

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176988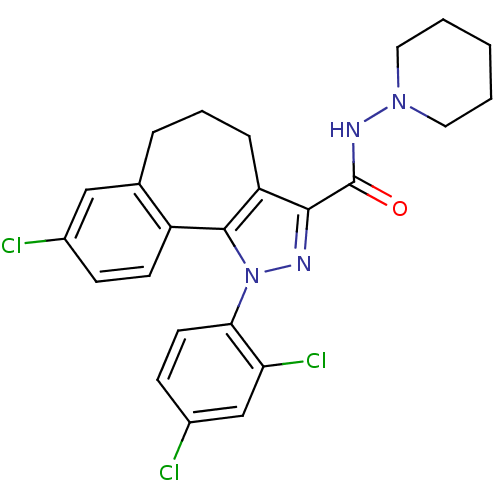 (8-Chloro-1-(2,4-dichloro-phenyl)-1,3a,4,5,6,10b-he...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | J Med Chem 51: 3526-39 (2008) Article DOI: 10.1021/jm8000778 BindingDB Entry DOI: 10.7270/Q29K4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521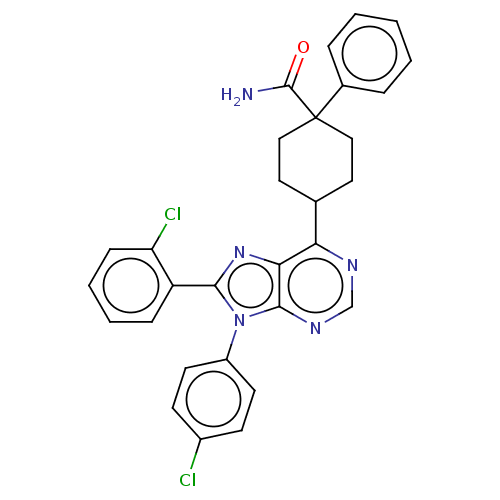 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170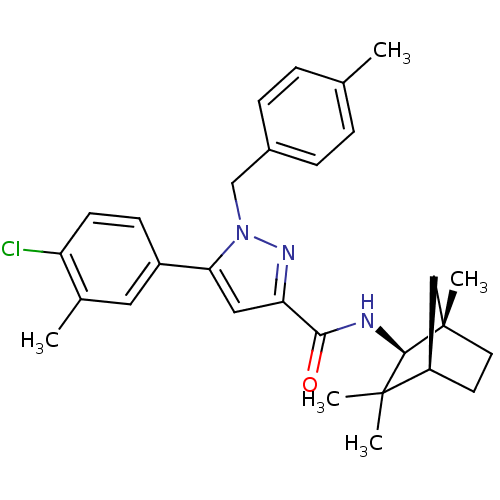 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534464 (CHEMBL4483714) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534464 (CHEMBL4483714) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.859 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534476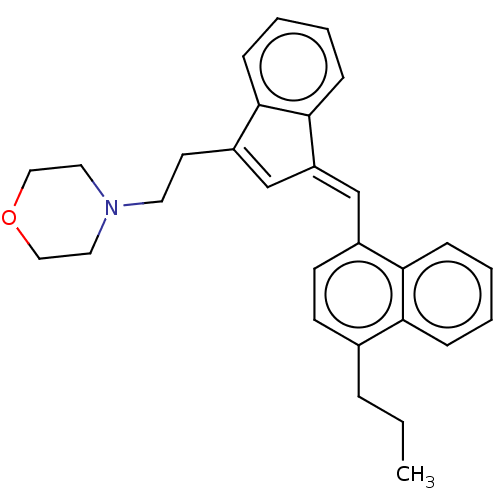 (CHEMBL4443660) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114673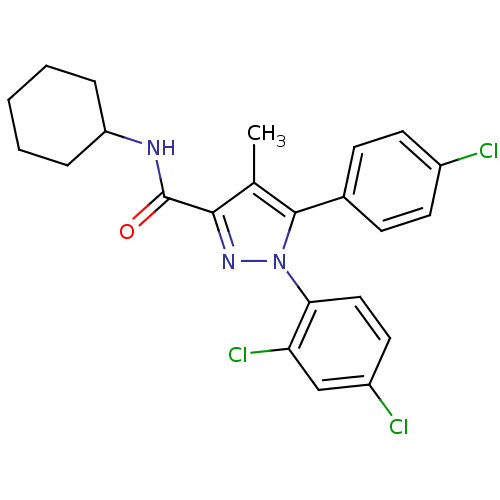 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 51: 3526-39 (2008) Article DOI: 10.1021/jm8000778 BindingDB Entry DOI: 10.7270/Q29K4B0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- SR-141,716 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534476 (CHEMBL4443660) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471438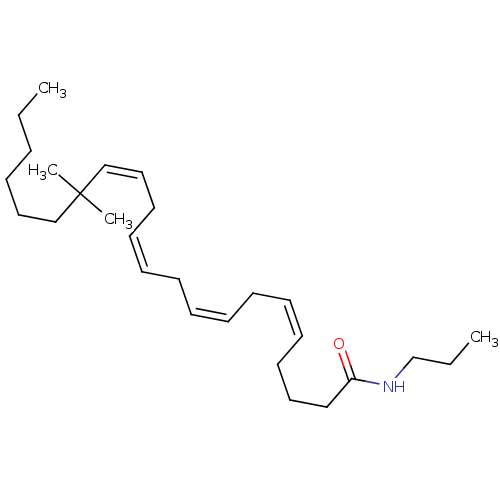 (CHEMBL122884) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane | J Med Chem 40: 3626-34 (1997) Article DOI: 10.1021/jm9702950 BindingDB Entry DOI: 10.7270/Q261131W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192536 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471436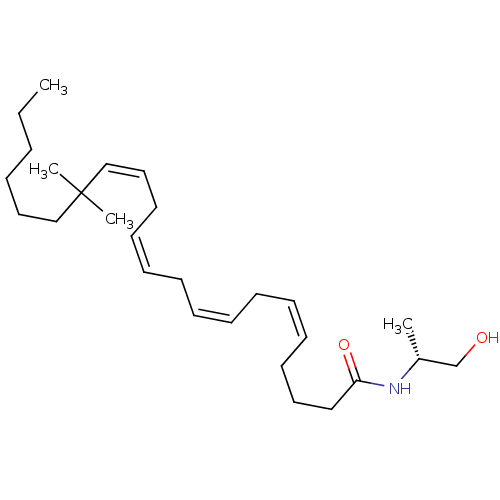 (CHEMBL123120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane | J Med Chem 40: 3626-34 (1997) Article DOI: 10.1021/jm9702950 BindingDB Entry DOI: 10.7270/Q261131W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192521 (US9187480, 4-[8-(2-chlorophenyl)-9-(4-chlorophenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50060605 ((5Z,8Z,11Z,14Z)-16,16-Dimethyl-docosa-5,8,11,14-te...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane | J Med Chem 40: 3626-34 (1997) Article DOI: 10.1021/jm9702950 BindingDB Entry DOI: 10.7270/Q261131W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192534 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192538 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50068669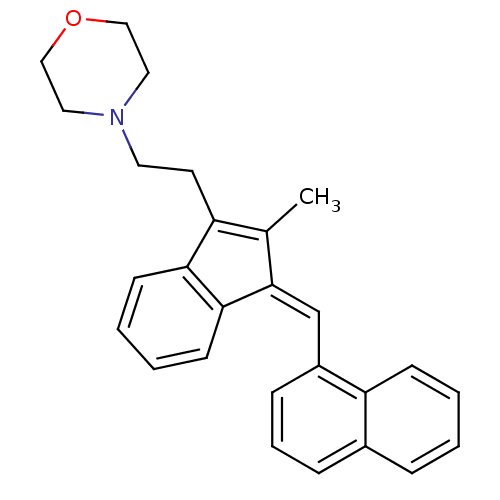 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor after 1 hr by competitive binding assay | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328956 (CHEMBL1269774 | N-{11-[(11-Aminoundecyl)amino]unde...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534485 (CHEMBL4435655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50212395 (CHEMBL123515) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50114673 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity in a competition assay by displacement of [3H]- CP 55 940 from Cannabinoid receptor 1 in rat whole brain membrane preparation | J Med Chem 45: 2708-19 (2002) BindingDB Entry DOI: 10.7270/Q2TX3DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192530 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50056468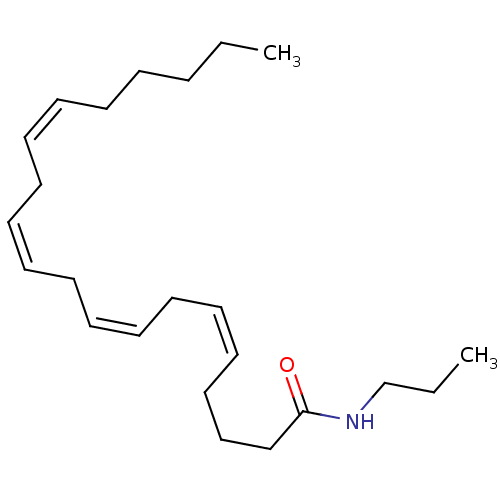 ((5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoic acid pr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane | J Med Chem 40: 3626-34 (1997) Article DOI: 10.1021/jm9702950 BindingDB Entry DOI: 10.7270/Q261131W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534457 (CHEMBL4444832) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192537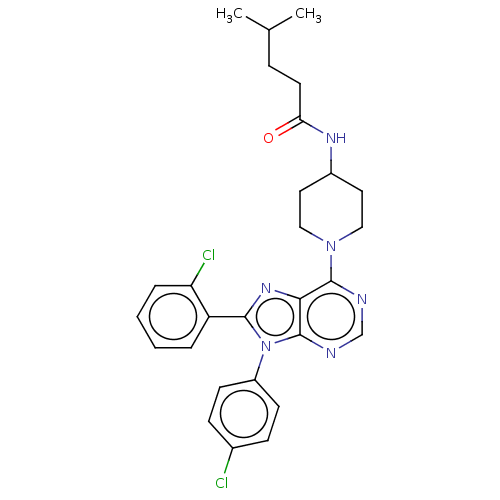 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50068666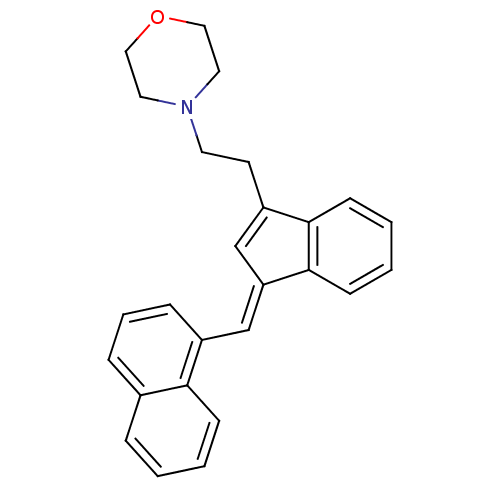 (4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068666 (4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534481 (CHEMBL4516360) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068669 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068669 (4-(2-{2-Methyl-3-[1-naphthalen-1-yl-meth-(Z)-ylide...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 1 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192532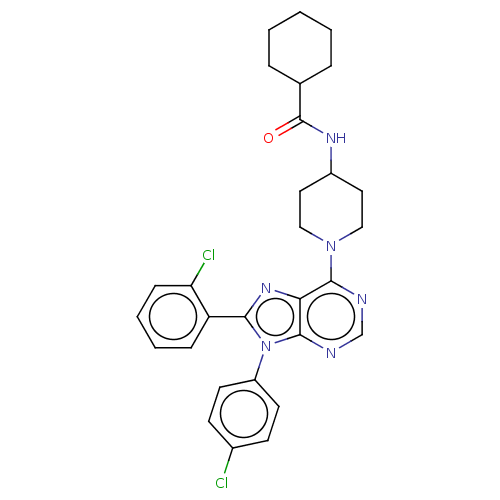 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192529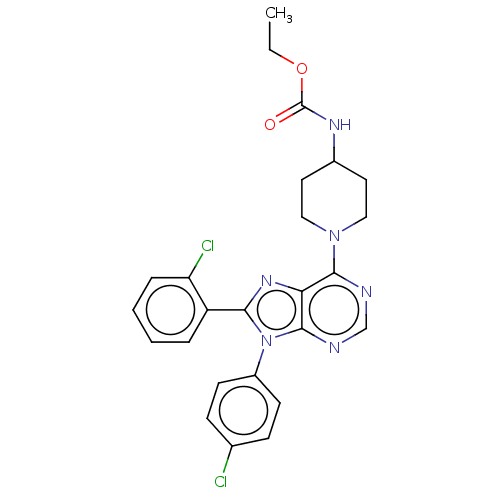 (US9187480, ethyl N-{1-[8-(2-chlorophenyl)-9-(4-chl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534481 (CHEMBL4516360) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471437 (CHEMBL121384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane | J Med Chem 40: 3626-34 (1997) Article DOI: 10.1021/jm9702950 BindingDB Entry DOI: 10.7270/Q261131W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192535 (US9187480, N-{1-[8-(2-chlorophenyl)-9-(4-chlorophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM192528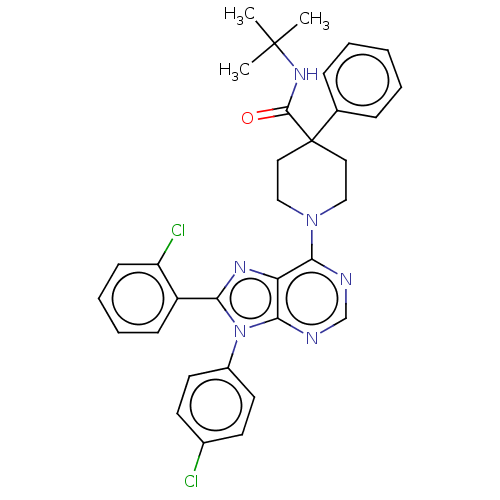 (US9187480, N-tert-butyl-1-[8-(2-chlorophenyl)-9-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute US Patent | Assay Description Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ... | US Patent US9187480 (2015) BindingDB Entry DOI: 10.7270/Q28051DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50212395 (CHEMBL123515) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534463 (CHEMBL4550629) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50353747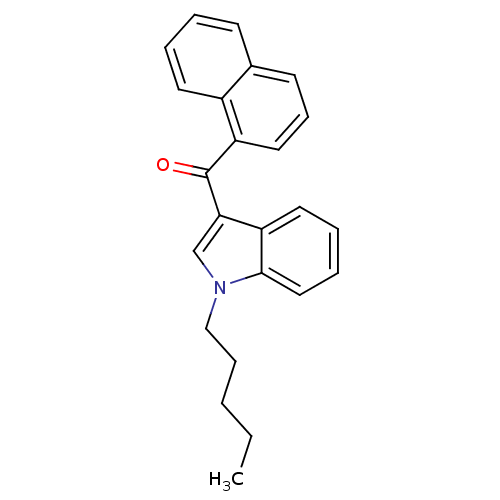 (CHEMBL561013 | JWH-018) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534498 (CHEMBL4474739) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328952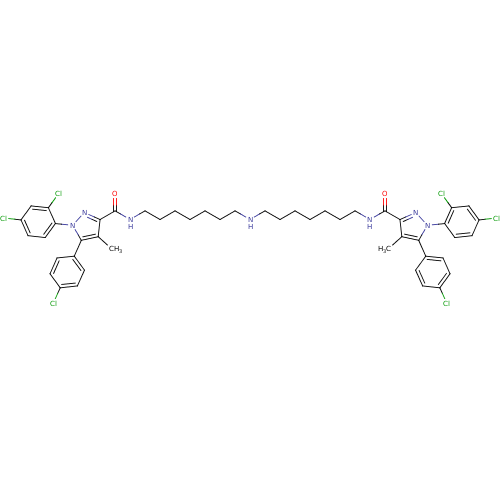 (CHEMBL1269766 | N,N'-(Iminodiheptane-7,1-diyl)bis[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328956 (CHEMBL1269774 | N-{11-[(11-Aminoundecyl)amino]unde...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50068666 (4-(2-{3-[1-Naphthalen-1-yl-meth-(E)-ylidene]-3H-in...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50471435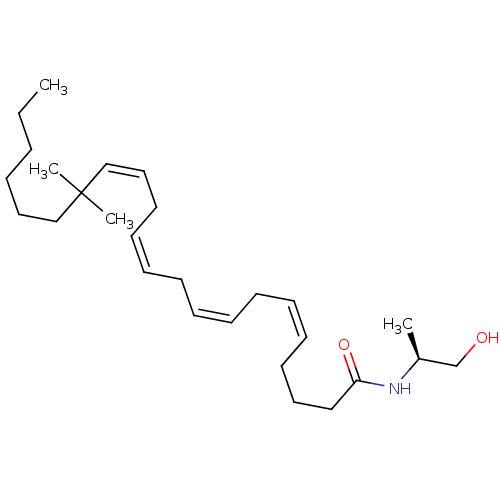 (CHEMBL120428) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards cannabinoid receptor using [3H]CP-55940 as radioligand in rat brain membrane | J Med Chem 40: 3626-34 (1997) Article DOI: 10.1021/jm9702950 BindingDB Entry DOI: 10.7270/Q261131W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50328957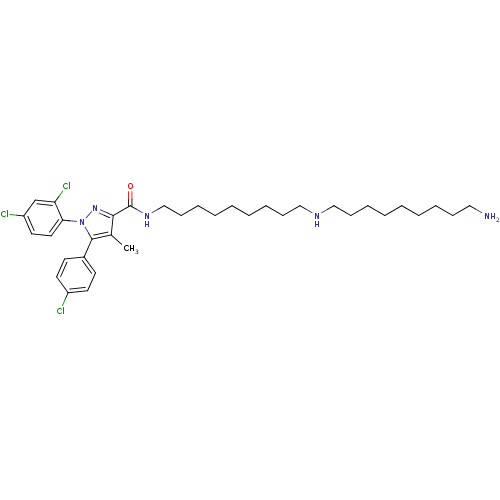 (CHEMBL1269773 | N-{9-[(9-Aminononyl)amino]nonyl}-5...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in HEK293 cells | J Med Chem 53: 7048-60 (2010) Article DOI: 10.1021/jm1006676 BindingDB Entry DOI: 10.7270/Q27P8ZMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 360 total ) | Next | Last >> |