InChI=1S/C24H32N6O4S/c1-14(2)30(15(3)4)11-16(31)12-34-18-7-6-17-20(21(18)33-5)27-24(29-9-8-26-22(17)29)28-23(32)19-10-25-13-35-19/h6-7,10,13-16,31H,8-9,11-12H2,1-5H3,(H,27,28,32)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139761
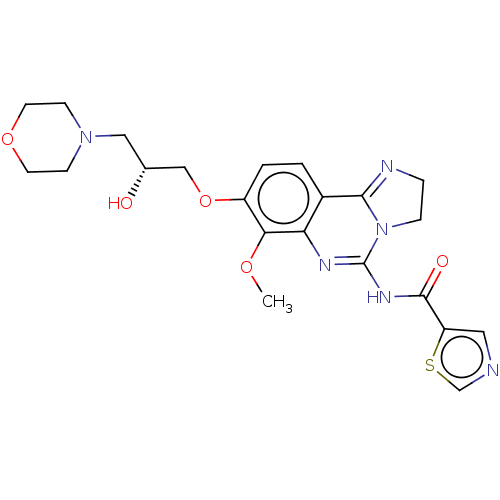
(US8895549, 32)Show SMILES COc1c(OC[C@H](O)CN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cncs3)=Nc12 |r,c:34,t:19| Show InChI InChI=1S/C22H26N6O5S/c1-31-19-16(33-12-14(29)11-27-6-8-32-9-7-27)3-2-15-18(19)25-22(28-5-4-24-20(15)28)26-21(30)17-10-23-13-34-17/h2-3,10,13-14,29H,4-9,11-12H2,1H3,(H,25,26,30)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50095409
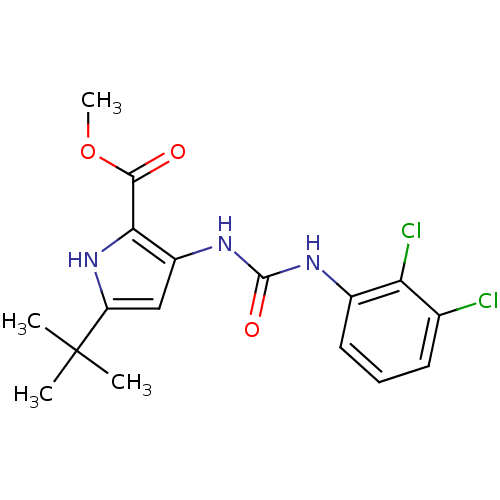
(5-tert-Butyl-3-[3-(2,3-dichloro-phenyl)-ureido]-1H...)Show SMILES COC(=O)c1[nH]c(cc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C17H19Cl2N3O3/c1-17(2,3)12-8-11(14(22-12)15(23)25-4)21-16(24)20-10-7-5-6-9(18)13(10)19/h5-8,22H,1-4H3,(H2,20,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coli |
Bioorg Med Chem Lett 11: 9-12 (2001)
BindingDB Entry DOI: 10.7270/Q2JM28WH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139721
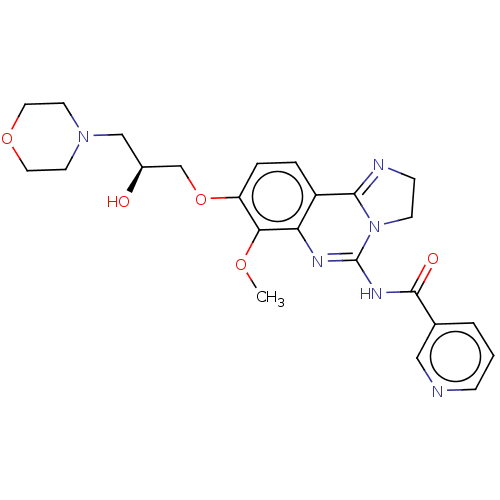
(US8895549, 3 | US9675616, 3 N-(8-{[(2S)-2-hydroxy-...)Show SMILES COc1c(OC[C@@H](O)CN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3cccnc3)=Nc12 |r,c:35,t:19| Show InChI InChI=1S/C24H28N6O5/c1-33-21-19(35-15-17(31)14-29-9-11-34-12-10-29)5-4-18-20(21)27-24(30-8-7-26-22(18)30)28-23(32)16-3-2-6-25-13-16/h2-6,13,17,31H,7-12,14-15H2,1H3,(H,27,28,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139733

(US8895549, 35)Show SMILES COc1c(OC[C@H](O)CN2CCC2)ccc2C3=NCCN3C(NC(=O)c3cncs3)=Nc12 |r,c:32,t:17| Show InChI InChI=1S/C21H24N6O4S/c1-30-18-15(31-11-13(28)10-26-6-2-7-26)4-3-14-17(18)24-21(27-8-5-23-19(14)27)25-20(29)16-9-22-12-32-16/h3-4,9,12-13,28H,2,5-8,10-11H2,1H3,(H,24,25,29)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139739
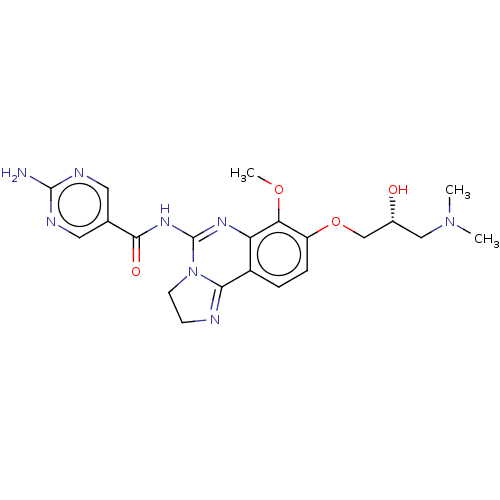
(US8895549, 29)Show SMILES COc1c(OC[C@H](O)CN(C)C)ccc2C3=NCCN3C(NC(=O)c3cnc(N)nc3)=Nc12 |r,c:32,t:15| Show InChI InChI=1S/C21H26N8O4/c1-28(2)10-13(30)11-33-15-5-4-14-16(17(15)32-3)26-21(29-7-6-23-18(14)29)27-19(31)12-8-24-20(22)25-9-12/h4-5,8-9,13,30H,6-7,10-11H2,1-3H3,(H2,22,24,25)(H,26,27,31)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50215770

(1-(5-((5-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)ca...)Show SMILES CCNC(=O)Nc1ccc(CNc2ncsc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)cn1 Show InChI InChI=1S/C20H18F2N6O4S/c1-2-23-19(30)28-15-6-3-11(8-24-15)9-25-17-16(33-10-26-17)18(29)27-12-4-5-13-14(7-12)32-20(21,22)31-13/h3-8,10,25H,2,9H2,1H3,(H,27,29)(H2,23,24,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215789
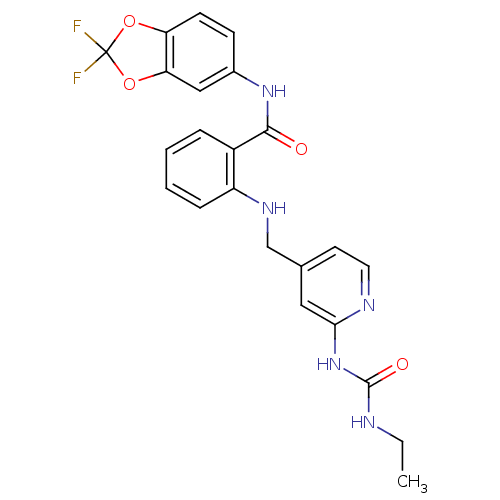
(1-(4-((2-((2,2-difluorobenzo[d][1,3]dioxol-5-yl)ca...)Show SMILES CCNC(=O)Nc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C23H21F2N5O4/c1-2-26-22(32)30-20-11-14(9-10-27-20)13-28-17-6-4-3-5-16(17)21(31)29-15-7-8-18-19(12-15)34-23(24,25)33-18/h3-12,28H,2,13H2,1H3,(H,29,31)(H2,26,27,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139732
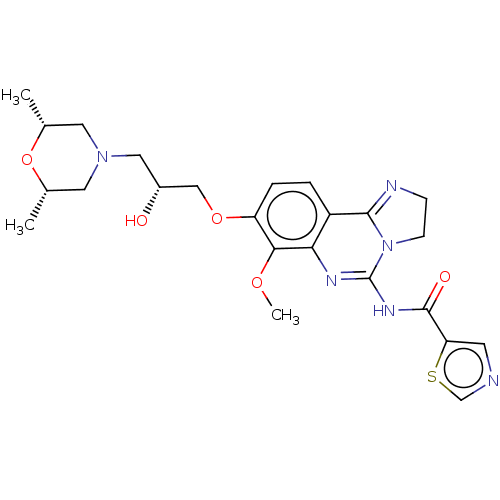
(US8895549, 34)Show SMILES COc1c(OC[C@H](O)CN2C[C@H](C)O[C@H](C)C2)ccc2C3=NCCN3C(NC(=O)c3cncs3)=Nc12 |r,c:36,t:21| Show InChI InChI=1S/C24H30N6O5S/c1-14-9-29(10-15(2)35-14)11-16(31)12-34-18-5-4-17-20(21(18)33-3)27-24(30-7-6-26-22(17)30)28-23(32)19-8-25-13-36-19/h4-5,8,13-16,31H,6-7,9-12H2,1-3H3,(H,27,28,32)/t14-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM139756
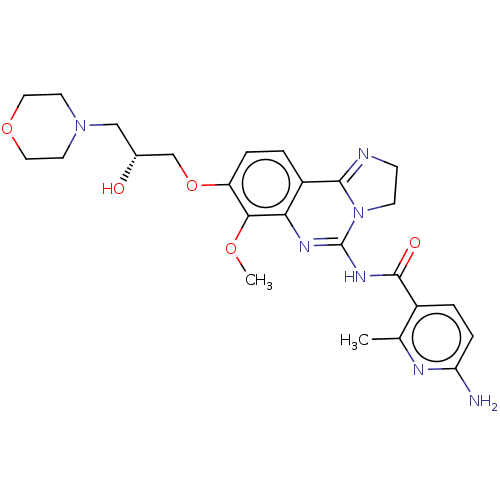
(US8895549, 23)Show SMILES COc1c(OC[C@H](O)CN2CCOCC2)ccc2C3=NCCN3C(NC(=O)c3ccc(N)nc3C)=Nc12 |r,c:37,t:19| Show InChI InChI=1S/C25H31N7O5/c1-15-17(4-6-20(26)28-15)24(34)30-25-29-21-18(23-27-7-8-32(23)25)3-5-19(22(21)35-2)37-14-16(33)13-31-9-11-36-12-10-31/h3-6,16,33H,7-14H2,1-2H3,(H2,26,28)(H,29,30,34)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH
US Patent
| Assay Description
As reagents for the kinase reaction itself and the quantification of the reaction product, the PI3-Kinase HTRF Assay kit from Millipore (#33-017) was... |
US Patent US8895549 (2014)
BindingDB Entry DOI: 10.7270/Q2NC5ZWF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50215782
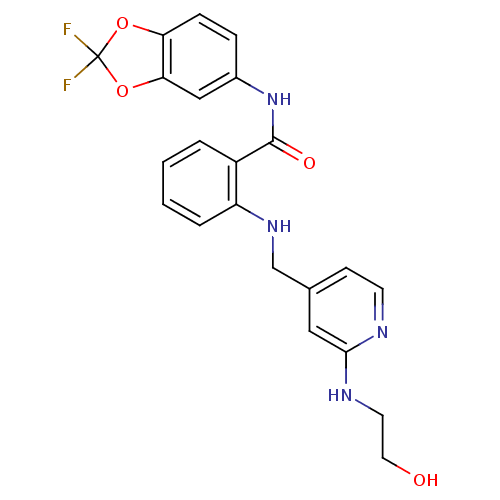
(CHEMBL398052 | N-(2,2-difluorobenzo[d][1,3]dioxol-...)Show SMILES OCCNc1cc(CNc2ccccc2C(=O)Nc2ccc3OC(F)(F)Oc3c2)ccn1 Show InChI InChI=1S/C22H20F2N4O4/c23-22(24)31-18-6-5-15(12-19(18)32-22)28-21(30)16-3-1-2-4-17(16)27-13-14-7-8-25-20(11-14)26-9-10-29/h1-8,11-12,27,29H,9-10,13H2,(H,25,26)(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cKit by FRET assay |
Bioorg Med Chem Lett 17: 4378-81 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.075
BindingDB Entry DOI: 10.7270/Q28K78S7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data