 Found 233 hits with Last Name = 'selby' and Initial = 'md'
Found 233 hits with Last Name = 'selby' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880
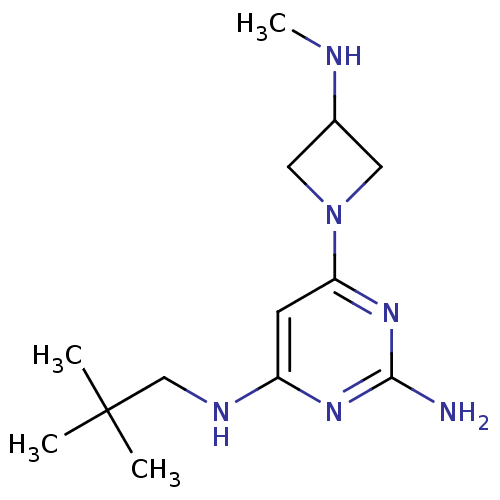
(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343152
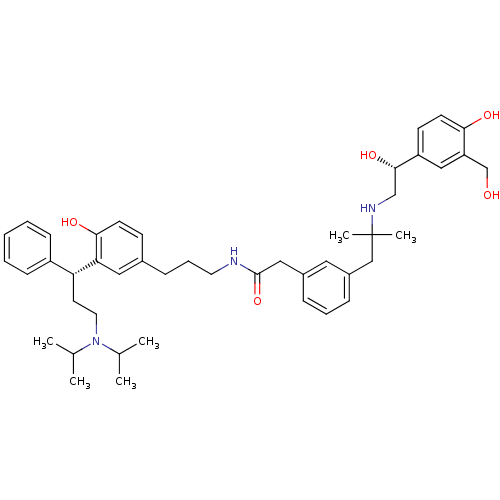
(CHEMBL1773196 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(CO)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H61N3O5/c1-31(2)48(32(3)4)23-21-39(36-15-8-7-9-16-36)40-25-33(17-19-42(40)51)14-11-22-46-44(53)26-34-12-10-13-35(24-34)28-45(5,6)47-29-43(52)37-18-20-41(50)38(27-37)30-49/h7-10,12-13,15-20,24-25,27,31-32,39,43,47,49-52H,11,14,21-23,26,28-30H2,1-6H3,(H,46,53)/t39-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50128835
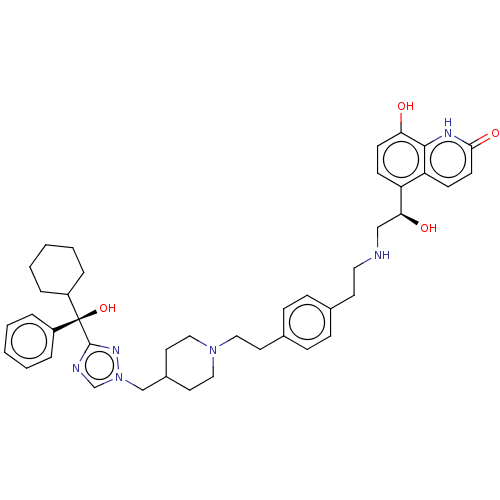
(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M2 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M1 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343153
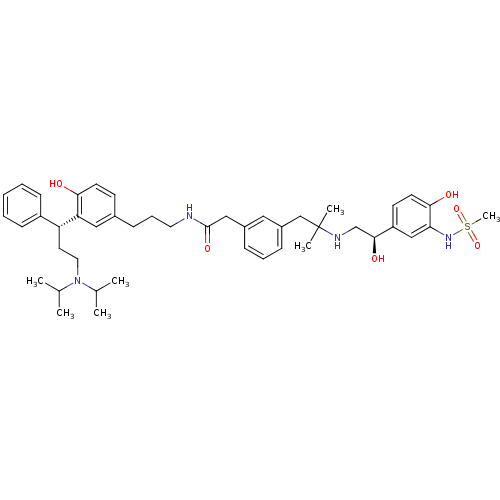
(CHEMBL1773197 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(CC(C)(C)NC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C45H62N4O6S/c1-31(2)49(32(3)4)24-22-38(36-16-9-8-10-17-36)39-26-33(18-20-41(39)50)15-12-23-46-44(53)27-34-13-11-14-35(25-34)29-45(5,6)47-30-43(52)37-19-21-42(51)40(28-37)48-56(7,54)55/h8-11,13-14,16-21,25-26,28,31-32,38,43,47-48,50-52H,12,15,22-24,27,29-30H2,1-7H3,(H,46,53)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343161
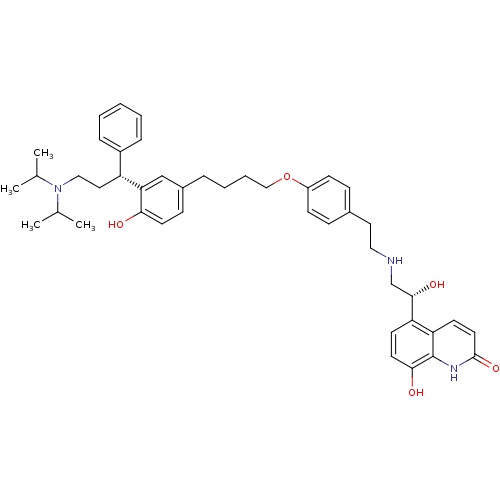
(5-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H55N3O5/c1-30(2)47(31(3)4)26-24-36(34-11-6-5-7-12-34)39-28-33(15-20-40(39)48)10-8-9-27-52-35-16-13-32(14-17-35)23-25-45-29-42(50)37-18-21-41(49)44-38(37)19-22-43(51)46-44/h5-7,11-22,28,30-31,36,42,45,48-50H,8-10,23-27,29H2,1-4H3,(H,46,51)/t36-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.305 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343161

(5-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c4[nH]c(=O)ccc34)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H55N3O5/c1-30(2)47(31(3)4)26-24-36(34-11-6-5-7-12-34)39-28-33(15-20-40(39)48)10-8-9-27-52-35-16-13-32(14-17-35)23-25-45-29-42(50)37-18-21-41(49)44-38(37)19-22-43(51)46-44/h5-7,11-22,28,30-31,36,42,45,48-50H,8-10,23-27,29H2,1-4H3,(H,46,51)/t36-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128837
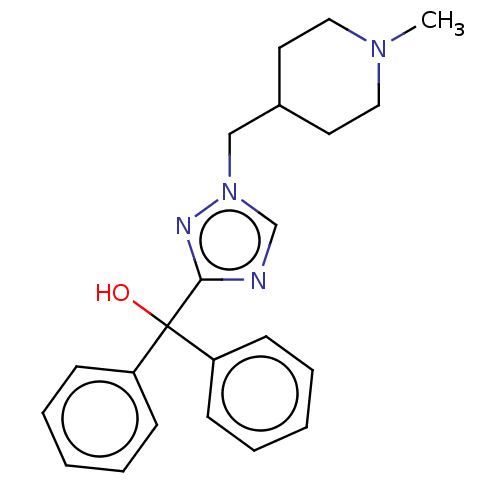
(CHEMBL3629354)Show SMILES CN1CCC(Cn2cnc(n2)C(O)(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C22H26N4O/c1-25-14-12-18(13-15-25)16-26-17-23-21(24-26)22(27,19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-11,17-18,27H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M4 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343154
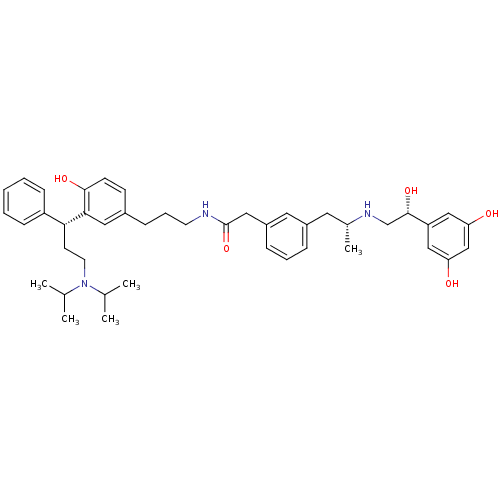
(2-(3-((R)-2-((R)-2-(3,5-dihydroxyphenyl)-2-hydroxy...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)Cc2cccc(C[C@@H](C)NC[C@H](O)c3cc(O)cc(O)c3)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C43H57N3O5/c1-29(2)46(30(3)4)20-18-39(35-14-7-6-8-15-35)40-23-32(16-17-41(40)49)13-10-19-44-43(51)24-34-12-9-11-33(22-34)21-31(5)45-28-42(50)36-25-37(47)27-38(48)26-36/h6-9,11-12,14-17,22-23,25-27,29-31,39,42,45,47-50H,10,13,18-21,24,28H2,1-5H3,(H,44,51)/t31-,39-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.397 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343157
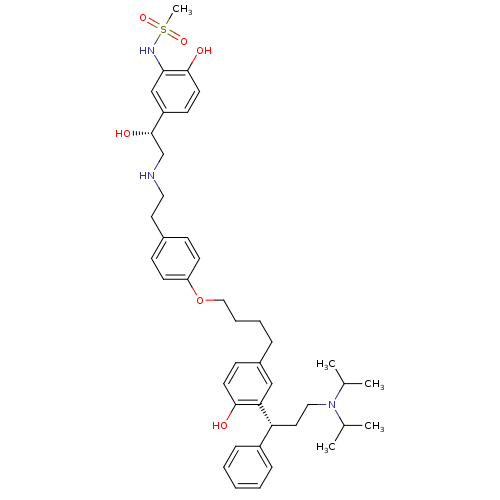
(CHEMBL1773264 | N-(5-((R)-2-(4-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(NS(C)(=O)=O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H57N3O6S/c1-30(2)45(31(3)4)25-23-37(34-12-7-6-8-13-34)38-27-33(16-20-40(38)46)11-9-10-26-51-36-18-14-32(15-19-36)22-24-43-29-42(48)35-17-21-41(47)39(28-35)44-52(5,49)50/h6-8,12-21,27-28,30-31,37,42-44,46-48H,9-11,22-26,29H2,1-5H3/t37-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.634 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128830

(CHEMBL3629353)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(CC1)OC(=O)Nc1ccccc1-c1ccc(O)c(Cl)c1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C38H47ClN4O6/c39-31-24-26(12-15-33(31)44)28-10-6-7-11-32(28)41-38(48)49-27-18-22-43(23-19-27)21-9-5-3-1-2-4-8-20-40-25-35(46)29-13-16-34(45)37-30(29)14-17-36(47)42-37/h6-7,10-17,24,27,35,40,44-46H,1-5,8-9,18-23,25H2,(H,41,48)(H,42,47)/t35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343158
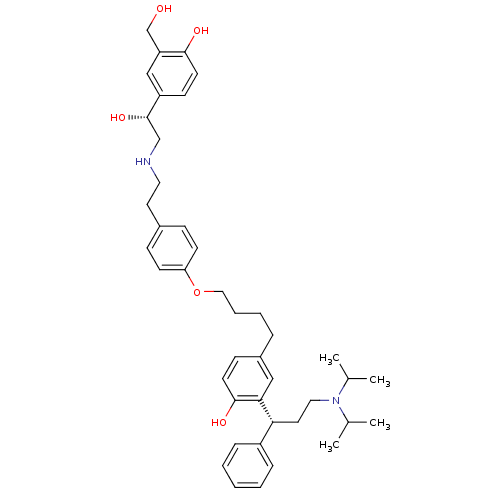
(2-((R)-3-(diisopropylamino)-1-phenylpropyl)-4-(4-(...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(CO)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C42H56N2O5/c1-30(2)44(31(3)4)24-22-38(34-11-6-5-7-12-34)39-26-33(15-19-41(39)47)10-8-9-25-49-37-17-13-32(14-18-37)21-23-43-28-42(48)35-16-20-40(46)36(27-35)29-45/h5-7,11-20,26-27,30-31,38,42-43,45-48H,8-10,21-25,28-29H2,1-4H3/t38-,42+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.765 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343160
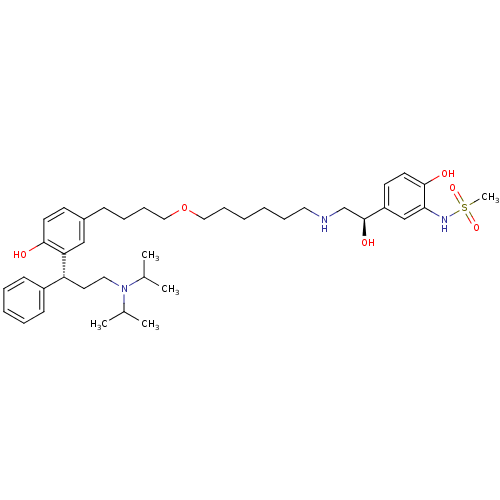
(CHEMBL1773266 | N-(5-((R)-2-(6-(4-(3-((R)-3-(diiso...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C40H61N3O6S/c1-30(2)43(31(3)4)24-22-35(33-16-9-8-10-17-33)36-27-32(18-20-38(36)44)15-11-14-26-49-25-13-7-6-12-23-41-29-40(46)34-19-21-39(45)37(28-34)42-50(5,47)48/h8-10,16-21,27-28,30-31,35,40-42,44-46H,6-7,11-15,22-26,29H2,1-5H3/t35-,40+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50128835

(CHEMBL3629360)Show SMILES O[C@@H](CNCCc1ccc(CCN2CCC(Cn3cnc(n3)[C@@](O)(C3CCCCC3)c3ccccc3)CC2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C42H52N6O4/c49-37-17-15-35(36-16-18-39(51)45-40(36)37)38(50)27-43-23-19-30-11-13-31(14-12-30)20-24-47-25-21-32(22-26-47)28-48-29-44-41(46-48)42(52,33-7-3-1-4-8-33)34-9-5-2-6-10-34/h1,3-4,7-8,11-18,29,32,34,38,43,49-50,52H,2,5-6,9-10,19-28H2,(H,45,51)/t38-,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Antagonist activity at muscarinic M5 receptor (unknown origin) |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128838
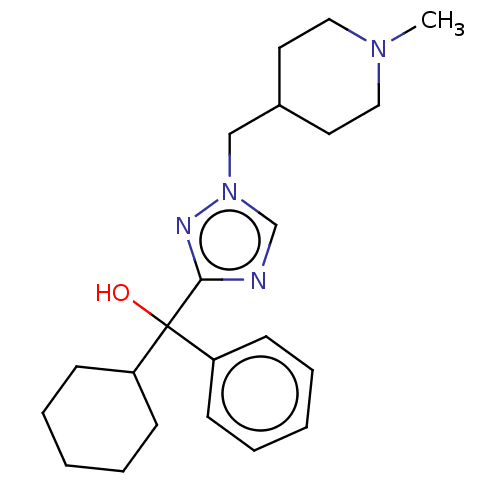
(CHEMBL3629355)Show SMILES CN1CCC(Cn2cnc(n2)C(O)(C2CCCCC2)c2ccccc2)CC1 Show InChI InChI=1S/C22H32N4O/c1-25-14-12-18(13-15-25)16-26-17-23-21(24-26)22(27,19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2,4-5,8-9,17-18,20,27H,3,6-7,10-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128836
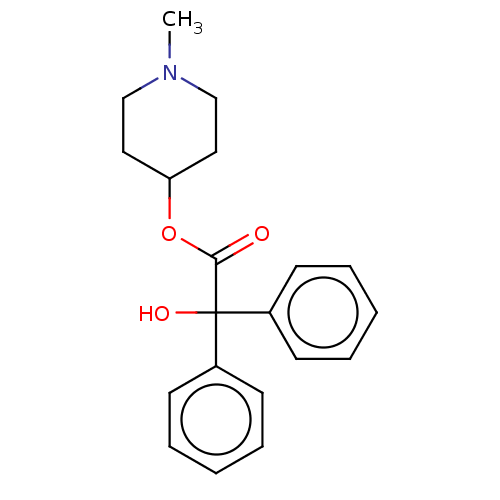
(CHEMBL143228)Show InChI InChI=1S/C20H23NO3/c1-21-14-12-18(13-15-21)24-19(22)20(23,16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18,23H,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356881

(CHEMBL1915537)Show InChI InChI=1S/C14H26N6/c1-14(2,3)5-6-17-11-7-12(19-13(15)18-11)20-8-10(9-20)16-4/h7,10,16H,5-6,8-9H2,1-4H3,(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343156
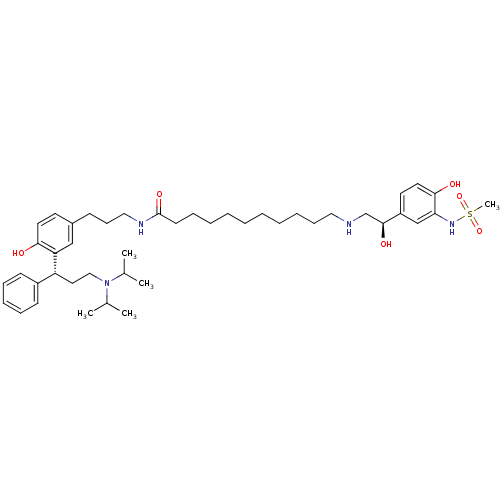
(CHEMBL1773263 | N-(3-(3-((R)-3-(diisopropylamino)-...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCNC(=O)CCCCCCCCCCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C44H68N4O6S/c1-33(2)48(34(3)4)29-26-38(36-19-13-12-14-20-36)39-30-35(22-24-41(39)49)18-17-28-46-44(52)21-15-10-8-6-7-9-11-16-27-45-32-43(51)37-23-25-42(50)40(31-37)47-55(5,53)54/h12-14,19-20,22-25,30-31,33-34,38,43,45,47,49-51H,6-11,15-18,21,26-29,32H2,1-5H3,(H,46,52)/t38-,43+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356880

(CHEMBL1915536)Show InChI InChI=1S/C13H24N6/c1-13(2,3)8-16-10-5-11(18-12(14)17-10)19-6-9(7-19)15-4/h5,9,15H,6-8H2,1-4H3,(H3,14,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50165008
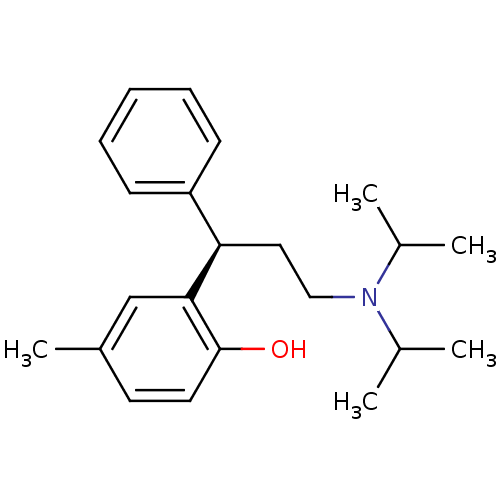
((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128831
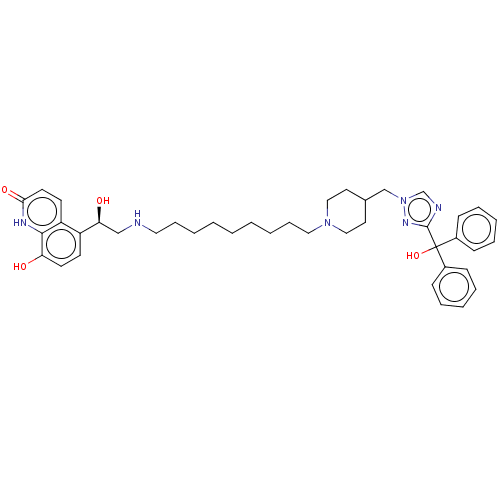
(CHEMBL3629356)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(Cn2cnc(n2)C(O)(c2ccccc2)c2ccccc2)CC1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H52N6O4/c48-36-20-18-34(35-19-21-38(50)44-39(35)36)37(49)28-42-24-12-4-2-1-3-5-13-25-46-26-22-31(23-27-46)29-47-30-43-40(45-47)41(51,32-14-8-6-9-15-32)33-16-10-7-11-17-33/h6-11,14-21,30-31,37,42,48-49,51H,1-5,12-13,22-29H2,(H,44,50)/t37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128834
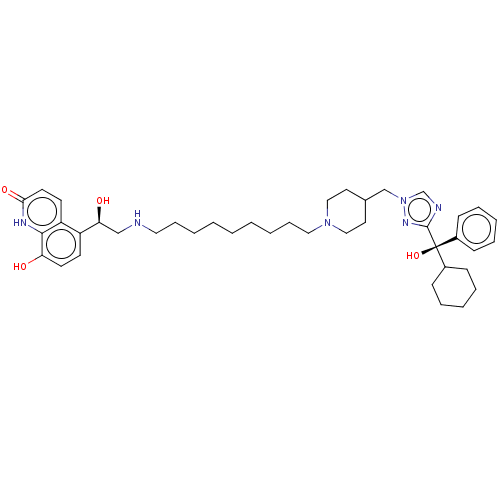
(CHEMBL3629359)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(Cn2cnc(n2)[C@@](O)(C2CCCCC2)c2ccccc2)CC1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H58N6O4/c48-36-20-18-34(35-19-21-38(50)44-39(35)36)37(49)28-42-24-12-4-2-1-3-5-13-25-46-26-22-31(23-27-46)29-47-30-43-40(45-47)41(51,32-14-8-6-9-15-32)33-16-10-7-11-17-33/h6,8-9,14-15,18-21,30-31,33,37,42,48-49,51H,1-5,7,10-13,16-17,22-29H2,(H,44,50)/t37-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Mus musculus (mouse)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from mouse H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H4 receptor |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362216

(CHEMBL1938977)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H18FN5/c1-20-5-9-7-21(8-10(9)6-20)14(17)15-18-12-3-2-11(16)4-13(12)19-15/h2-4,9-10,17H,5-8H2,1H3,(H,18,19)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362214
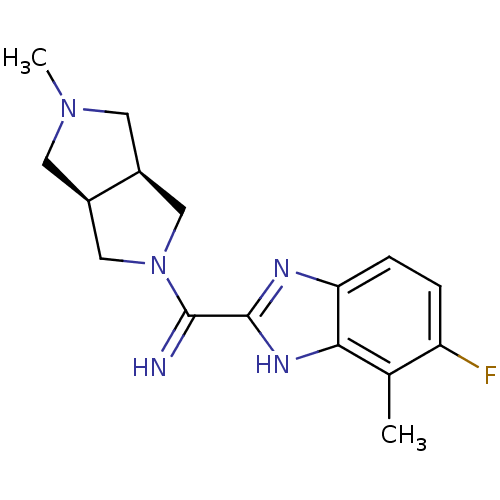
(CHEMBL1938975)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)c(C)c2[nH]1 |r| Show InChI InChI=1S/C16H20FN5/c1-9-12(17)3-4-13-14(9)20-16(19-13)15(18)22-7-10-5-21(2)6-11(10)8-22/h3-4,10-11,18H,5-8H2,1-2H3,(H,19,20)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356881

(CHEMBL1915537)Show InChI InChI=1S/C14H26N6/c1-14(2,3)5-6-17-11-7-12(19-13(15)18-11)20-8-10(9-20)16-4/h7,10,16H,5-6,8-9H2,1-4H3,(H3,15,17,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343159

(4-((R)-2-(4-(4-(3-((R)-3-(diisopropylamino)-1-phen...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCCCOc2ccc(CCNC[C@H](O)c3ccc(O)c(O)c3)cc2)ccc1O)C(C)C |r| Show InChI InChI=1S/C41H54N2O5/c1-29(2)43(30(3)4)24-22-36(33-11-6-5-7-12-33)37-26-32(15-19-38(37)44)10-8-9-25-48-35-17-13-31(14-18-35)21-23-42-28-41(47)34-16-20-39(45)40(46)27-34/h5-7,11-20,26-27,29-30,36,41-42,44-47H,8-10,21-25,28H2,1-4H3/t36-,41+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50343155
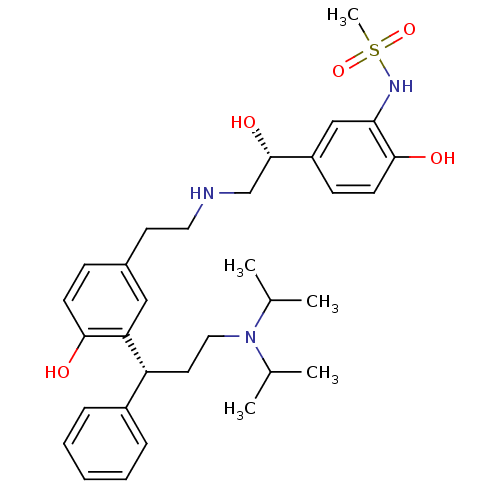
(CHEMBL1773262 | N-(5-((R)-2-(3-((R)-3-(diisopropyl...)Show SMILES CC(C)N(CC[C@H](c1ccccc1)c1cc(CCNC[C@H](O)c2ccc(O)c(NS(C)(=O)=O)c2)ccc1O)C(C)C |r| Show InChI InChI=1S/C32H45N3O5S/c1-22(2)35(23(3)4)18-16-27(25-9-7-6-8-10-25)28-19-24(11-13-30(28)36)15-17-33-21-32(38)26-12-14-31(37)29(20-26)34-41(5,39)40/h6-14,19-20,22-23,27,32-34,36-38H,15-18,21H2,1-5H3/t27-,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay |
Bioorg Med Chem Lett 21: 2759-63 (2011)
Article DOI: 10.1016/j.bmcl.2010.10.132
BindingDB Entry DOI: 10.7270/Q2G73F1B |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362218
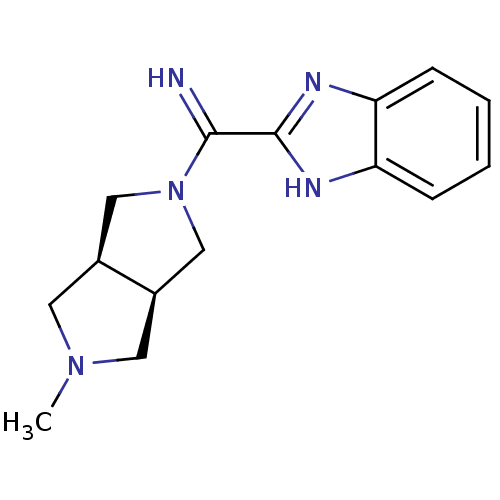
(CHEMBL1938979)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccccc2[nH]1 |r| Show InChI InChI=1S/C15H19N5/c1-19-6-10-8-20(9-11(10)7-19)14(16)15-17-12-4-2-3-5-13(12)18-15/h2-5,10-11,16H,6-9H2,1H3,(H,17,18)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor by functional assay |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50128833
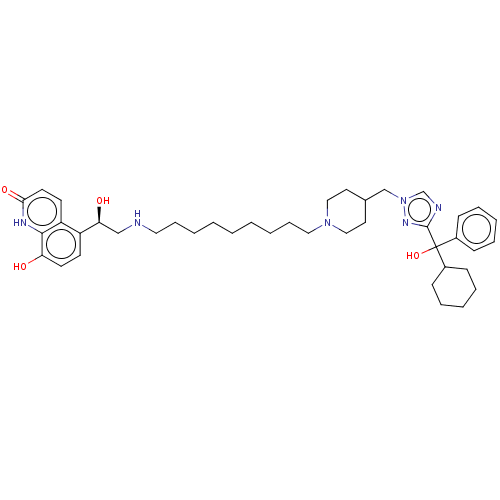
(CHEMBL3629358)Show SMILES O[C@@H](CNCCCCCCCCCN1CCC(Cn2cnc(n2)C(O)(C2CCCCC2)c2ccccc2)CC1)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C41H58N6O4/c48-36-20-18-34(35-19-21-38(50)44-39(35)36)37(49)28-42-24-12-4-2-1-3-5-13-25-46-26-22-31(23-27-46)29-47-30-43-40(45-47)41(51,32-14-8-6-9-15-32)33-16-10-7-11-17-33/h6,8-9,14-15,18-21,30-31,33,37,42,48-49,51H,1-5,7,10-13,16-17,22-29H2,(H,44,50)/t37-,41?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution method |
Bioorg Med Chem Lett 25: 5121-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.008
BindingDB Entry DOI: 10.7270/Q2JQ12V7 |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human histamine H4 receptor expressed in CHO cells after 90 mins by scintillation counting technique |
Bioorg Med Chem Lett 21: 6591-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.114
BindingDB Entry DOI: 10.7270/Q2DF6RNQ |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22566

(5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...)Show InChI InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362216

(CHEMBL1938977)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H18FN5/c1-20-5-9-7-21(8-10(9)6-20)14(17)15-18-12-3-2-11(16)4-13(12)19-15/h2-4,9-10,17H,5-8H2,1H3,(H,18,19)/t9-,10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50362214

(CHEMBL1938975)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2ccc(F)c(C)c2[nH]1 |r| Show InChI InChI=1S/C16H20FN5/c1-9-12(17)3-4-13-14(9)20-16(19-13)15(18)22-7-10-5-21(2)6-11(10)8-22/h3-4,10-11,18H,5-8H2,1-2H3,(H,19,20)/t10-,11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in HEK293 cells assessed as rev inhibition of forskolin-stimulated cAMP accumulation by ... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888
(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]histamine from human recombinant histamine H4 receptor expressed in CHO cells coexpressing Ga15 by radioligand filtration binding... |
Bioorg Med Chem Lett 22: 1156-9 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.098
BindingDB Entry DOI: 10.7270/Q2Q81DJD |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888

(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50356888

(CHEMBL1915348)Show SMILES CN1C[C@H]2CN(C[C@H]2C1)C(=N)c1nc2cc(F)c(F)cc2[nH]1 |r| Show InChI InChI=1S/C15H17F2N5/c1-21-4-8-6-22(7-9(8)5-21)14(18)15-19-12-2-10(16)11(17)3-13(12)20-15/h2-3,8-9,18H,4-7H2,1H3,(H,19,20)/t8-,9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from full length human H4R expressed in HEK293 cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Rattus norvegicus (rat)) | BDBM50356884

(CHEMBL1915540)Show InChI InChI=1S/C13H22N6/c1-15-10-4-5-19(8-10)12-6-11(17-13(14)18-12)16-7-9-2-3-9/h6,9-10,15H,2-5,7-8H2,1H3,(H3,14,16,17,18)/t10-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-histamine from rat H4R expressed in HEK293T cells |
Bioorg Med Chem Lett 21: 6596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.125
BindingDB Entry DOI: 10.7270/Q20C4W6C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data