

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523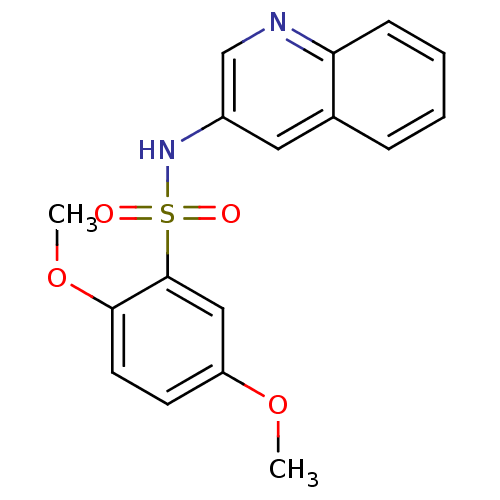 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP using CDP-star as substrate by non-competitive Lineweaver-Burk plot | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50299523 (2,5-Dimethoxy-N-(quinolin-3-yl)benzenesulfonamide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNAP using DEA as substrate by non-competitive Lineweaver-Burk plot | J Med Chem 52: 6919-25 (2009) Article DOI: 10.1021/jm900383s BindingDB Entry DOI: 10.7270/Q2WS8T9M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 7 (Homo sapiens (Human)) | BDBM88776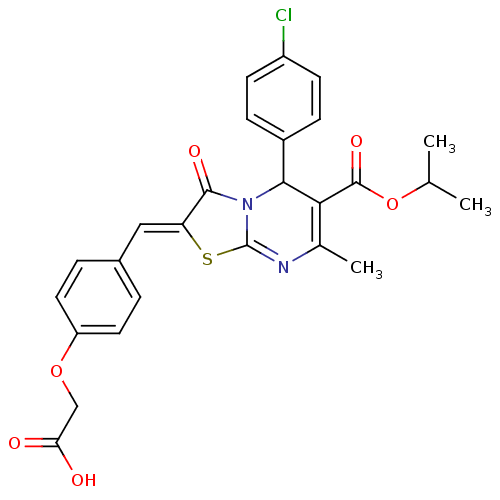 (2-[4-[(Z)-[5-(4-chlorophenyl)-6-isopropoxycarbonyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of recombinant HePTP expressed in Escherichia coli by Michaelis-Menten kinetic analysis | ACS Med Chem Lett 2: 113-118 (2011) Article DOI: 10.1021/ml100103p BindingDB Entry DOI: 10.7270/Q2319WWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Mus musculus) | BDBM50447413 (CHEMBL3115157) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of mouse duodenal-specific FLAG-tagged IAP expressed in African green monkey COS1 cells using p-nitrophenyl phosphate as subst... | Bioorg Med Chem Lett 24: 1000-4 (2014) Article DOI: 10.1016/j.bmcl.2013.12.043 BindingDB Entry DOI: 10.7270/Q2ST7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50241179 ((S)-6-phenyl-2,3,5,6-tetrahydroimidazo[2,1-b]thiaz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNSALP (unknown origin) | Bioorg Med Chem Lett 19: 222-5 (2008) Article DOI: 10.1016/j.bmcl.2008.10.107 BindingDB Entry DOI: 10.7270/Q2CR5T64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM10847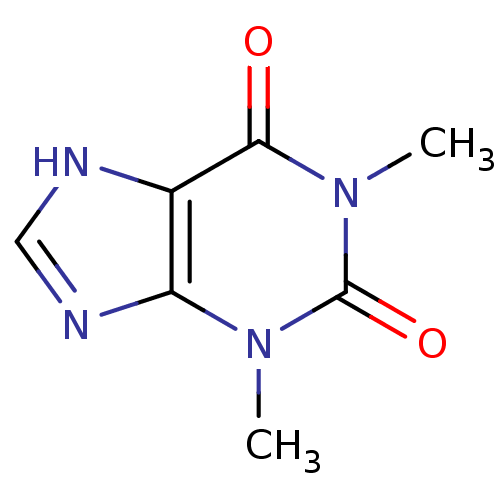 (1,3-dimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-dion...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNSALP (unknown origin) | Bioorg Med Chem Lett 19: 222-5 (2008) Article DOI: 10.1016/j.bmcl.2008.10.107 BindingDB Entry DOI: 10.7270/Q2CR5T64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50253982 (CHEMBL461194 | N-(2-hydroxyethyl)-3-(2,3,4-trichlo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Inhibition of TNSALP (unknown origin) | Bioorg Med Chem Lett 19: 222-5 (2008) Article DOI: 10.1016/j.bmcl.2008.10.107 BindingDB Entry DOI: 10.7270/Q2CR5T64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332599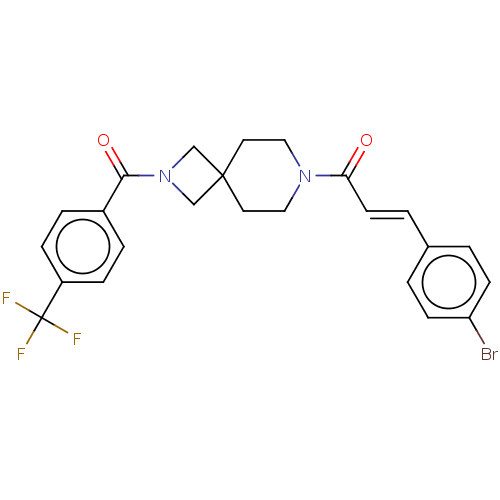 ((2E)-3-(4-bromophenyl)-1-(2-{[4-(trifluoromethyl)p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332614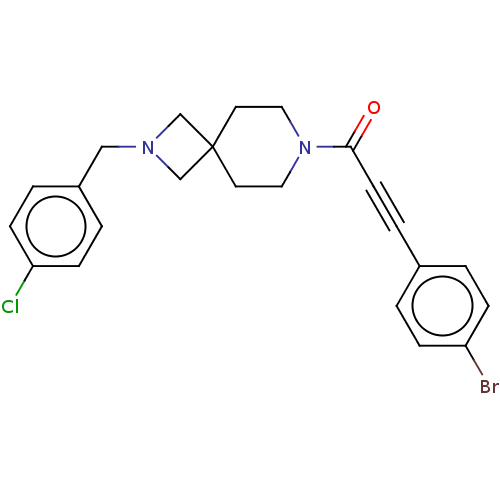 (3-(4-bromophenyl)-1-{2-[(4-chlorophenyl)methyl]-2,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332616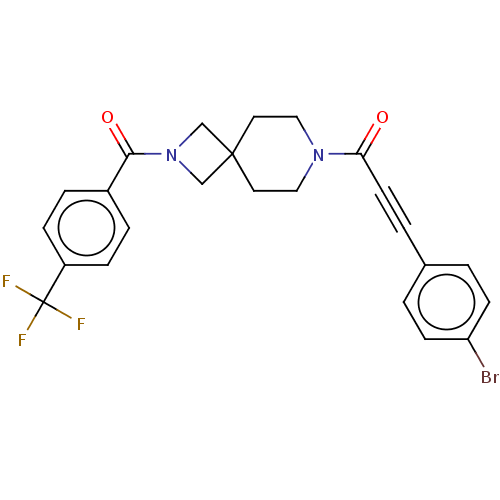 (3-(4-bromophenyl)-1-(2-{[4-(trifluoromethyl)phenyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332617 (3-(4-bromophenyl)-1-{2-[(4-bromophenyl)methyl]-2,7...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332618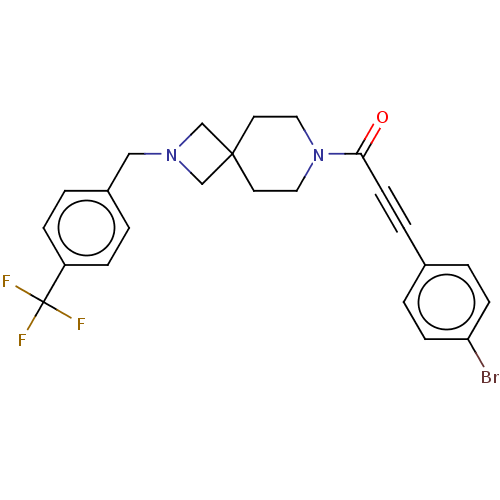 (3-(4-bromophenyl)-1-(2-{[4-(trifluoromethyl)phenyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332619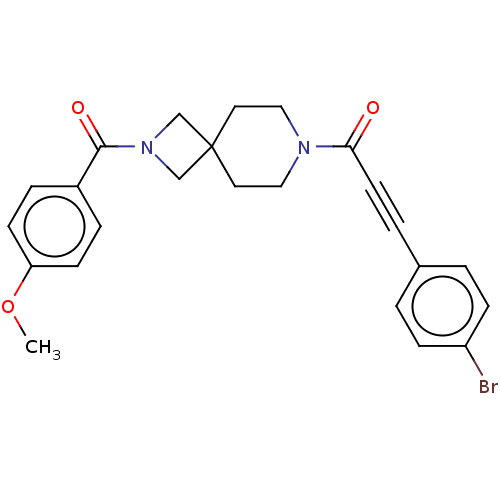 (3-(4-bromophenyl)-1-{2-[(4-methoxyphenyl)carbonyl]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332621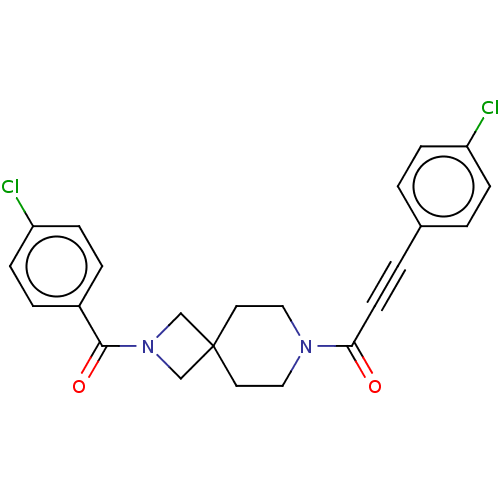 (3-(4-chlorophenyl)-1-{2-[(4-chlorophenyl)carbonyl]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332622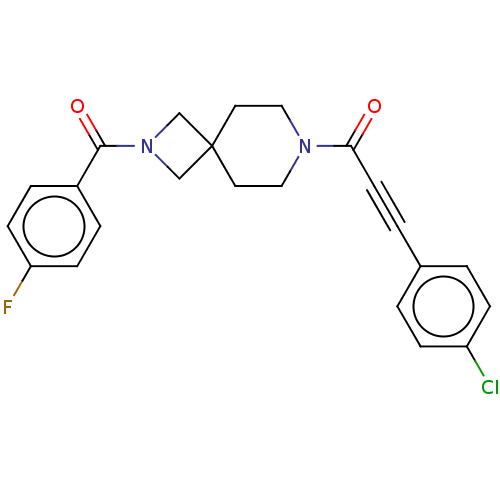 (3-(4-chlorophenyl)-1-{2-[(4-fluorophenyl)carbonyl]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332623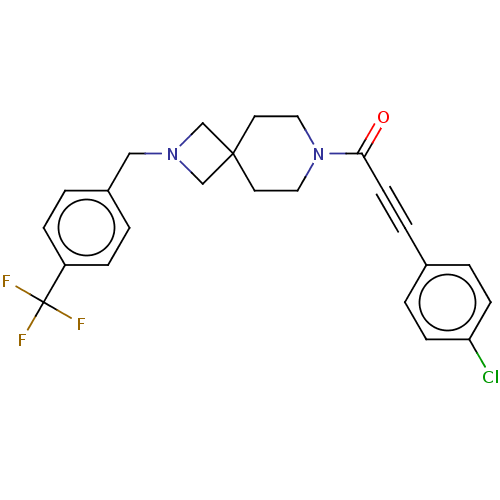 (3-(4-chlorophenyl)-1-(2-{[4-(trifluoromethyl)pheny...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332624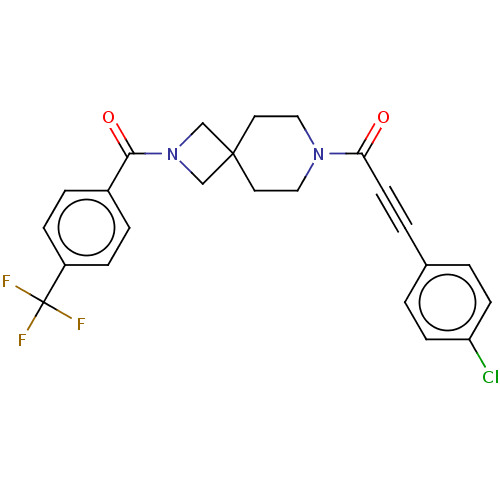 (3-(4-chlorophenyl)-1-(2-{[4-(trifluoromethyl)pheny...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332625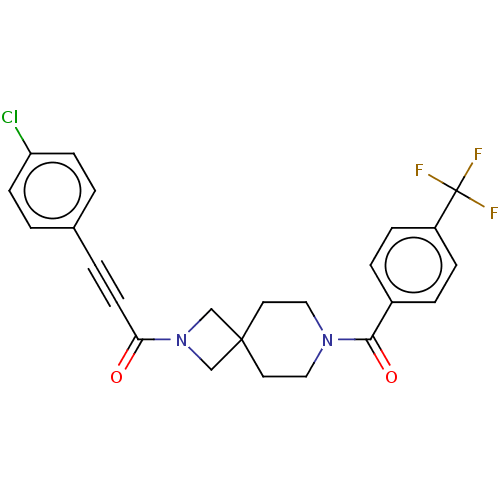 (3-(4-chlorophenyl)-1-(7-{[4-(trifluoromethyl)pheny...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332627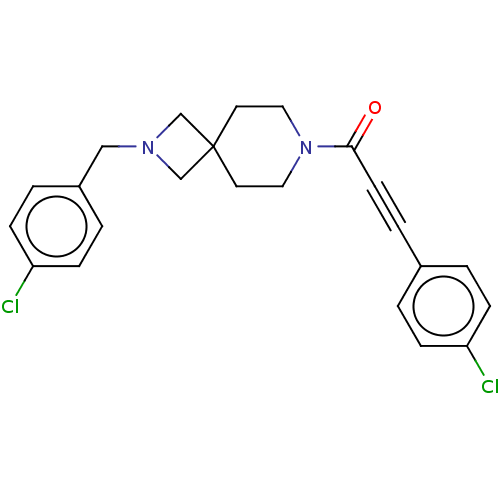 (3-(4-chlorophenyl)-1-{2-[(4-chlorophenyl)methyl]-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332629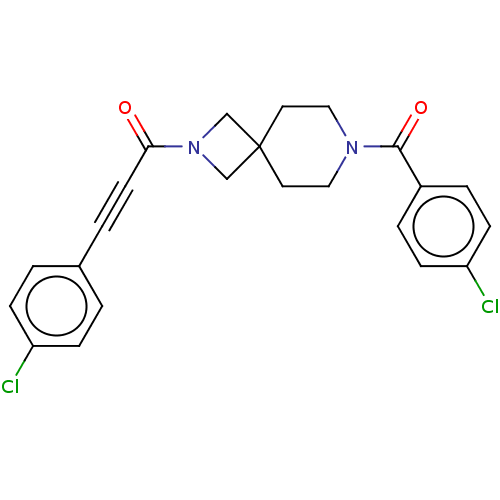 (3-(4-chlorophenyl)-1-{7-[(4-chlorophenyl)carbonyl]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332632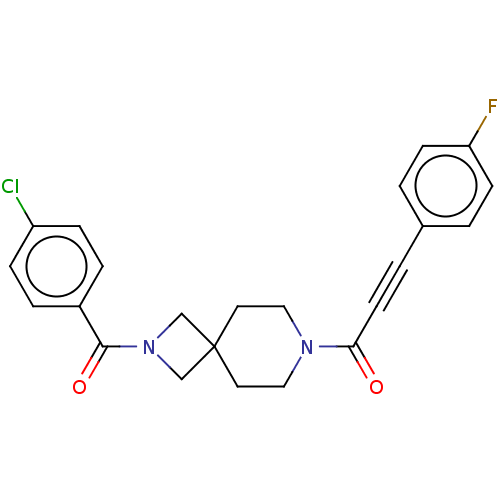 (1-{2-[(4-chlorophenyl)carbonyl]-2,7-diazaspiro[3.5...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332633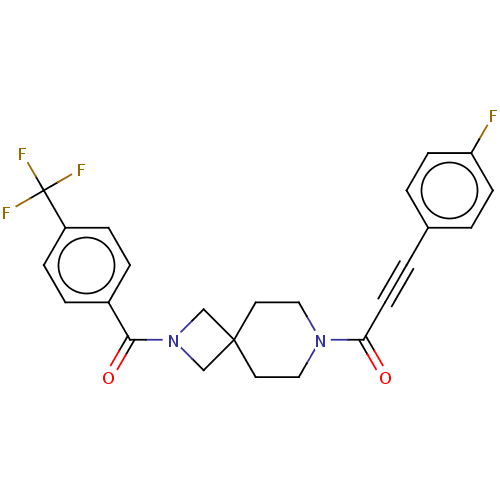 (3-(4-fluorophenyl)-1-(2-{[4-(trifluoromethyl)pheny...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332636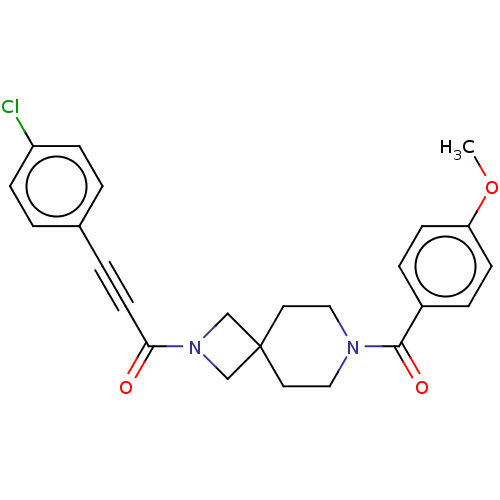 (3-(4-chlorophenyl)-1-{7-[(4-methoxyphenyl)carbonyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332640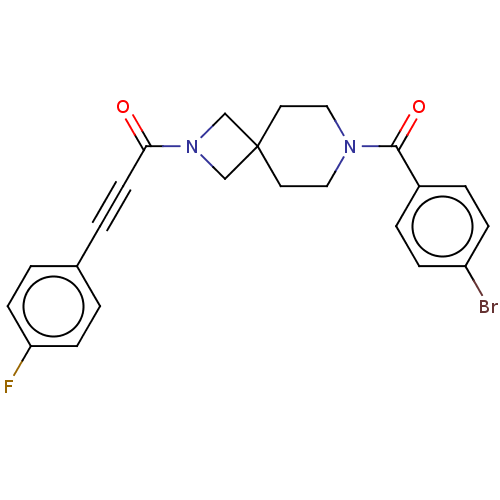 (1-{7-[(4-bromophenyl)carbonyl]-2,7-diazaspiro[3.5]...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332641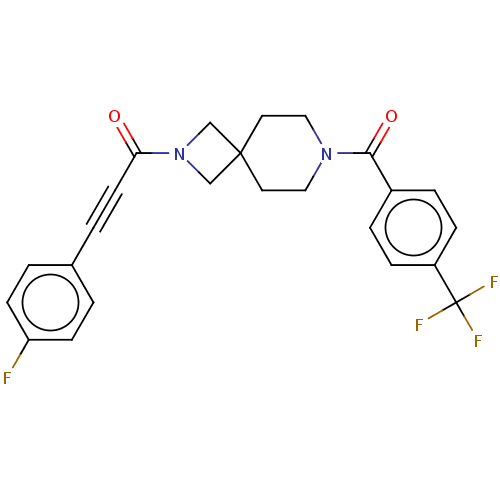 (3-(4-fluorophenyl)-1-(7-{[4-(trifluoromethyl)pheny...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332546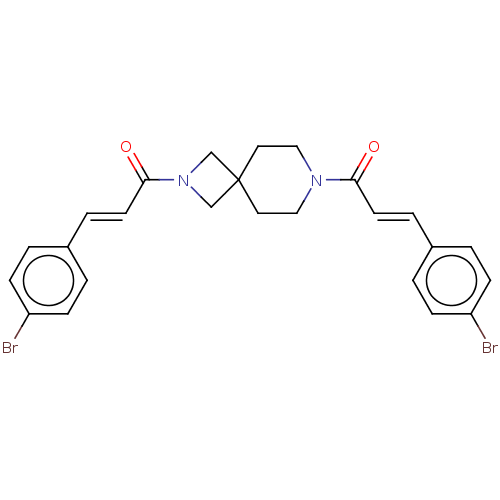 ((2E)-1-{7-[(2E)-3-(4-bromophenyl)prop-2-enoyl]-2,7...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332547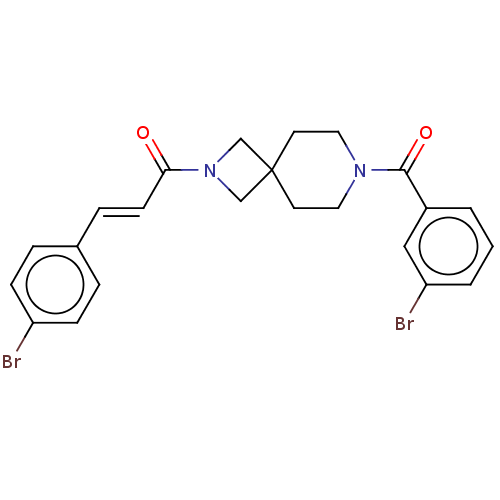 ((2E)-3-(4-bromophenyl)-1-{7-[(3-bromophenyl)carbon...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332549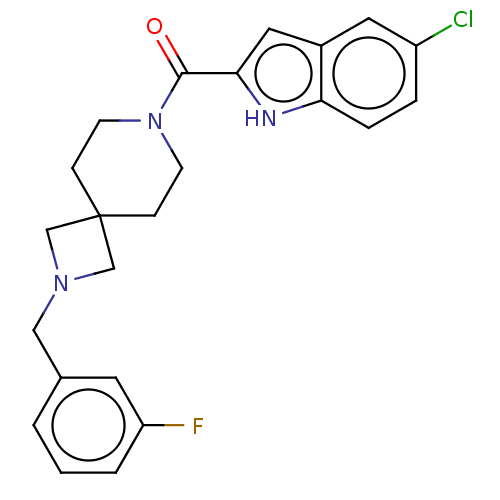 (5-chloroindol-2-yl 2-[(3-fluorophenyl)methyl]-2,7-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332551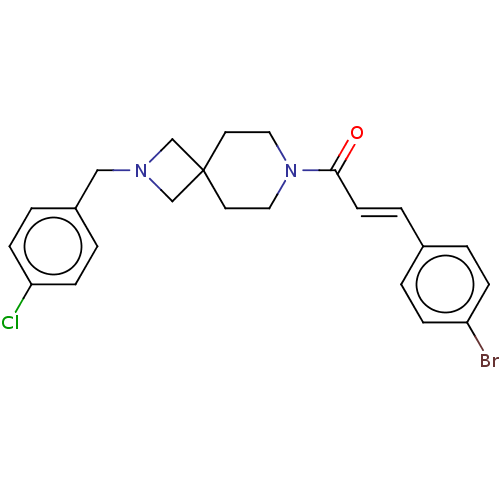 ((2E)-3-(4-bromophenyl)-1-{2-[(4-chlorophenyl)methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332552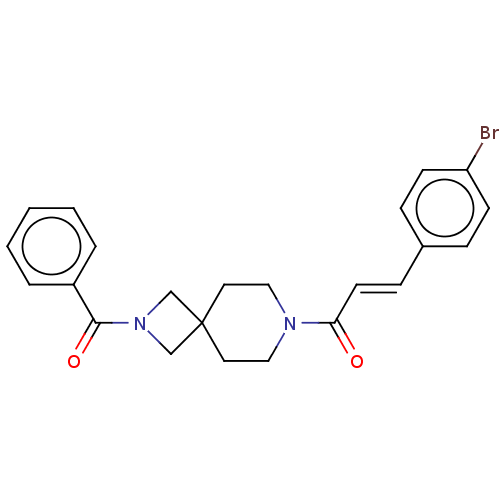 ((2E)-3-(4-bromophenyl)-1-[2-(phenylcarbonyl)-2,7-d...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332553 ((2E)-3-(4-bromophenyl)-1-{2-[(4-fluorophenyl)methy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332554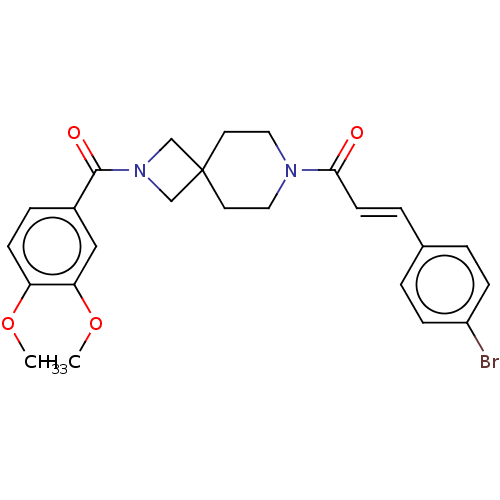 ((2E)-1-{2-[(3,4-dimethoxyphenyl)carbonyl]-2,7-diaz...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332555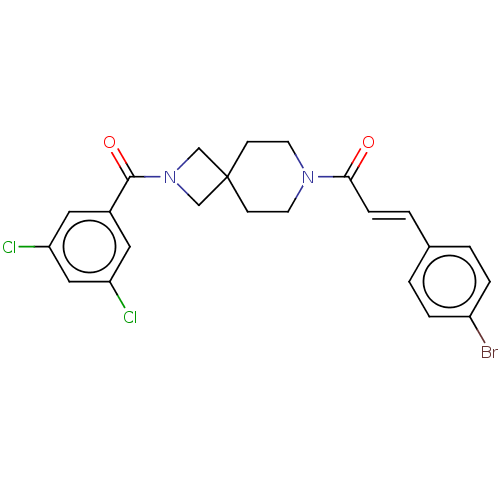 ((2E)-1-{2-[(3,5-dichlorophenyl)carbonyl]-2,7-diaza...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332556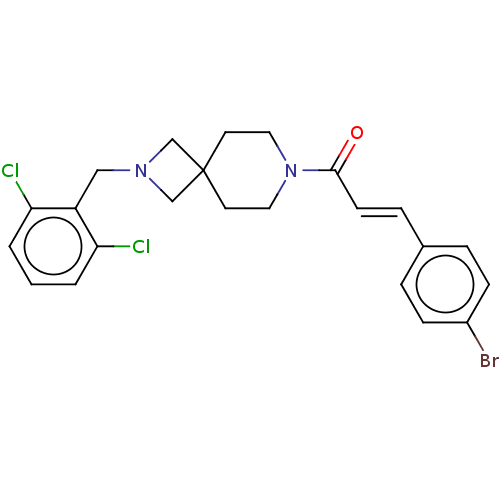 ((2E)-1-{2-[(2,6-dichlorophenyl)methyl]-2,7-diazasp...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332557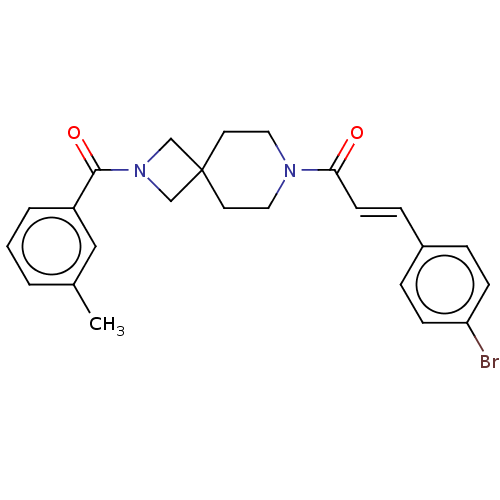 ((2E)-3-(4-bromophenyl)-1-{2-[(3-methylphenyl)carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332562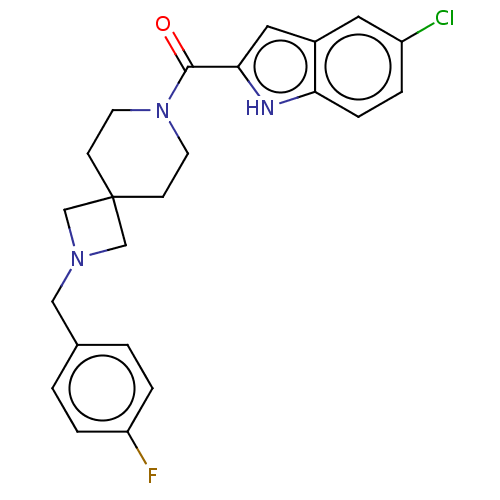 (5-chloroindol-2-yl 2-[(4-fluorophenyl)methyl]-2,7-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332563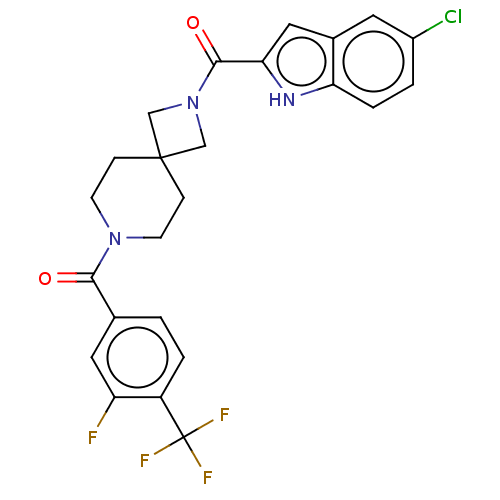 (5-chloroindol-2-yl 7-{[3-fluoro-4-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332564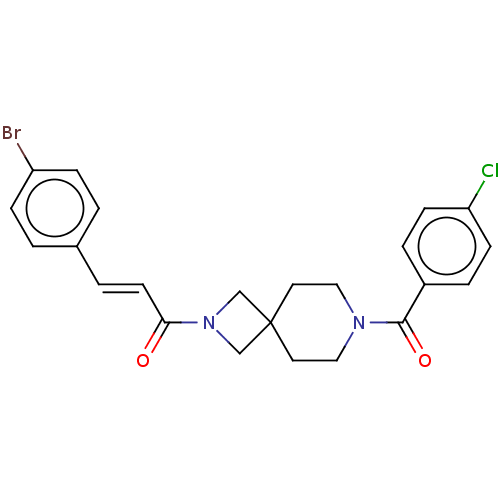 ((2E)-3-(4-bromophenyl)-1-{7-[(4-chlorophenyl)carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332565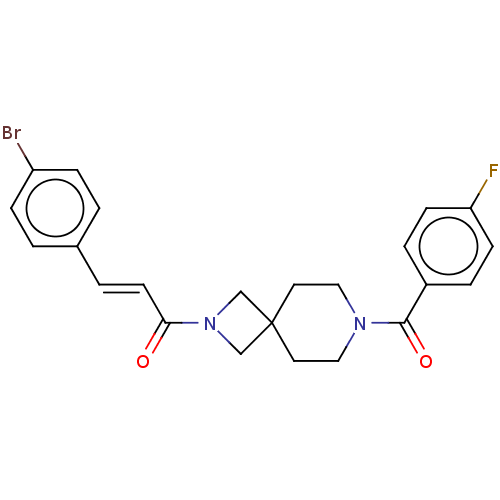 ((2E)-3-(4-bromophenyl)-1-{7-[(4-fluorophenyl)carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332566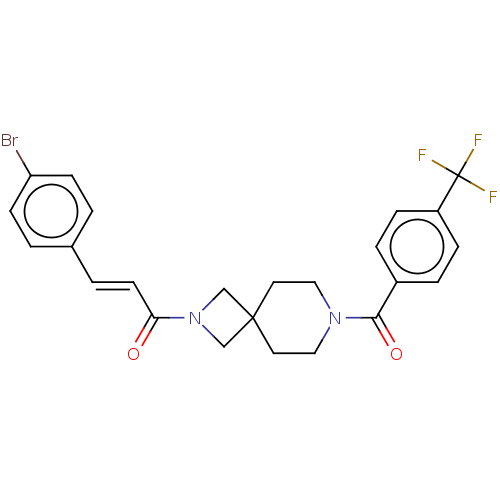 ((2E)-3-(4-bromophenyl)-1-(7-{[4-(trifluoromethyl)p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332567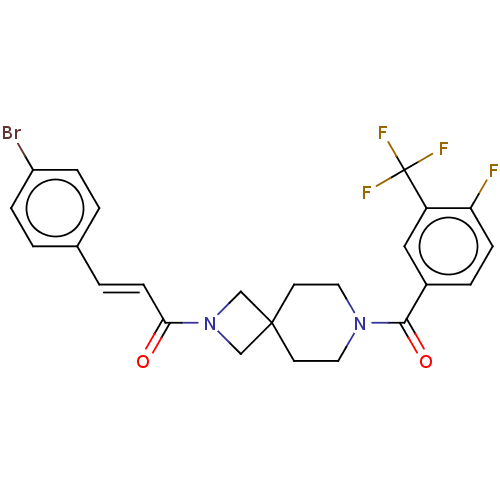 ((2E)-3-(4-bromophenyl)-1-(7-{[4-fluoro-3-(trifluor...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332573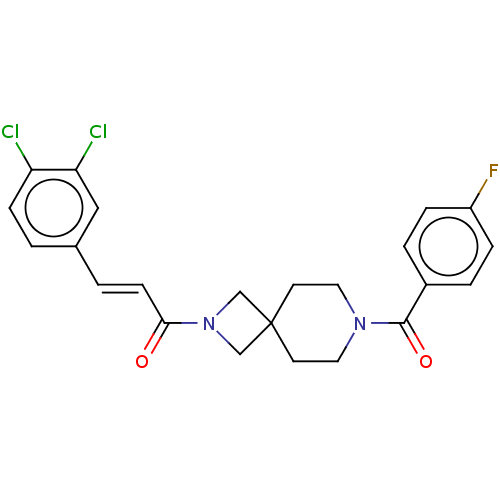 ((2E)-3-(3,4-dichlorophenyl)-1-{7-[(4-fluorophenyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332574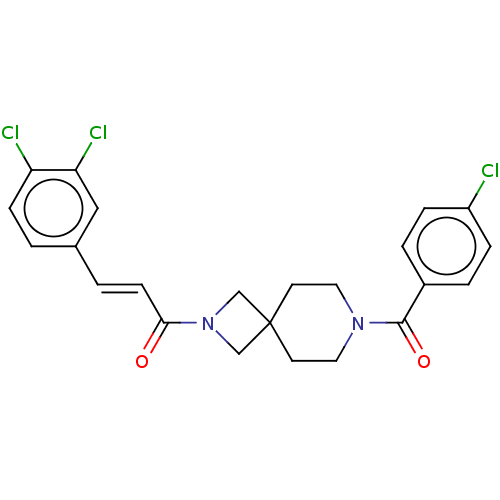 ((2E)-3-(3,4-dichlorophenyl)-1-{7-[(4-chlorophenyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332550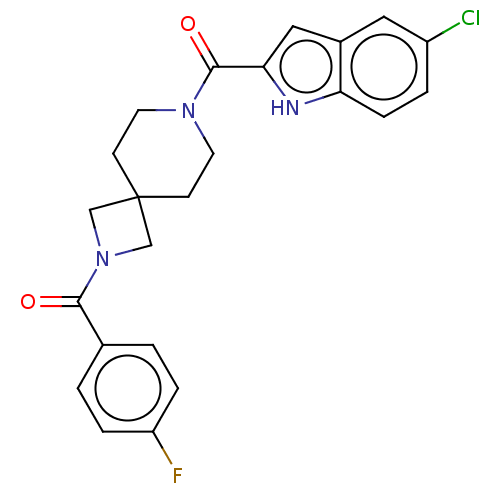 (5-chloroindol-2-yl 2-[(4-fluorophenyl)carbonyl]-2,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332645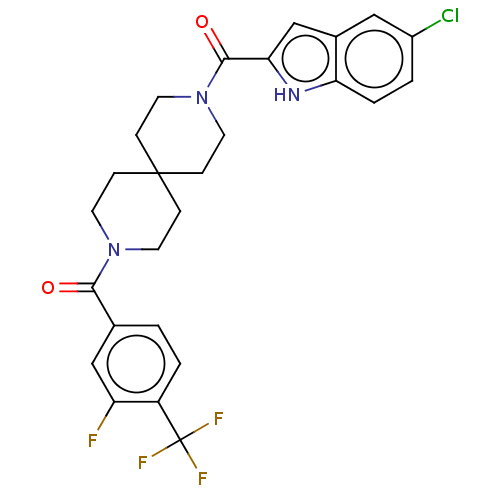 (5-chloroindol-2-yl 9-{[3-fluoro-4-(trifluoromethyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332646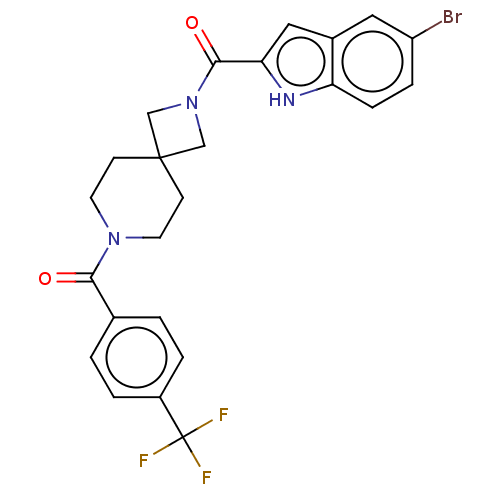 (5-bromoindol-2-yl 7-{[4-(trifluoromethyl)phenyl]ca...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332648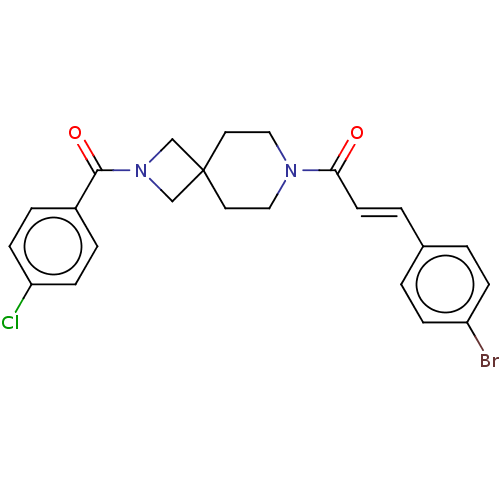 ((2E)-3-(4-bromophenyl)-1-{2-[(4-chlorophenyl)carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332613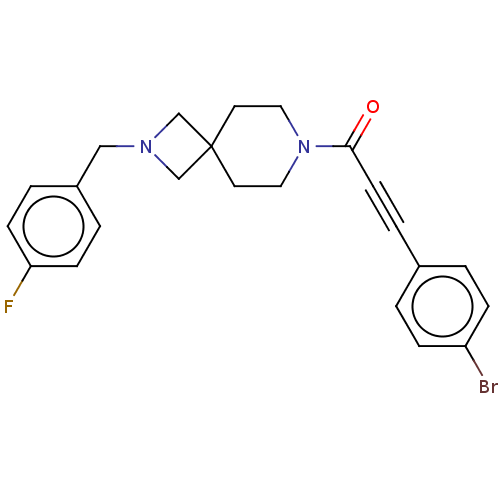 (3-(4-bromophenyl)-1-{2-[(4-fluorophenyl)methyl]-2,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332598 ((2E)-3-(4-bromophenyl)-1-{2-[(4-fluorophenyl)carbo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM332575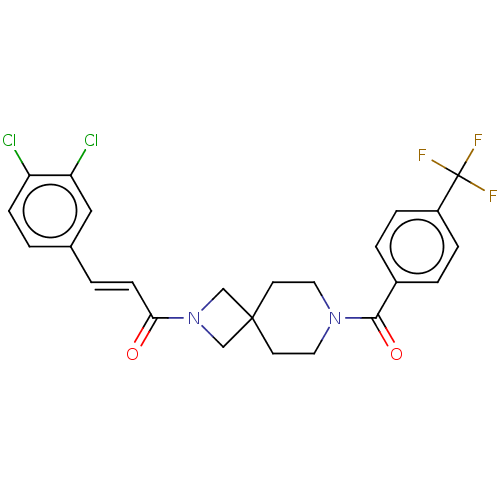 ((2E)-3-(3,4-dichlorophenyl)-1-(7-{[4-(trifluoromet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INSTITUTE US Patent | Assay Description The test compounds that demonstrated a corrected % activity of >=50% were defined as inhibitors of the reaction. The experimental values were normali... | US Patent US10196369 (2019) BindingDB Entry DOI: 10.7270/Q2BR8V88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1641 total ) | Next | Last >> |