

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267969 (Sargahydroquinoic Acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241345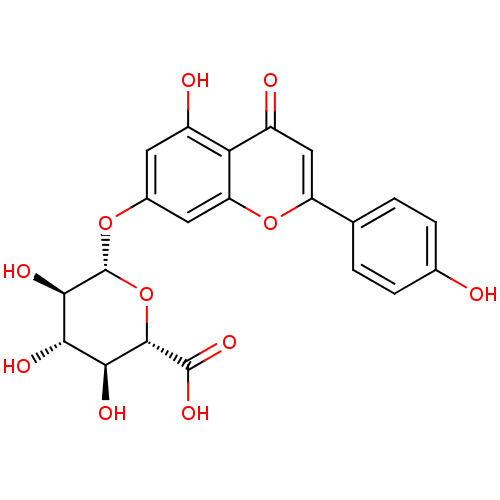 (Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Catholic University of Daegu | Assay Description The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM4078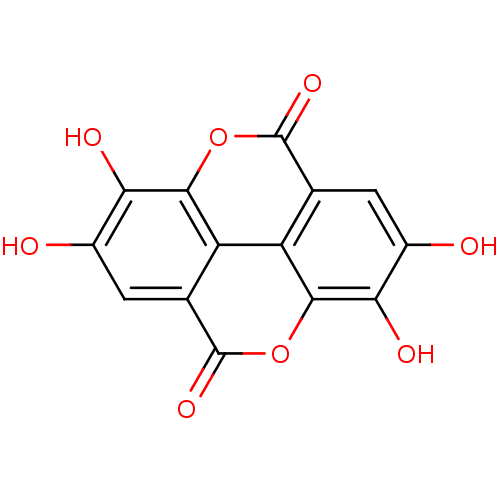 (6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Catholic University of Daegu | Assay Description The reaction mixtures contained in various different concentrations of p-NPP as a PTP1B substrate in the presence or absence of the active compound. ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267968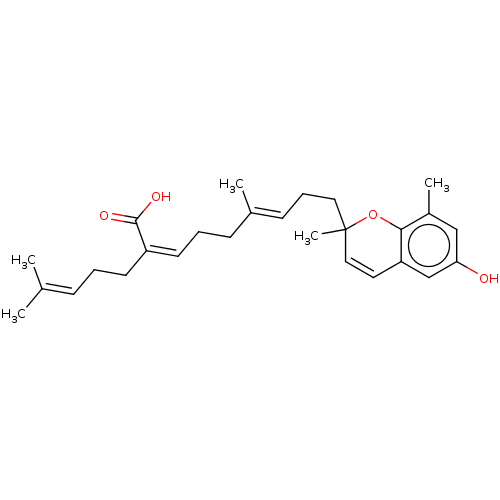 (CHEMBL4085945) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Noncompetitive inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226157 (PTP1B spring 7 (7)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 3.00E+3 | -32.8 | 4.80E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267967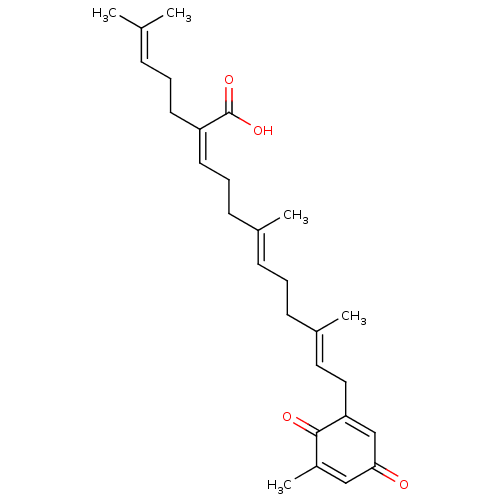 (CHEMBL4064412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by Dixon plot analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226153 (Selaginellin U (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 9.70E+3 | -29.8 | 1.38E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226155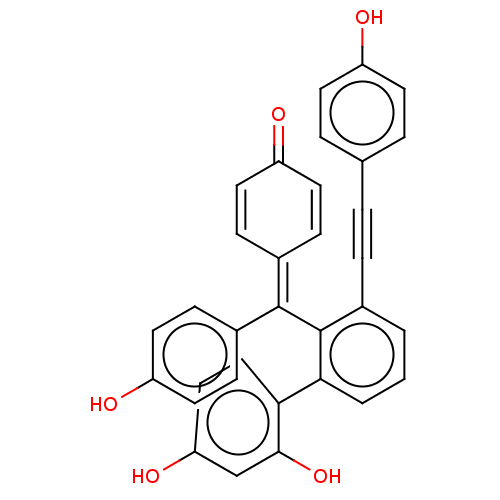 (Selaginellin W (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.11E+4 | -29.4 | 1.46E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226154 (Selaginellin V (3)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 1.13E+4 | -29.4 | 1.45E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226156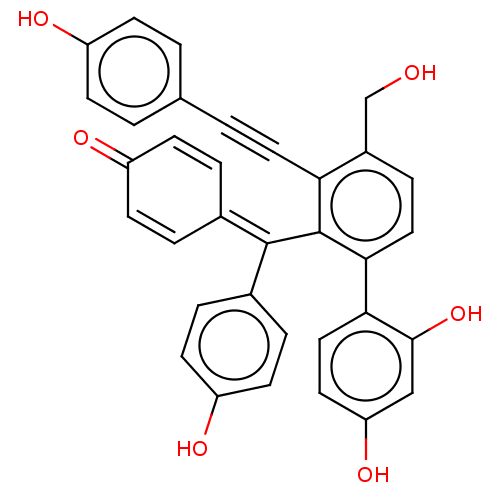 (PTP1B spring 5 (5)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | 1.39E+4 | -28.8 | 1.59E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093523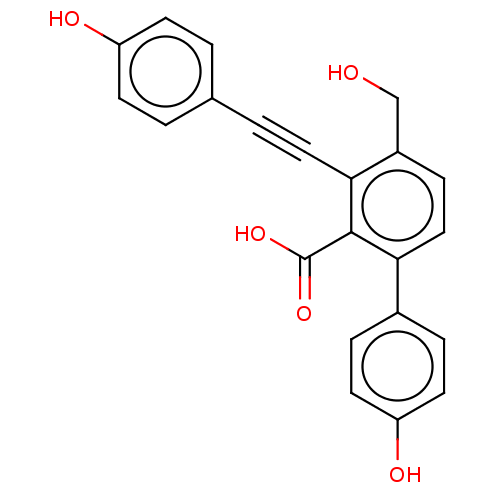 (CHEMBL3585679 | PTP1B spring 6 (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 1.45E+4 | -28.7 | 1.32E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50100436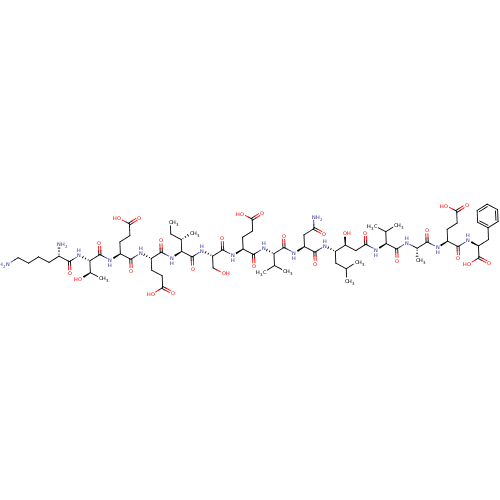 (CHEMBL412768 | NH2-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by FRET assay | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50100436 (CHEMBL412768 | NH2-Lys-Thr-Glu-Glu-Ile-Ser-Glu-Val...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50203126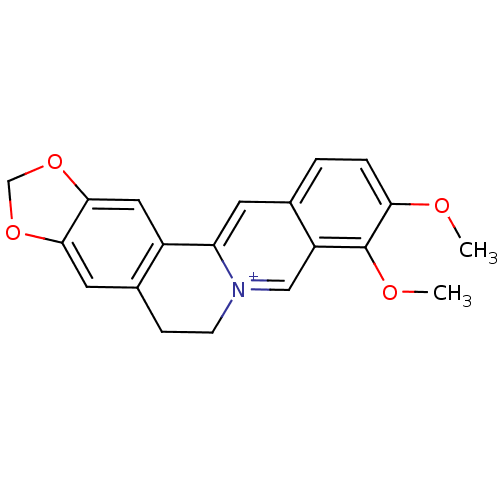 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | n/a | n/a | 770 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometric analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267969 (Sargahydroquinoic Acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by FRET assay | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonbuk National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate pretreated for 10 mins followed by substrate addition measured after 20 mins by spectrop... | Bioorg Med Chem Lett 27: 2274-2280 (2017) Article DOI: 10.1016/j.bmcl.2017.04.054 BindingDB Entry DOI: 10.7270/Q2ZK5K3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by FRET assay | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267968 (CHEMBL4085945) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by FRET assay | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241345 (Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM4078 (6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 7.73E+3 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50203126 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate incubated for 15 mins by spectrophotometric analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50267968 (CHEMBL4085945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate incubated for 15 mins by spectrophotometric analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 9.43E+3 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50267967 (CHEMBL4064412) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate incubated for 15 mins by spectrophotometric analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50267967 (CHEMBL4064412) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK-Quencher as substrate after 60 mins by FRET assay | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50267969 (Sargahydroquinoic Acid) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine chloride as substrate incubated for 15 mins by spectrophotometric analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226177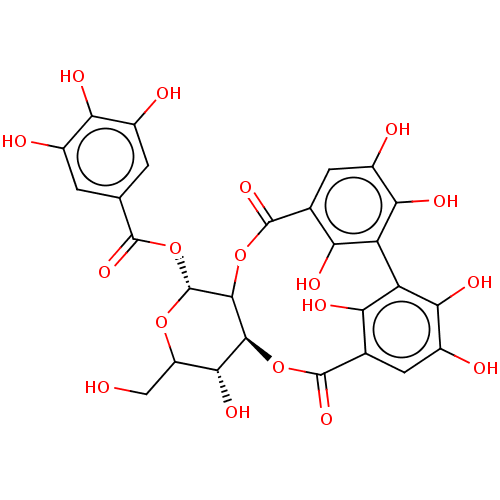 (Agritannin (1)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50249470 (CHEBI:28327 | Prunin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonbuk National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate pretreated for 10 mins followed by substrate addition measured after 20 mins by spectrop... | Bioorg Med Chem Lett 27: 2274-2280 (2017) Article DOI: 10.1016/j.bmcl.2017.04.054 BindingDB Entry DOI: 10.7270/Q2ZK5K3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226184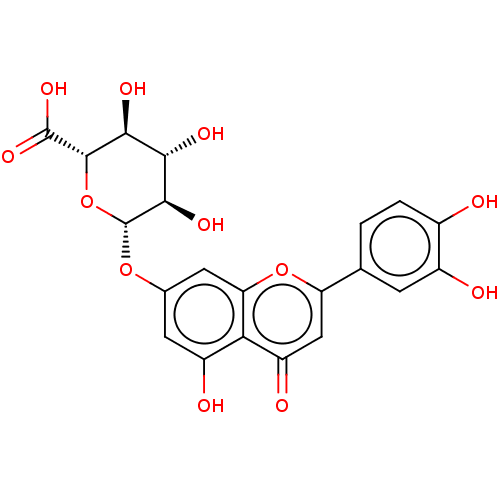 (Luteolin 7-O-β-D-glucuronide (14)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226181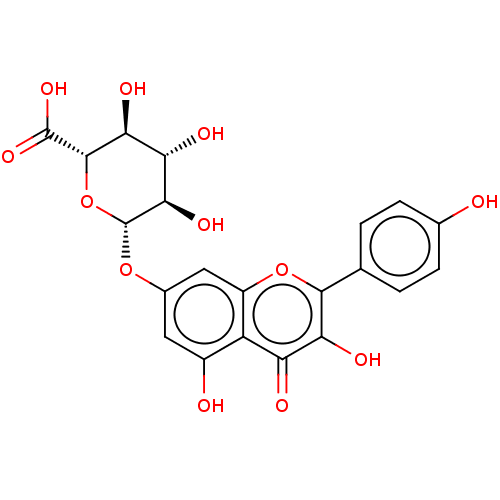 (Kaempferol 7-O-β-D-glucuronide (5)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | n/a | n/a | 4.83E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226185 (Luteolin 7-O-β-D-glucuronide methyl ester (15...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4.91E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50249471 (CHEBI:28705 | Narirutin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chonbuk National University Curated by ChEMBL | Assay Description Inhibition of PTP1B (unknown origin) using pNPP as substrate pretreated for 10 mins followed by substrate addition measured after 20 mins by spectrop... | Bioorg Med Chem Lett 27: 2274-2280 (2017) Article DOI: 10.1016/j.bmcl.2017.04.054 BindingDB Entry DOI: 10.7270/Q2ZK5K3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226152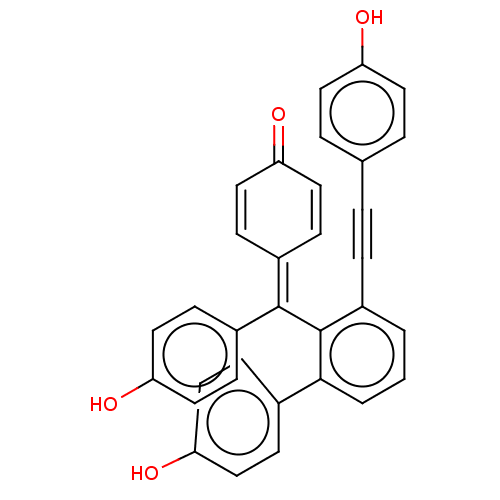 (Selaginellin T (1)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | 6.0 | 37 | |
Catholic University of Daegu | Assay Description In each 96-well plates (total 200 μL of volume), there were 2 mM p-NPP and PTP1B (0.05-0.1 μg) in a buffer containing 50 mM citrate (pH 6.0... | Bioorg Chem 72: 273-281 (2017) BindingDB Entry DOI: 10.7270/Q2ZP450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226177 (Agritannin (1)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6.02E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM226182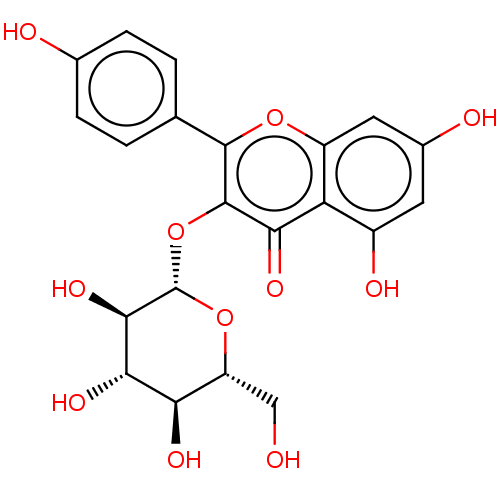 (Kaempferol 3-O-β-D-glucoside (6)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | n/a | n/a | 6.07E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241345 (Apigenin 7-O-β-D-glucuronide (9) | CHEMBL2542...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226178 (Agriflavone (2)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6.34E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226180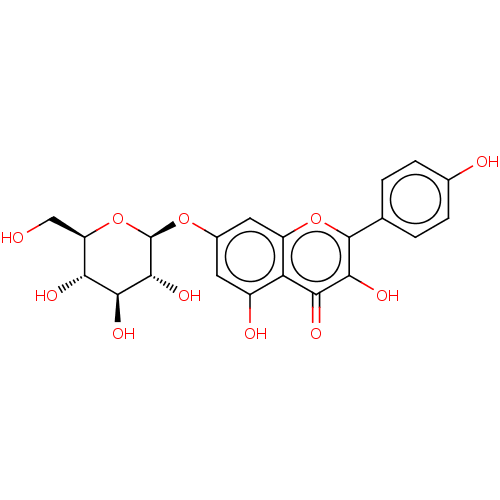 (Kaempferol 7-O-β-D-glucoside (4)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | n/a | n/a | 6.39E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226181 (Kaempferol 7-O-β-D-glucuronide (5)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 6.43E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226185 (Luteolin 7-O-β-D-glucuronide methyl ester (15...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 6.59E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50267967 (CHEMBL4064412) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Food and Life Science, Pukyong National University, Busan 608-737, Republic of Korea. Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometric analysis | Bioorg Med Chem 25: 3964-3970 (2017) Article DOI: 10.1016/j.bmc.2017.05.033 BindingDB Entry DOI: 10.7270/Q2K076RW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50241242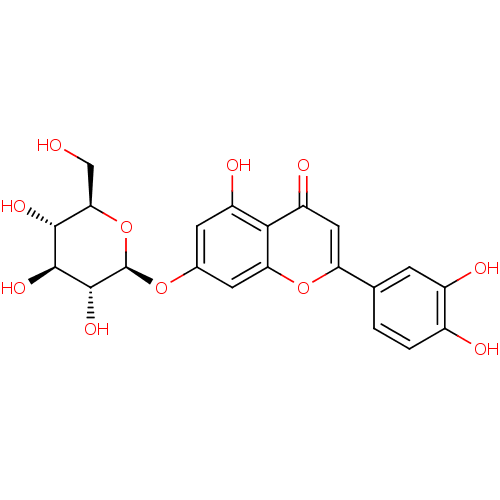 (2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxo-4H-chromen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | n/a | n/a | 7.16E+4 | n/a | n/a | n/a | n/a | 6.0 | n/a | |
Catholic University of Daegu | Assay Description PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | n/a | n/a | 7.28E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226187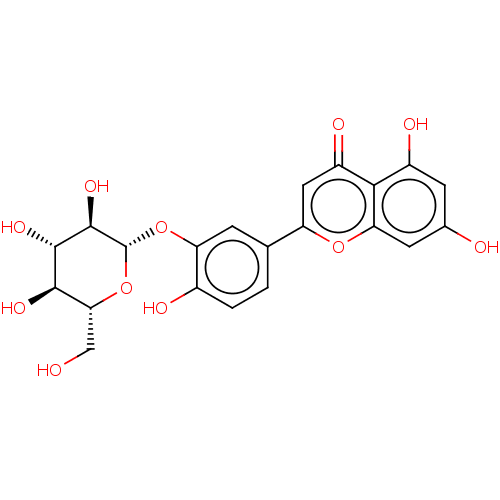 (Luteolin 3'-O-β-D-glucoside (17)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | n/a | n/a | 7.46E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50241242 (2-(3,4-dihydroxyphenyl)-5-hydroxy-4-oxo-4H-chromen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | n/a | n/a | 7.47E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM226186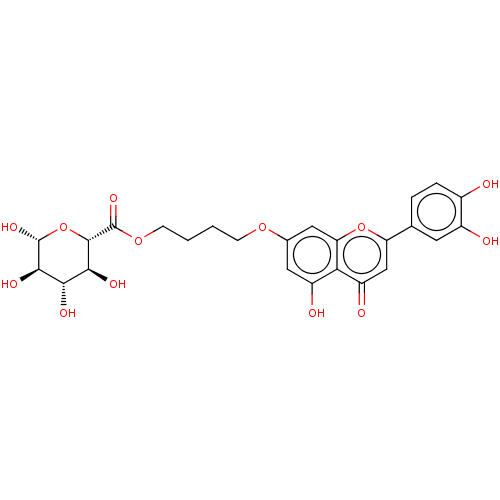 (Luteolin 7-O-β-D-glucuronide butyl ester (16)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7.69E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 | |
Catholic University of Daegu | Assay Description Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a... | Bioorg Chem 72: 293-300 (2017) BindingDB Entry DOI: 10.7270/Q2Q52NH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |