

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073754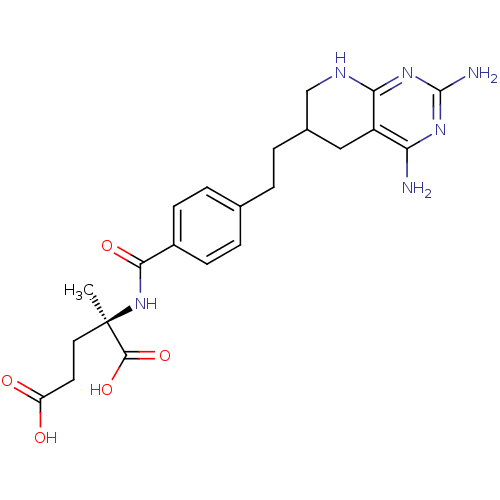 ((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073752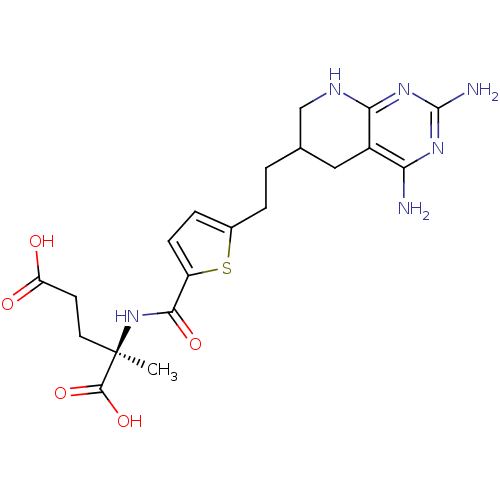 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50073753 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human dihydrofolate reductase (DHFR) | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50073753 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50073754 ((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Thymidylate synthase | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50073753 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Thymidylate synthase enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50073752 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human Thymidylate synthase enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50073752 ((R)-2-({5-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50073754 ((R)-2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pyrido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against murine glycinamide ribonucleotide formyltransferase (GARFT) enzyme | Bioorg Med Chem Lett 9: 75-8 (1999) BindingDB Entry DOI: 10.7270/Q2SX6CCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174854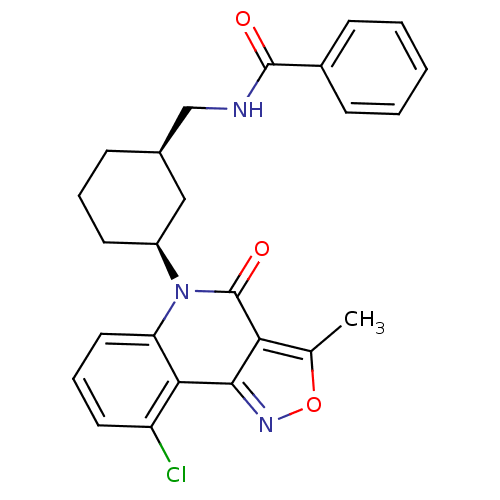 (CHEMBL200216 | N-(((1R,3S)-3-(9-chloro-3-methyl-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1-mediated LTC4 uptake into membrane vesicles from HeLa-T5 cells expressing MRP1 | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174852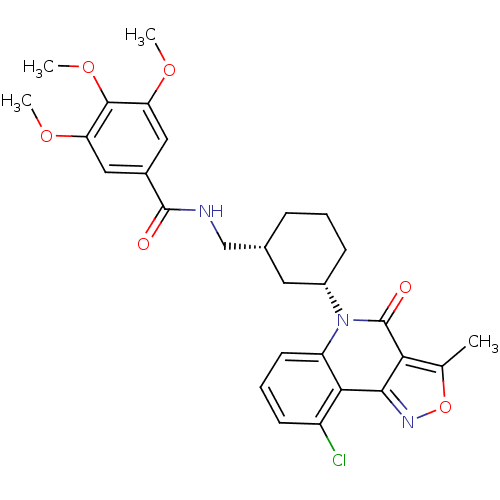 (CHEMBL198648 | rac-N-((3-(9-chloro-3-methyl-4-oxoi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1-mediated LTC4 uptake into membrane vesicles from HeLa-T5 cells expressing MRP1 | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174853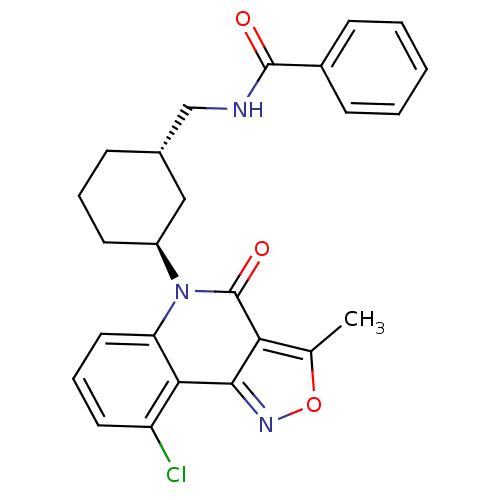 (CHEMBL199217 | rac-N-((3-(9-chloro-3-methyl-4-oxoi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1-mediated LTC4 uptake into membrane vesicles from HeLa-T5 cells expressing MRP1 | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111918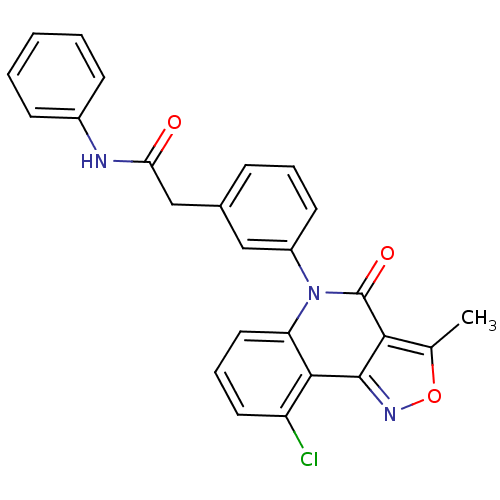 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111919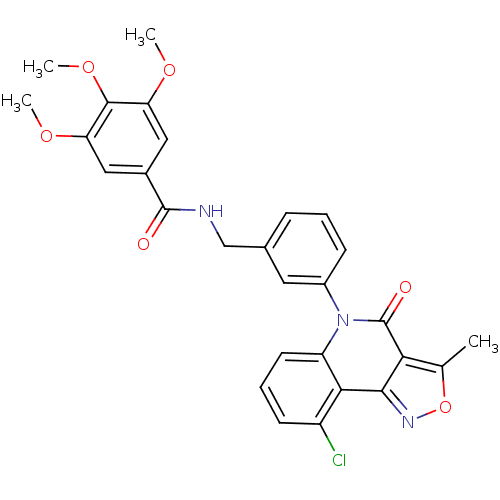 (CHEMBL554924 | N-[3-(9-Chloro-3-methyl-4-oxo-4H-is...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111920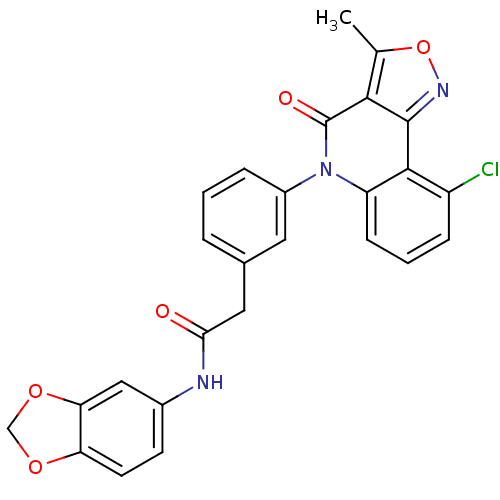 (CHEMBL354544 | N-Benzo[1,3]dioxol-5-yl-2-[3-(9-chl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111921 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111915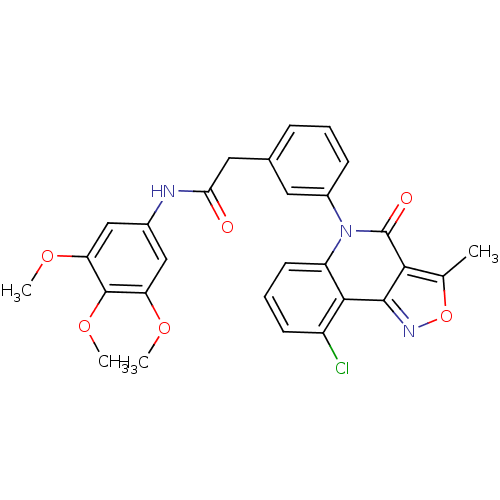 (2-(3-(9-chloro-3-methyl-4-oxoisoxazolo[4,3-c]quino...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111914 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111922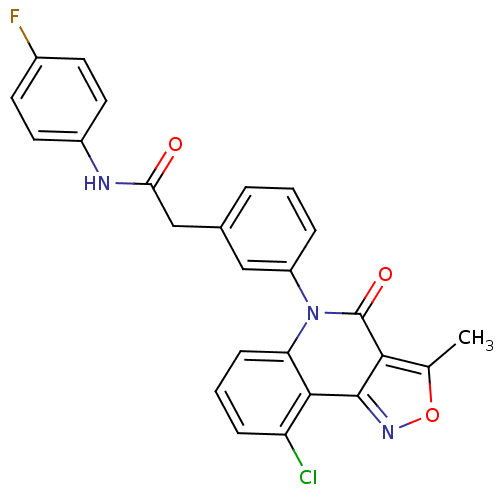 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111923 (9-Chloro-3-methyl-5-{3-[(3,4,5-trimethoxy-benzylam...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111924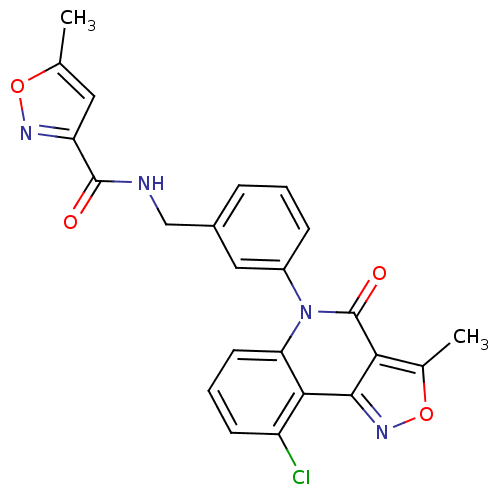 (5-Methyl-isoxazole-3-carboxylic acid 3-(9-chloro-3...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111925 (4-{2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111926 (5-Methyl-isoxazole-3-carboxylic acid [(1R,2R)-6-(9...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111927 (CHEMBL355843 | N-(4-tert-Butyl-phenyl)-2-[3-(9-chl...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111916 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111917 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111913 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111911 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111912 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111908 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111909 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111907 (2-[3-(9-Chloro-3-methyl-4-oxo-4H-isoxazolo[4,3-c]q...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174853 (CHEMBL199217 | rac-N-((3-(9-chloro-3-methyl-4-oxoi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 640 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1 in HeLa-T5 cells | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174855 (CHEMBL437892 | rac-N-((3-(9-chloro-3-methyl-4-oxoi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 420 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1 in HeLa-T5 cells | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174852 (CHEMBL198648 | rac-N-((3-(9-chloro-3-methyl-4-oxoi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1 in HeLa-T5 cells | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Multidrug resistance-associated protein 1 (Homo sapiens (Human)) | BDBM50174854 (CHEMBL200216 | N-(((1R,3S)-3-(9-chloro-3-methyl-4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibitory activity against MRP1 in HeLa-T5 cells | Bioorg Med Chem Lett 15: 5526-30 (2005) Article DOI: 10.1016/j.bmcl.2005.08.075 BindingDB Entry DOI: 10.7270/Q2VX0G1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50111910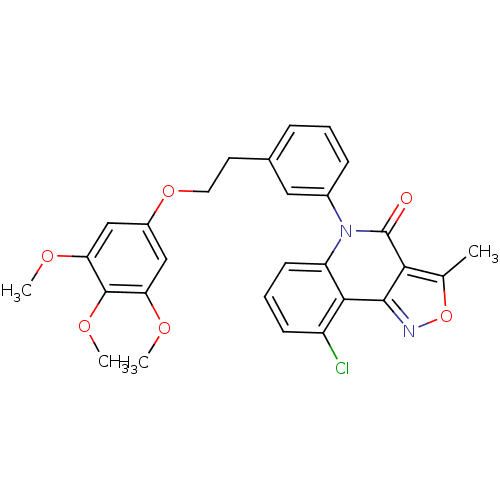 (9-Chloro-3-methyl-5-{3-[2-(3,4,5-trimethoxy-phenox...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description In vitro for its inhibitory activity against MRP1-transfected HeLa-T5 cell line in the presence of doxorubicin | Bioorg Med Chem Lett 12: 883-6 (2002) BindingDB Entry DOI: 10.7270/Q2SB4534 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||