

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014174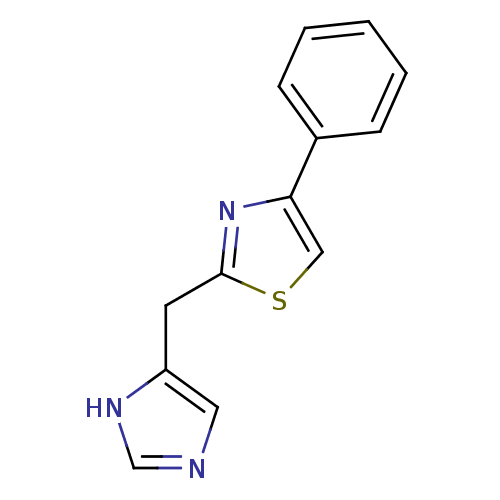 (2-(1H-Imidazol-4-ylmethyl)-4-phenyl-thiazole | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013040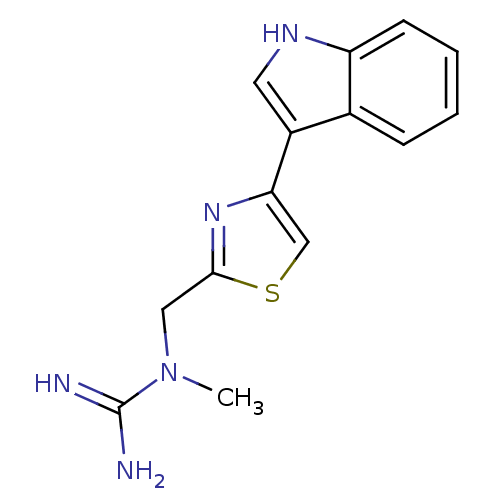 (CHEMBL93244 | N-[4-(1H-Indol-3-yl)-thiazol-2-ylmet...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013044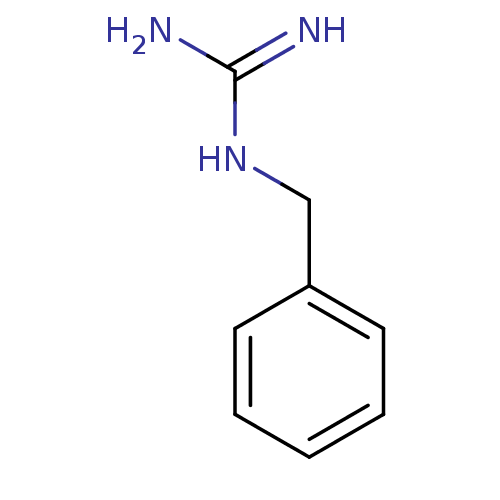 (1-Benzylguanidine | CHEMBL288640 | N-Benzyl-guanid...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013045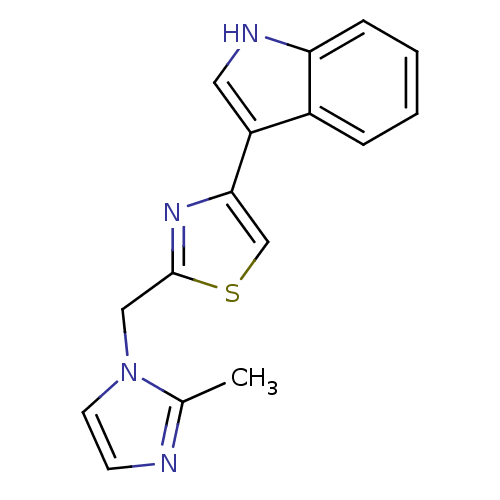 (3-[2-(2-Methyl-imidazol-1-ylmethyl)-thiazol-4-yl]-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013046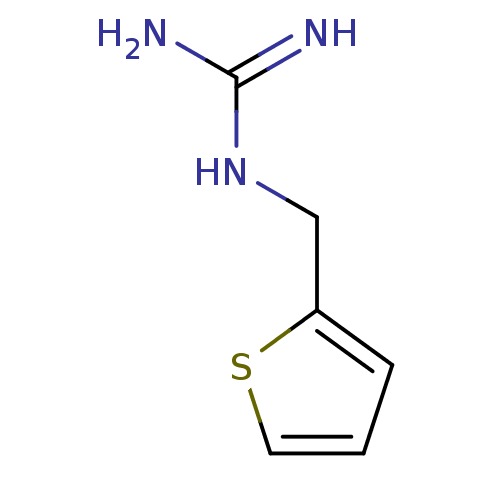 (1-(Thiophen-2-ylmethyl)guanidine | CHEMBL93064 | N...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | >1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Mus musculus (house mouse)) | BDBM50000492 ((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Antibacterial activity against Escherichia coli DHFR | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007692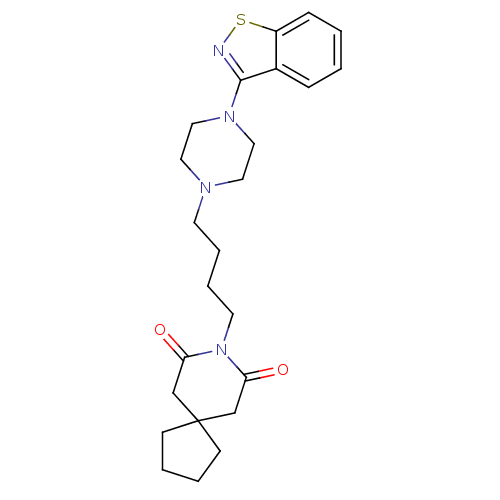 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048806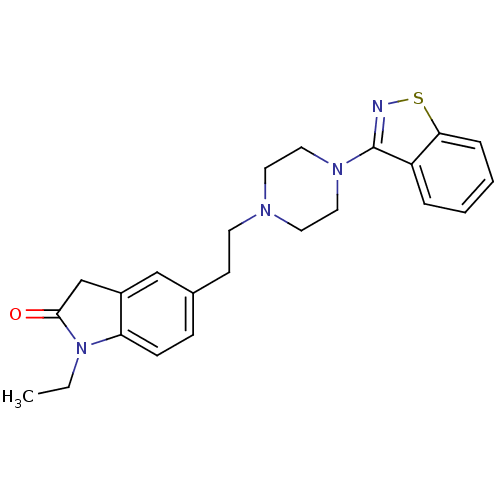 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048807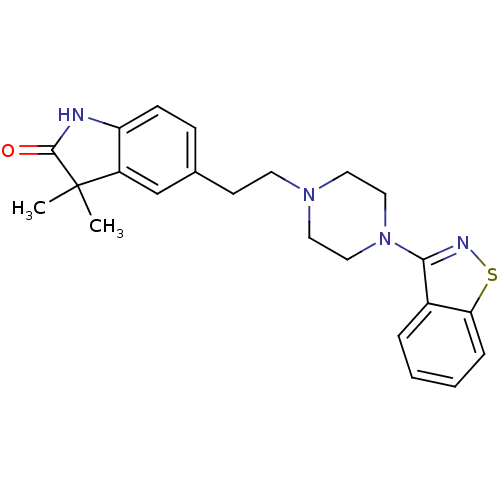 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50013039 (CHEMBL40260 | N-[4-(1H-Indol-3-yl)-thiazol-2-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013039 (CHEMBL40260 | N-[4-(1H-Indol-3-yl)-thiazol-2-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of dihydrofolate reductase of Escherichia coli | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048804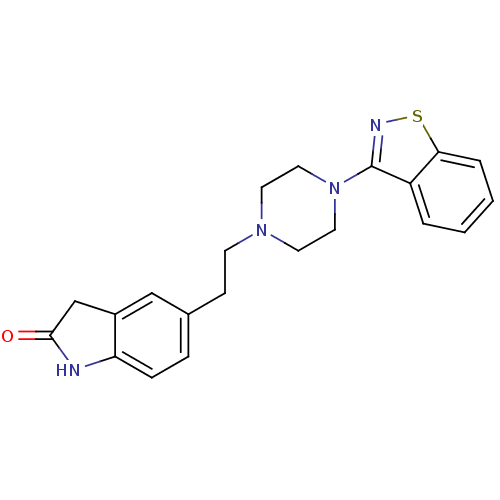 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048802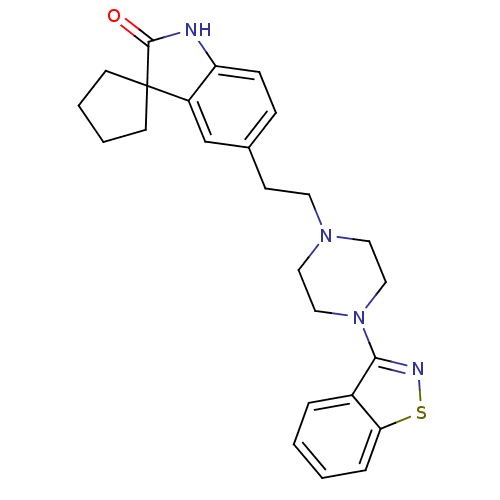 (5'-[2-(4-benzo[d]isothiazol-3-ylhexahydro-1-pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048805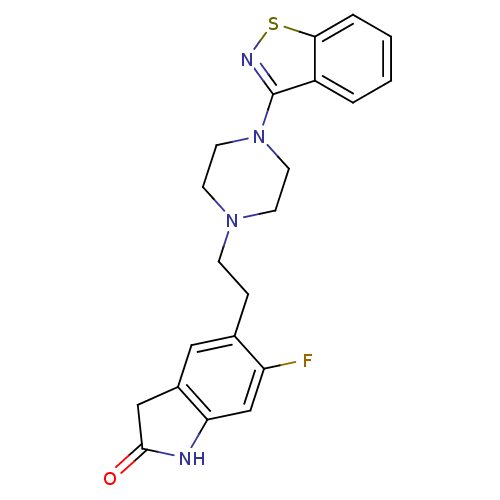 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048800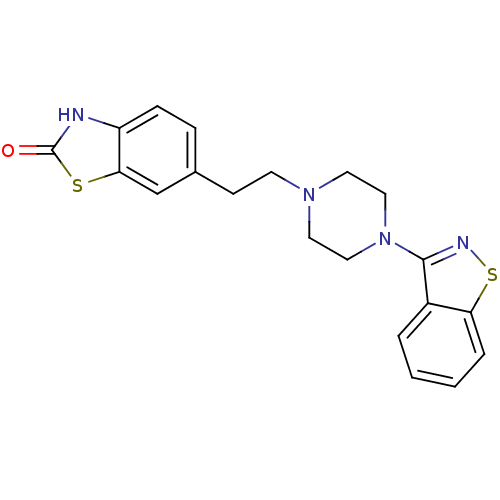 (6-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048801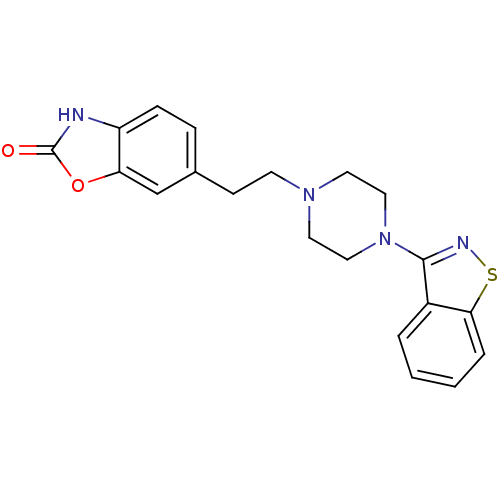 (6-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50014407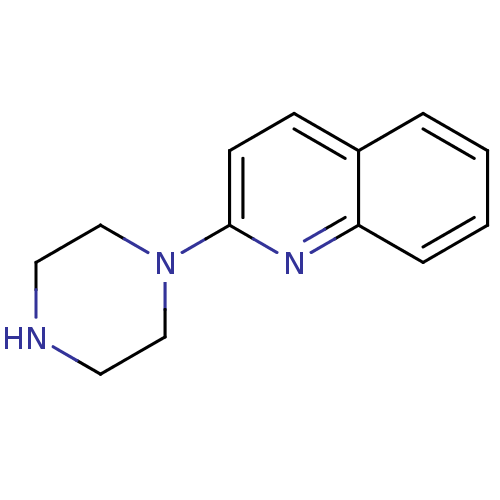 (2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013043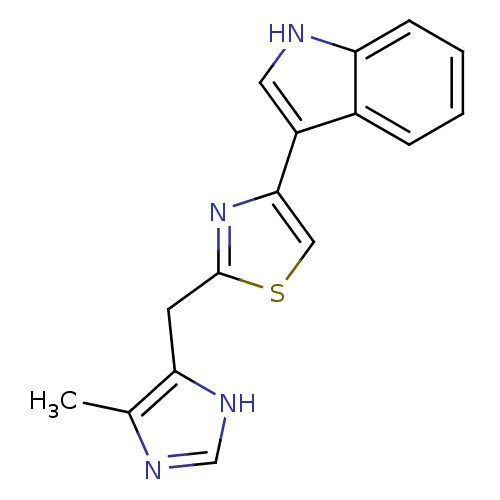 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013043 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014600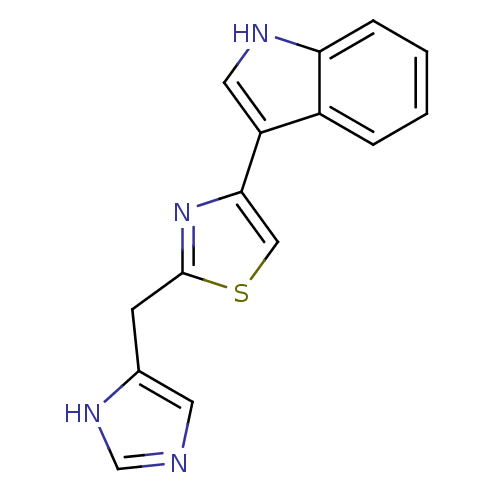 (3-[2-(1H-Imidazol-4-ylmethyl)-thiazol-4-yl]-1H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50033658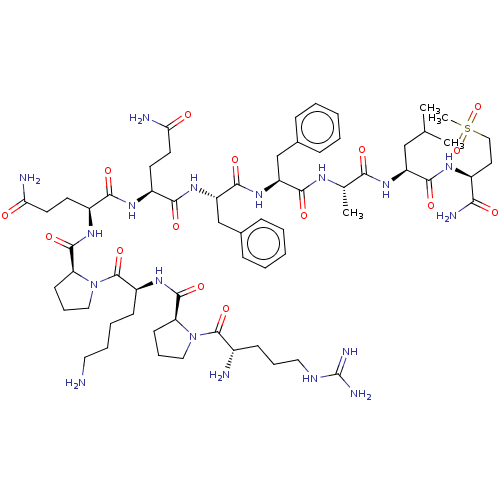 (Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Sarcosine-Leu-Met(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity against NK1 receptor | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to displace [3H]-ketanserin from 5-hydroxytryptamine 2A receptor in rat brain. | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity against NK1 receptor | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013038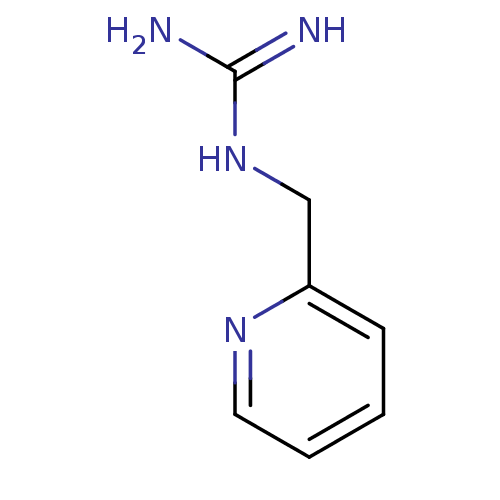 (1-(Pyridin-3-ylmethyl)guanidine | CHEMBL93964 | N-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3B (Mus musculus) | BDBM50013042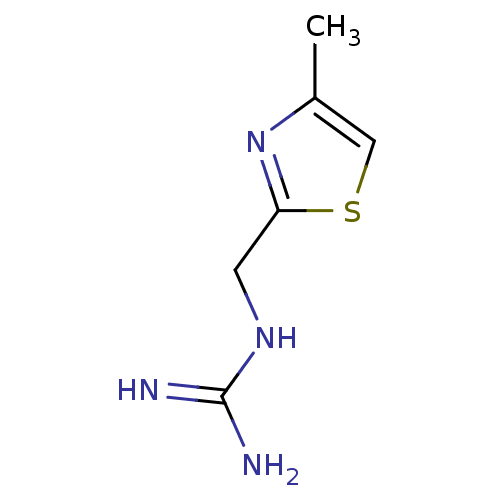 (CHEMBL92180 | N-(4-Methyl-thiazol-2-ylmethyl)-guan...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro binding affinity for the 5-hydroxytryptamine 3 receptor was determined with NG-108-15 mouse neuroblastoma-glioma cells | J Med Chem 33: 2721-5 (1990) BindingDB Entry DOI: 10.7270/Q2CJ8F2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014601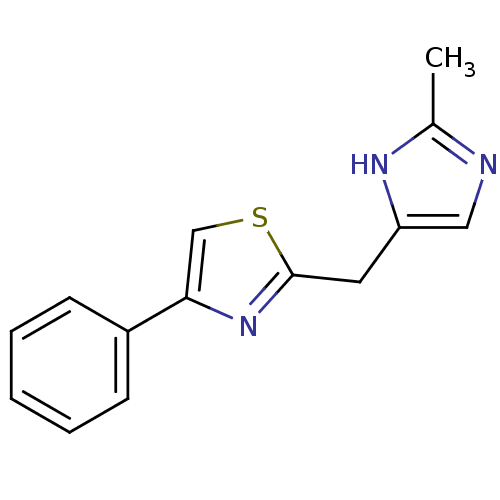 (2-(2-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50222218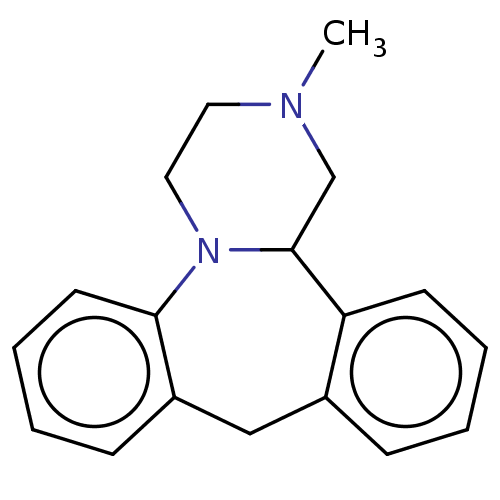 (CHEBI:51137 | Mianserin) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 361 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013045 (3-[2-(2-Methyl-imidazol-1-ylmethyl)-thiazol-4-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014406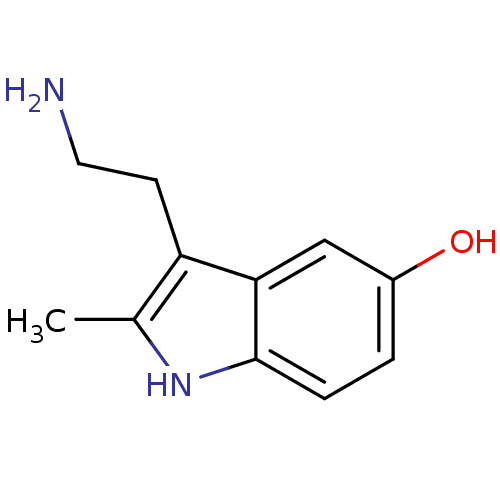 (2-Me 5-HT | 2-Methyl-5-hydroxytryptamine | 2-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Mus musculus (house mouse)) | BDBM50014406 (2-Me 5-HT | 2-Methyl-5-hydroxytryptamine | 2-methy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description The binding affinity was measured for 5-hydroxytryptamine 3 receptor on NG 108-15 cell line of mouse neuroblastoma-glioma cells in presence of [3H]5 ... | J Med Chem 33: 3020-3 (1990) BindingDB Entry DOI: 10.7270/Q2VM4FGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50001447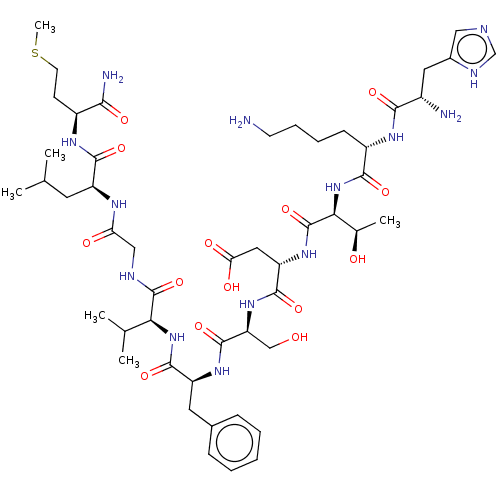 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity against NK2 receptor | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20954 (2-(4-methoxy-2,6-dimethylphenoxy)-3,6-dimethyl-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007558 (6-[2-(4-Naphthalen-1-yl-piperazin-1-yl)-ethyl]-3H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Rattus norvegicus (rat)) | BDBM20950 (2-(4-bromo-2,6-dimethylphenoxy)-3,6-dimethyl-4-(pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Pfizer | Assay Description The IC50 values were measured by monitoring the inhibition effects of test compounds on [125I]-o-CRF binding to rat cortex in vitro. | J Med Chem 51: 1377-84 (2008) Article DOI: 10.1021/jm070578k BindingDB Entry DOI: 10.7270/Q2F47MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007563 (1-Ethyl-5-[2-(4-naphthalen-1-yl-piperazin-1-yl)-et...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5-hydroxytryptamine 1A receptor in the rat brain using [3H]-8-hydroxy-2-(di-n-propylamine)tetralin as radioligand. | J Med Chem 34: 1860-6 (1991) BindingDB Entry DOI: 10.7270/Q2Q52NK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |