

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50007965 (CHEMBL312194 | Tyr(psi)CH2O-Gly-Gly-Phe-Leu-NH2 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Binding affinity of the compound to opioid receptor mu was determined in rat brain using [3H]-DAGO as radioligand | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015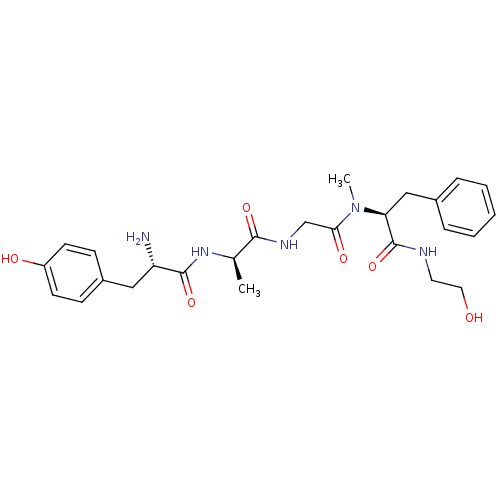 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Binding affinity of the compound to opioid receptor mu was determined in rat brain using [3H]-DAGO as radioligand | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50007965 (CHEMBL312194 | Tyr(psi)CH2O-Gly-Gly-Phe-Leu-NH2 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Binding affinity of the compound to opioid receptor delta was determined in rat brain using [3H]-DSTBULET as radioligand | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 629 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Binding affinity of the compound to opioid receptor delta was determined in rat brain using [3H]-DSTBULET as radioligand | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50007966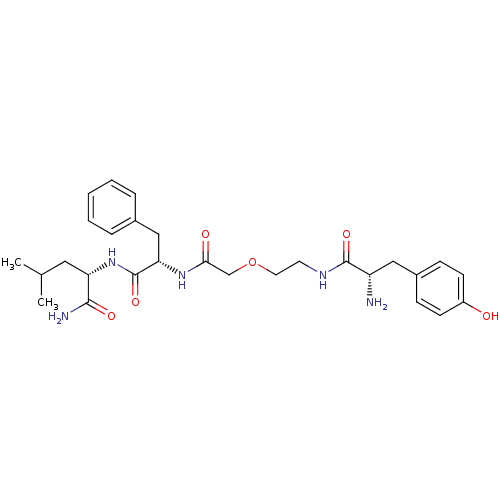 (CHEMBL420022 | Tyr-Gly(psi)CH2O-Gly-PheLeu-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Binding affinity of the compound to opioid receptor mu was determined in rat brain using [3H]-DAGO as radioligand | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50007966 (CHEMBL420022 | Tyr-Gly(psi)CH2O-Gly-PheLeu-NH2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Binding affinity of the compound to opioid receptor delta was determined in rat brain using [3H]-DSTBULET as radioligand | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004434 (3-Acetylamino-N-[1-(1-{2-[1-{1-[1-(1-carbamoyl-2-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004434 (3-Acetylamino-N-[1-(1-{2-[1-{1-[1-(1-carbamoyl-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004436 (3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004436 (3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004437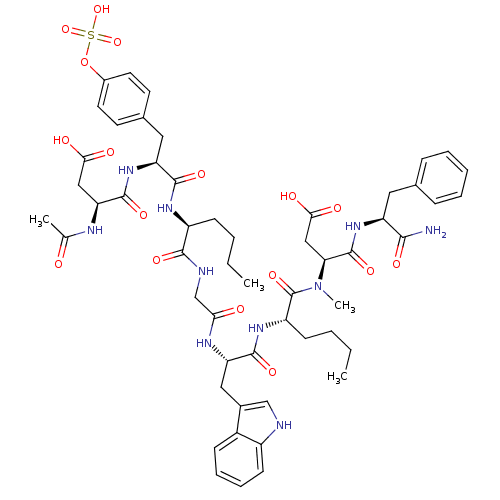 (3-Acetylamino-N-[1-[1-({[1-(1-{[1-(1-carbamoyl-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004433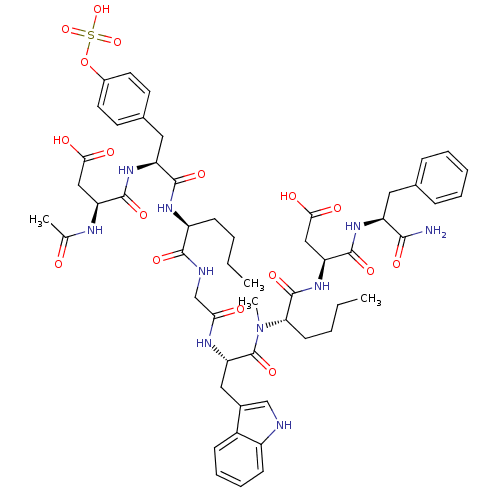 (3-Acetylamino-N-[1-[1-({[1-({1-[1-(1-carbamoyl-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004435 (3-Acetylamino-N-[1-{1-[({[1-{1-[1-(1-carbamoyl-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 265 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004439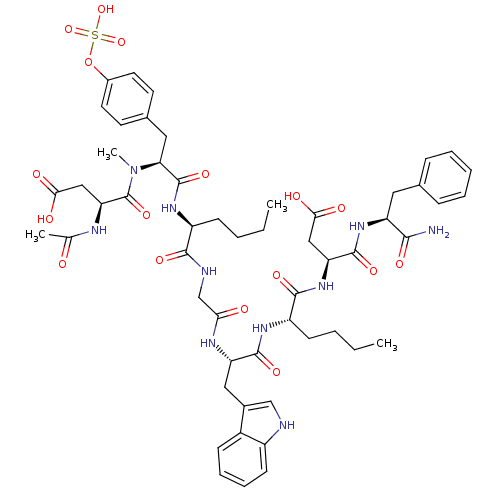 (3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004438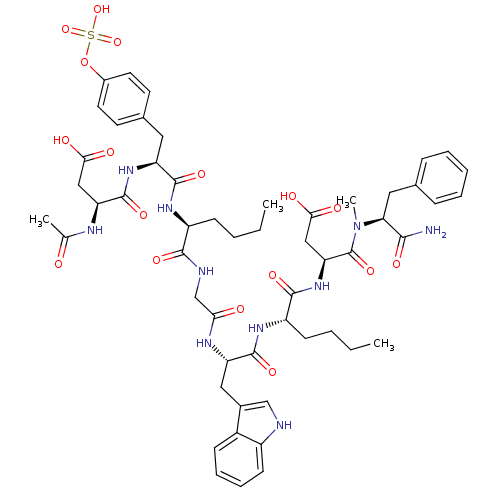 (3-Acetylamino-N-[1-[1-({[1-(1-{1-[(1-carbamoyl-2-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004440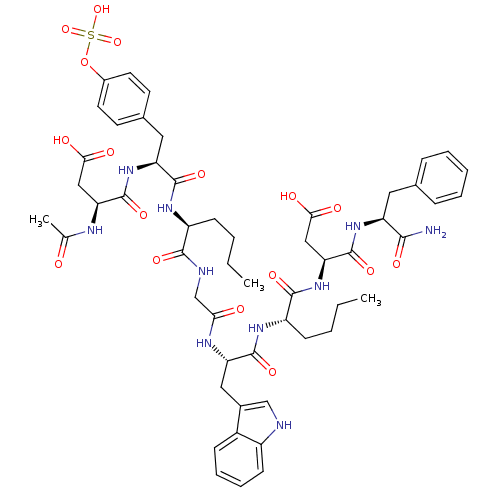 (3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004433 (3-Acetylamino-N-[1-[1-({[1-({1-[1-(1-carbamoyl-2-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004437 (3-Acetylamino-N-[1-[1-({[1-(1-{[1-(1-carbamoyl-2-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004438 (3-Acetylamino-N-[1-[1-({[1-(1-{1-[(1-carbamoyl-2-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004440 (3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50004435 (3-Acetylamino-N-[1-{1-[({[1-{1-[1-(1-carbamoyl-2-p...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig gall bladder cholecystokinin type A receptor | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50004439 (3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital Curated by ChEMBL | Assay Description In vitro smooth muscle contraction activity in guinea pig stomach consisting of CCK/gastrin receptor subtype | J Med Chem 35: 2806-11 (1992) BindingDB Entry DOI: 10.7270/Q28C9V6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50007962 (CHEMBL81919 | pGlu6-Phe-NMePhe-Gly-Leu-Met-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective dose of the compound was measured on NK3 receptors of guinea pig ileum for maximal contraction in the presence of 10e-7 M substance P methy... | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50007972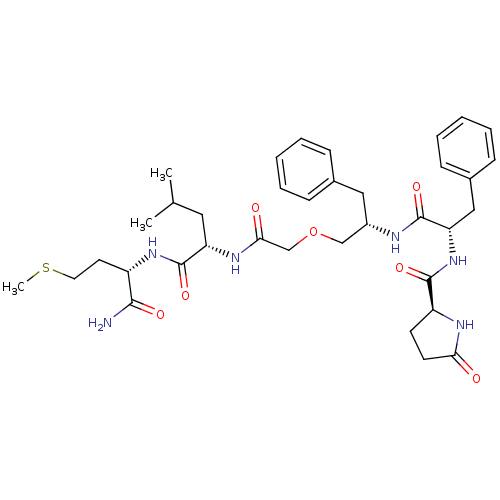 (CHEMBL311917 | pGlu6-Phe-Phe8(psi)CH2O-Gly-Leu-Met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective dose of the compound was measured on NK3 receptors of guinea pig ileum for maximal contraction in the presence of 10e-7 M substance P methy... | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50007963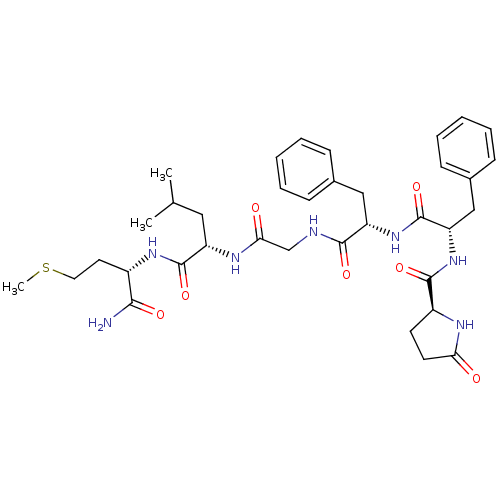 (CHEMBL441061 | pGlu6-Phe-Phe-Gly-Leu-Met-NH2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective dose of the compound was measured on NK3 receptors of guinea pig ileum for maximal contraction in the presence of 10e-7 M substance P methy... | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50007962 (CHEMBL81919 | pGlu6-Phe-NMePhe-Gly-Leu-Met-NH2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective dose of the compound was measured on NK1 receptors of guinea pig ileum for maximal contraction in the presence of 3*10e-7 M atropine | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50452295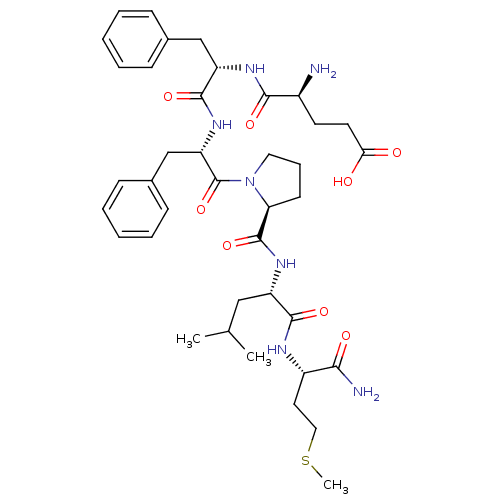 (CHEMBL2371799) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for effective dose against muscular receptor in guinea pig ileum | J Med Chem 29: 1284-8 (1987) BindingDB Entry DOI: 10.7270/Q2930S56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50016358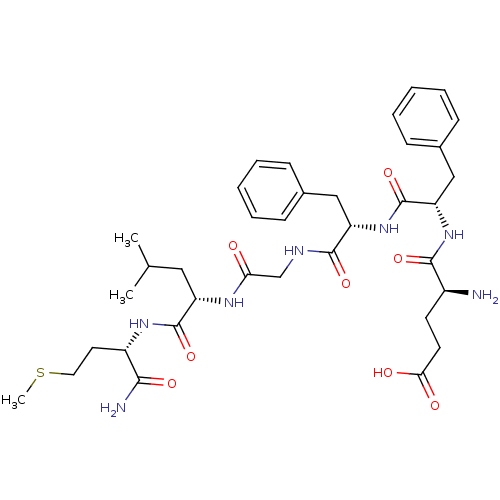 (4-Amino-4-{1-[1-({[1-(1-carbamoyl-3-methylsulfanyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for effective dose against Neuronal receptor in guinea pig ileum | J Med Chem 29: 1284-8 (1987) BindingDB Entry DOI: 10.7270/Q2930S56 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50007972 (CHEMBL311917 | pGlu6-Phe-Phe8(psi)CH2O-Gly-Leu-Met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective dose of the compound was measured on NK1 receptors of guinea pig ileum for maximal contraction in the presence of 3*10e-7 M atropine | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50007963 (CHEMBL441061 | pGlu6-Phe-Phe-Gly-Leu-Met-NH2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective dose of the compound was measured on NK1 receptors of guinea pig ileum for maximal contraction in the presence of 3*10e-7 M atropine | J Med Chem 34: 2430-8 (1991) BindingDB Entry DOI: 10.7270/Q2P55MFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052515 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052516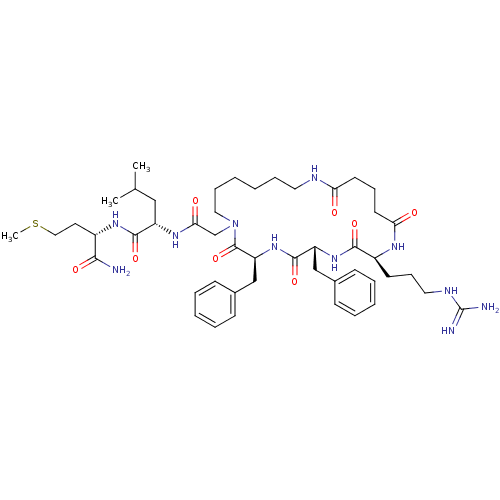 ((S)-2-{2-[(3S,6R,9S)-3,6-Dibenzyl-9-(3-guanidino-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052517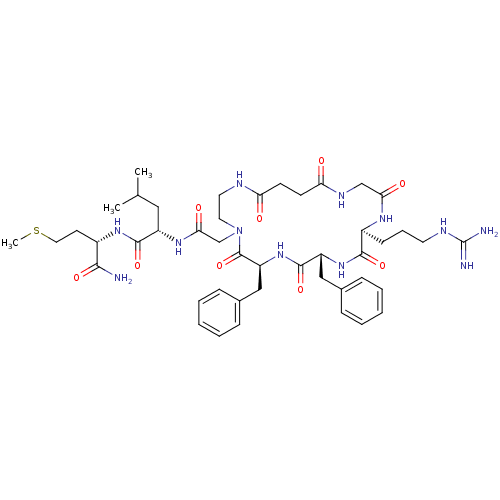 ((S)-2-{2-[(6S,9R,12S)-6,9-Dibenzyl-12-(3-guanidino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052518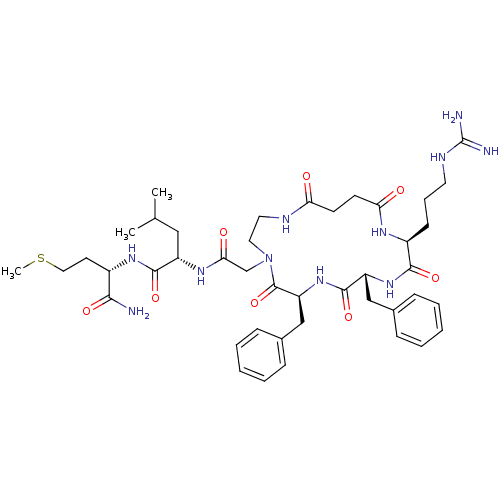 ((S)-2-{2-[(6S,9R,12S)-6,9-Dibenzyl-12-(3-guanidino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein (RPV) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052519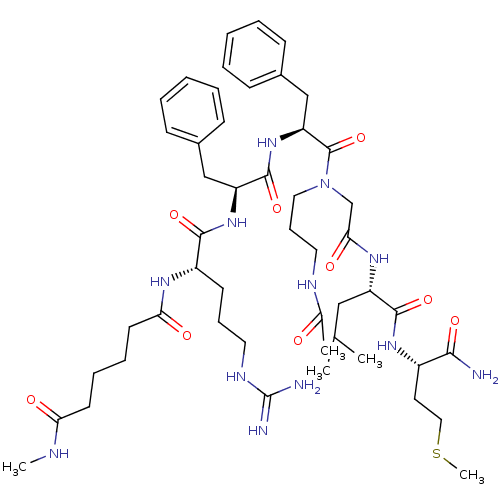 (CHEMBL265115 | Hexanedioic acid (1-{1-[1-((3-acety...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens(RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052520 ((S)-2-{2-[(3S,6R,9S)-3,6-Dibenzyl-9-(3-guanidino-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein (RPV) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052518 ((S)-2-{2-[(6S,9R,12S)-6,9-Dibenzyl-12-(3-guanidino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052519 (CHEMBL265115 | Hexanedioic acid (1-{1-[1-((3-acety...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein(RPV). | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052521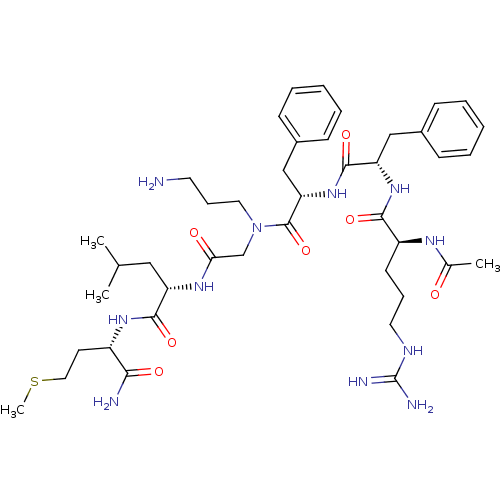 ((S)-2-{2-[{(S)-2-[(S)-2-((S)-2-Acetylamino-5-guani...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein (RPV) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052522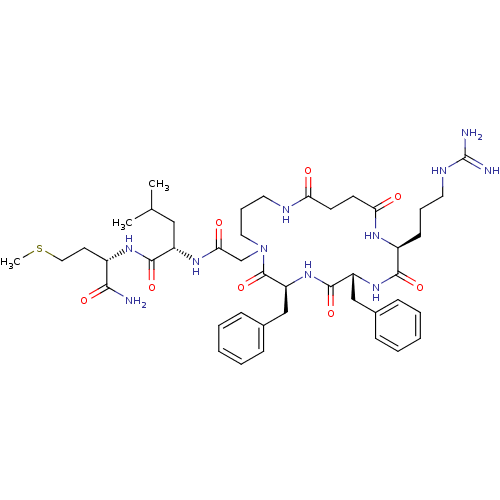 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052524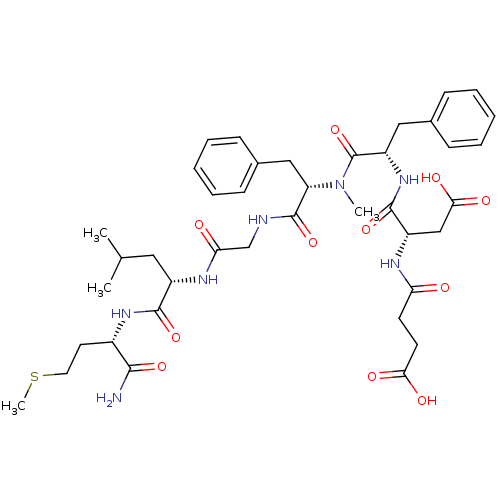 ((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052523 ((R)-1-{(S)-2-[(S)-2-((S)-2-Acetylamino-5-guanidino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein(RPV). | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052516 ((S)-2-{2-[(3S,6R,9S)-3,6-Dibenzyl-9-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052522 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein (RPV) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052519 (CHEMBL265115 | Hexanedioic acid (1-{1-[1-((3-acety...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052525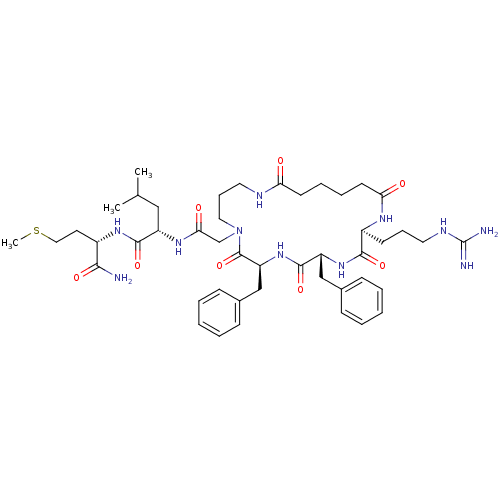 ((S)-2-{2-[(2S,5R,8S)-5,8-Dibenzyl-2-(3-guanidino-p...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052523 ((R)-1-{(S)-2-[(S)-2-((S)-2-Acetylamino-5-guanidino...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50052524 ((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 2 in rat vas deferens (RVD) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Rattus norvegicus) | BDBM50052524 ((S)-N-((S)-1-{[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 3 in rat portal vein (RPV) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50052523 ((R)-1-{(S)-2-[(S)-2-((S)-2-Acetylamino-5-guanidino...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Hebrew University of Jerusalem Curated by ChEMBL | Assay Description Effective concentration to activate Tachykinin receptor 1 in guinea pig ileum(GPI) | J Med Chem 39: 3174-8 (1996) Article DOI: 10.1021/jm960154i BindingDB Entry DOI: 10.7270/Q27943SZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |