 Found 11110 hits with Last Name = 'sha' and Initial = 'd'
Found 11110 hits with Last Name = 'sha' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50493155
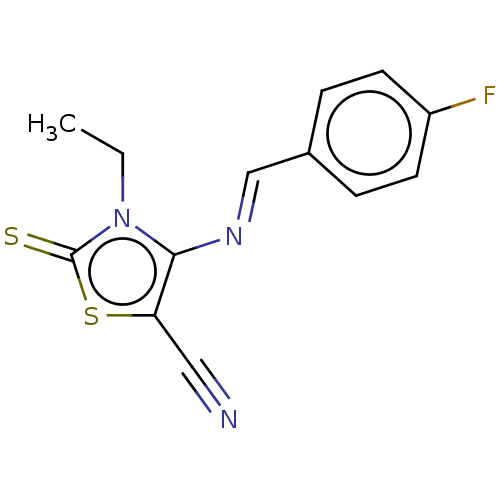
(CHEMBL2419148)Show InChI InChI=1S/C13H10FN3S2/c1-2-17-12(11(7-15)19-13(17)18)16-8-9-3-5-10(14)6-4-9/h3-6,8H,2H2,1H3/b16-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50493144
(CHEMBL2419144)Show InChI InChI=1S/C17H10FN3S2/c18-13-8-6-12(7-9-13)11-20-16-15(10-19)23-17(22)21(16)14-4-2-1-3-5-14/h1-9,11H/b20-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50493151
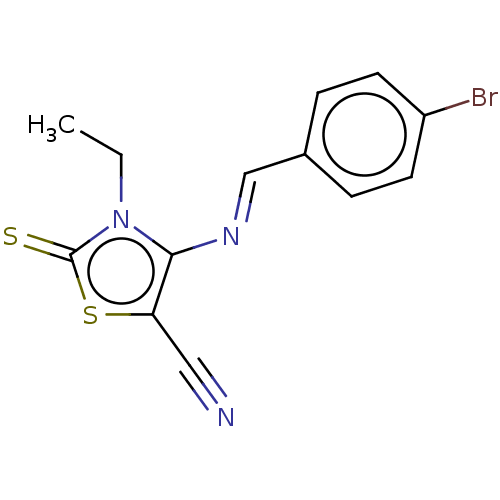
(CHEMBL2419139)Show InChI InChI=1S/C13H10BrN3S2/c1-2-17-12(11(7-15)19-13(17)18)16-8-9-3-5-10(14)6-4-9/h3-6,8H,2H2,1H3/b16-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493145
(CHEMBL2419149)Show InChI InChI=1S/C17H10BrN3S2/c18-13-8-6-12(7-9-13)11-20-16-15(10-19)23-17(22)21(16)14-4-2-1-3-5-14/h1-9,11H/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493144

(CHEMBL2419144)Show InChI InChI=1S/C17H10FN3S2/c18-13-8-6-12(7-9-13)11-20-16-15(10-19)23-17(22)21(16)14-4-2-1-3-5-14/h1-9,11H/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493151

(CHEMBL2419139)Show InChI InChI=1S/C13H10BrN3S2/c1-2-17-12(11(7-15)19-13(17)18)16-8-9-3-5-10(14)6-4-9/h3-6,8H,2H2,1H3/b16-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793
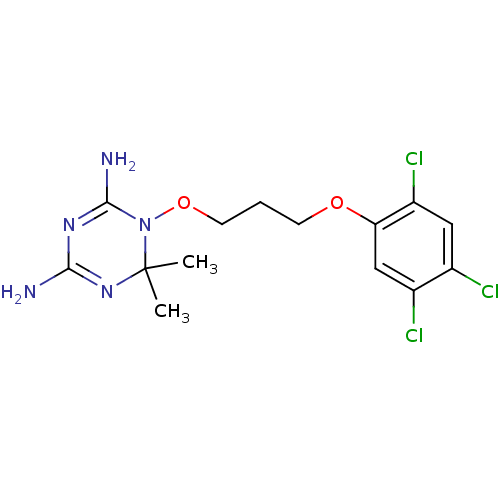
(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0110 | -62.5 | 0.570 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793

(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0200 | -61.1 | 2.30 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493157
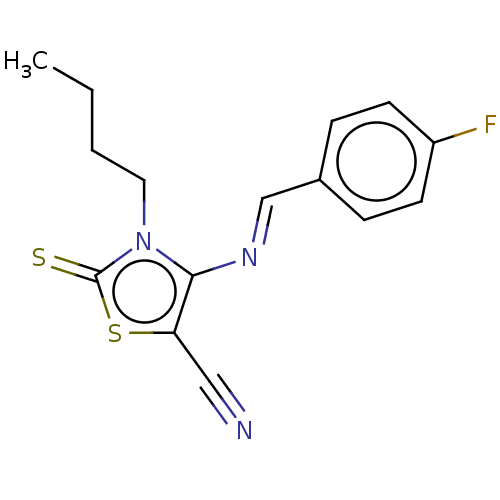
(CHEMBL2419146)Show InChI InChI=1S/C15H14FN3S2/c1-2-3-8-19-14(13(9-17)21-15(19)20)18-10-11-4-6-12(16)7-5-11/h4-7,10H,2-3,8H2,1H3/b18-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493154

(CHEMBL2419147)Show InChI InChI=1S/C14H12FN3S2/c1-2-7-18-13(12(8-16)20-14(18)19)17-9-10-3-5-11(15)6-4-10/h3-6,9H,2,7H2,1H3/b17-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase [N51I,C59R,S108N,I164L]
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793

(6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...)Show SMILES CC1(C)N=C(N)N=C(N)N1OCCCOc1cc(Cl)c(Cl)cc1Cl |t:3,6| Show InChI InChI=1S/C14H18Cl3N5O2/c1-14(2)21-12(18)20-13(19)22(14)24-5-3-4-23-11-7-9(16)8(15)6-10(11)17/h6-7H,3-5H2,1-2H3,(H4,18,19,20,21) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0370 | -59.5 | 18 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948
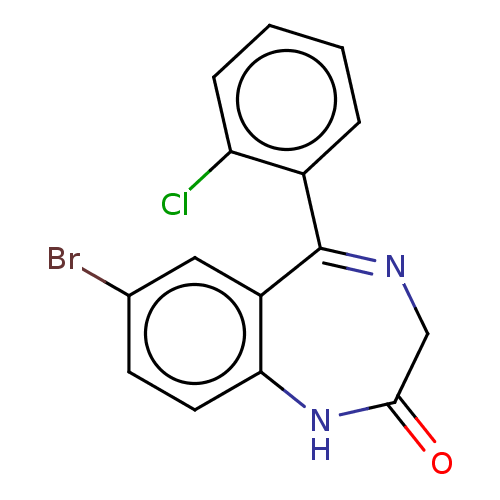
(PHENAZEPAM) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948

(PHENAZEPAM) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004193
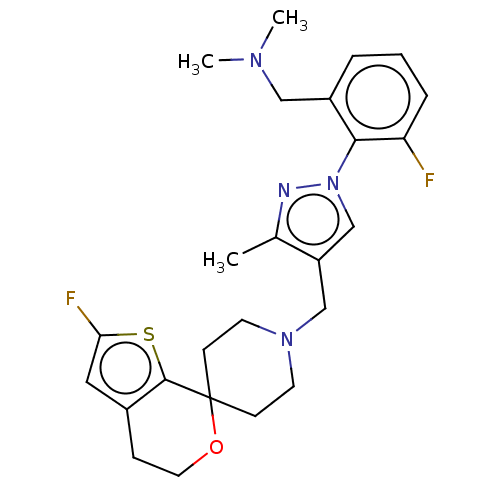
(CHEMBL3236476)Show SMILES OC(C(O)C(O)=O)C(O)=O.CN(C)Cc1cccc(F)c1-n1cc(CN2CCC3(CC2)OCCc2cc(F)sc32)c(C)n1 Show InChI InChI=1S/C25H30F2N4OS.C4H6O6/c1-17-20(16-31(28-17)23-19(14-29(2)3)5-4-6-21(23)26)15-30-10-8-25(9-11-30)24-18(7-12-32-25)13-22(27)33-24;5-1(3(7)8)2(6)4(9)10/h4-6,13,16H,7-12,14-15H2,1-3H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0648 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493156
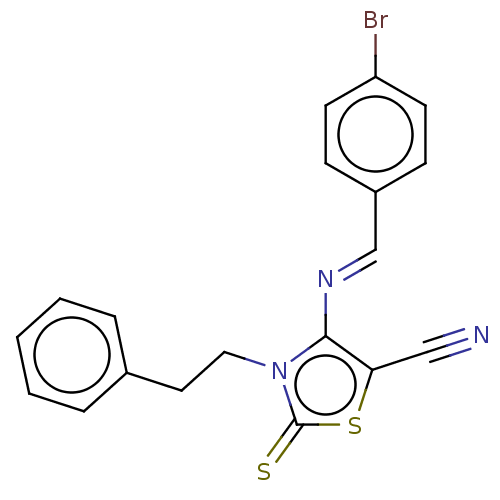
(CHEMBL2419150)Show SMILES Brc1ccc(\C=N\c2c(sc(=S)n2CCc2ccccc2)C#N)cc1 Show InChI InChI=1S/C19H14BrN3S2/c20-16-8-6-15(7-9-16)13-22-18-17(12-21)25-19(24)23(18)11-10-14-4-2-1-3-5-14/h1-9,13H,10-11H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004180
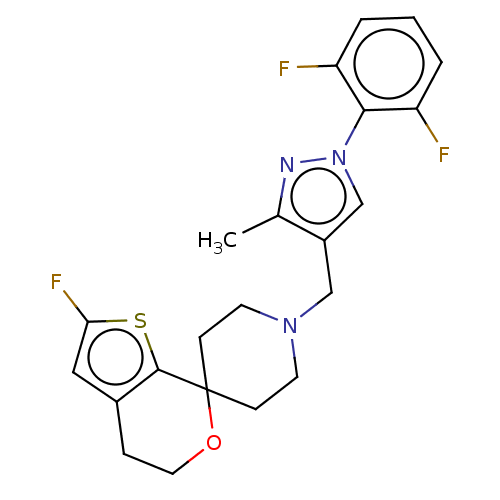
(CHEMBL3236474)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCCc1cc(F)sc21)-c1c(F)cccc1F Show InChI InChI=1S/C22H22F3N3OS.C4H6O6/c1-14-16(13-28(26-14)20-17(23)3-2-4-18(20)24)12-27-8-6-22(7-9-27)21-15(5-10-29-22)11-19(25)30-21;5-1(3(7)8)2(6)4(9)10/h2-4,11,13H,5-10,12H2,1H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0711 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004195
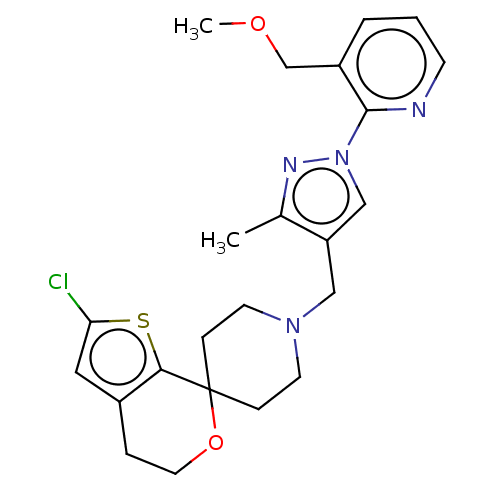
(CHEMBL3236478)Show SMILES OC(C(O)C(O)=O)C(O)=O.COCc1cccnc1-n1cc(CN2CCC3(CC2)OCCc2cc(Cl)sc32)c(C)n1 Show InChI InChI=1S/C23H27ClN4O2S.C4H6O6/c1-16-19(14-28(26-16)22-18(15-29-2)4-3-8-25-22)13-27-9-6-23(7-10-27)21-17(5-11-30-23)12-20(24)31-21;5-1(3(7)8)2(6)4(9)10/h3-4,8,12,14H,5-7,9-11,13,15H2,1-2H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0908 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004175

(CHEMBL3236486)Show SMILES Cc1nn(cc1CN1CCC2(CC1)OCC(F)(F)c1cc(Cl)sc21)-c1ncccc1-c1ncc[nH]1 Show InChI InChI=1S/C24H23ClF2N6OS/c1-15-16(13-33(31-15)22-17(3-2-6-30-22)21-28-7-8-29-21)12-32-9-4-23(5-10-32)20-18(11-19(25)35-20)24(26,27)14-34-23/h2-3,6-8,11,13H,4-5,9-10,12,14H2,1H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0916 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004196
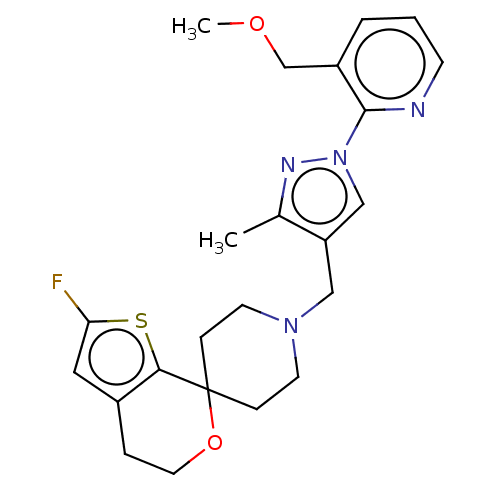
(CHEMBL3236479)Show SMILES OC(C(O)C(O)=O)C(O)=O.COCc1cccnc1-n1cc(CN2CCC3(CC2)OCCc2cc(F)sc32)c(C)n1 Show InChI InChI=1S/C23H27FN4O2S.C4H6O6/c1-16-19(14-28(26-16)22-18(15-29-2)4-3-8-25-22)13-27-9-6-23(7-10-27)21-17(5-11-30-23)12-20(24)31-21;5-1(3(7)8)2(6)4(9)10/h3-4,8,12,14H,5-7,9-11,13,15H2,1-2H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0939 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004181
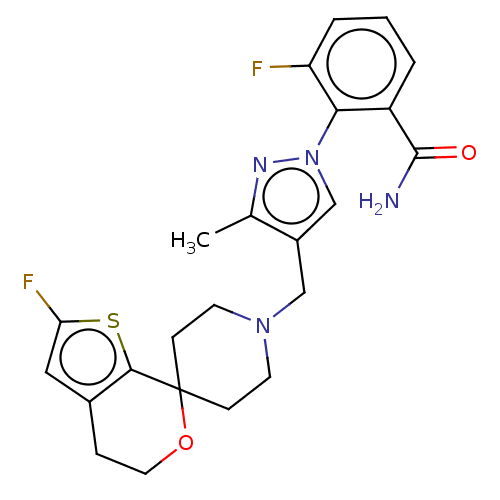
(CHEMBL3236475)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCCc1cc(F)sc21)-c1c(F)cccc1C(N)=O Show InChI InChI=1S/C23H24F2N4O2S.C4H6O6/c1-14-16(13-29(27-14)20-17(22(26)30)3-2-4-18(20)24)12-28-8-6-23(7-9-28)21-15(5-10-31-23)11-19(25)32-21;5-1(3(7)8)2(6)4(9)10/h2-4,11,13H,5-10,12H2,1H3,(H2,26,30);1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0954 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004174
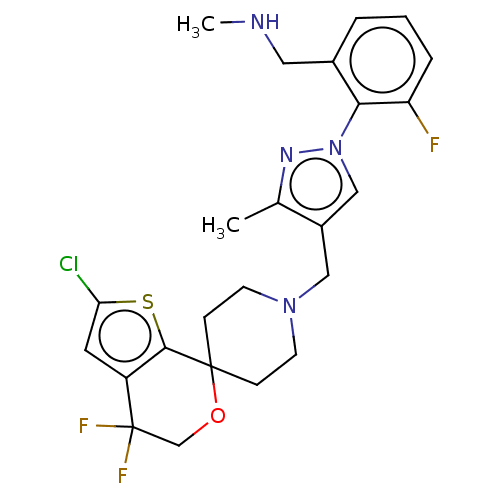
(CHEMBL3236485)Show SMILES OC(C(O)C(O)=O)C(O)=O.CNCc1cccc(F)c1-n1cc(CN2CCC3(CC2)OCC(F)(F)c2cc(Cl)sc32)c(C)n1 Show InChI InChI=1S/C24H26ClF3N4OS.C4H6O6/c1-15-17(13-32(30-15)21-16(11-29-2)4-3-5-19(21)26)12-31-8-6-23(7-9-31)22-18(10-20(25)34-22)24(27,28)14-33-23;5-1(3(7)8)2(6)4(9)10/h3-5,10,13,29H,6-9,11-12,14H2,1-2H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50493148
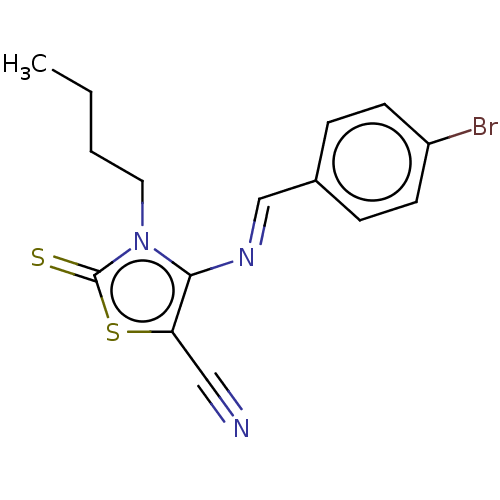
(CHEMBL2419137)Show InChI InChI=1S/C15H14BrN3S2/c1-2-3-8-19-14(13(9-17)21-15(19)20)18-10-11-4-6-12(16)7-5-11/h4-7,10H,2-3,8H2,1H3/b18-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004176
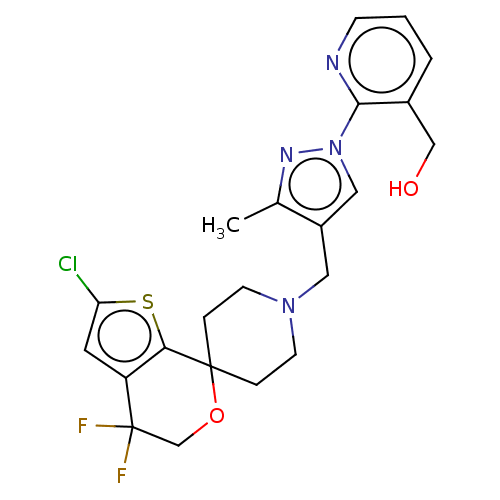
(CHEMBL3236487)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCC(F)(F)c1cc(Cl)sc21)-c1ncccc1CO Show InChI InChI=1S/C22H23ClF2N4O2S.C4H6O6/c1-14-16(11-29(27-14)20-15(12-30)3-2-6-26-20)10-28-7-4-21(5-8-28)19-17(9-18(23)32-19)22(24,25)13-31-21;5-1(3(7)8)2(6)4(9)10/h2-3,6,9,11,30H,4-5,7-8,10,12-13H2,1H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004173
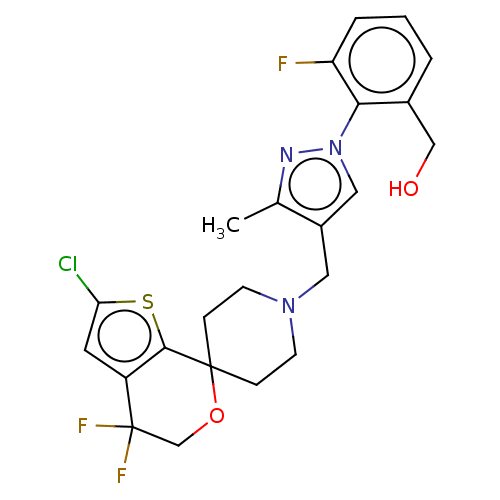
(CHEMBL3236484)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCC(F)(F)c1cc(Cl)sc21)-c1c(F)cccc1CO Show InChI InChI=1S/C23H23ClF3N3O2S.C4H6O6/c1-14-16(11-30(28-14)20-15(12-31)3-2-4-18(20)25)10-29-7-5-22(6-8-29)21-17(9-19(24)33-21)23(26,27)13-32-22;5-1(3(7)8)2(6)4(9)10/h2-4,9,11,31H,5-8,10,12-13H2,1H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599948

(PHENAZEPAM) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 5
(Homo sapiens (Human)) | BDBM50533413
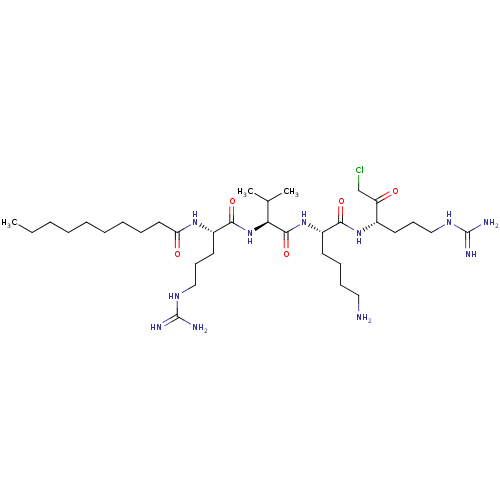
(CHEMBL3126388)Show SMILES CCCCCCCCCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl |r| Show InChI InChI=1S/C34H66ClN11O5/c1-4-5-6-7-8-9-10-18-28(48)43-25(17-14-21-42-34(39)40)31(50)46-29(23(2)3)32(51)45-26(15-11-12-19-36)30(49)44-24(27(47)22-35)16-13-20-41-33(37)38/h23-26,29H,4-22,36H2,1-3H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H4,37,38,41)(H4,39,40,42)/t24-,25-,26-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal truncated human SPC6 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry |
J Med Chem 59: 7719-37 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01516
BindingDB Entry DOI: 10.7270/Q2M048X4 |
More data for this
Ligand-Target Pair | |
Proprotein convertase subtilisin/kexin type 7
(Homo sapiens (Human)) | BDBM50533413

(CHEMBL3126388)Show SMILES CCCCCCCCCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl |r| Show InChI InChI=1S/C34H66ClN11O5/c1-4-5-6-7-8-9-10-18-28(48)43-25(17-14-21-42-34(39)40)31(50)46-29(23(2)3)32(51)45-26(15-11-12-19-36)30(49)44-24(27(47)22-35)16-13-20-41-33(37)38/h23-26,29H,4-22,36H2,1-3H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H4,37,38,41)(H4,39,40,42)/t24-,25-,26-,29-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal truncated human SPC7 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry |
J Med Chem 59: 7719-37 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01516
BindingDB Entry DOI: 10.7270/Q2M048X4 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004194
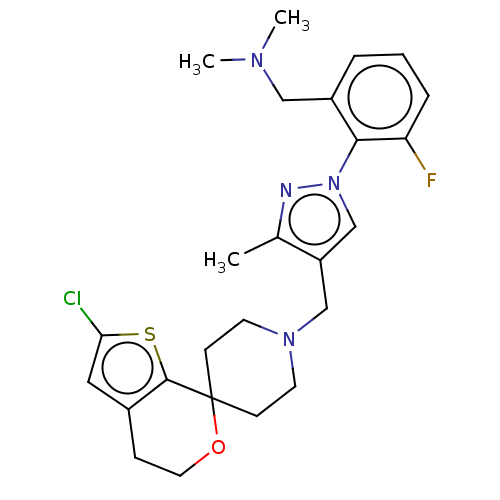
(CHEMBL3236477)Show SMILES OC(C(O)C(O)=O)C(O)=O.CN(C)Cc1cccc(F)c1-n1cc(CN2CCC3(CC2)OCCc2cc(Cl)sc32)c(C)n1 Show InChI InChI=1S/C25H30ClFN4OS.C4H6O6/c1-17-20(16-31(28-17)23-19(14-29(2)3)5-4-6-21(23)27)15-30-10-8-25(9-11-30)24-18(7-12-32-25)13-22(26)33-24;5-1(3(7)8)2(6)4(9)10/h4-6,13,16H,7-12,14-15H2,1-3H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004179
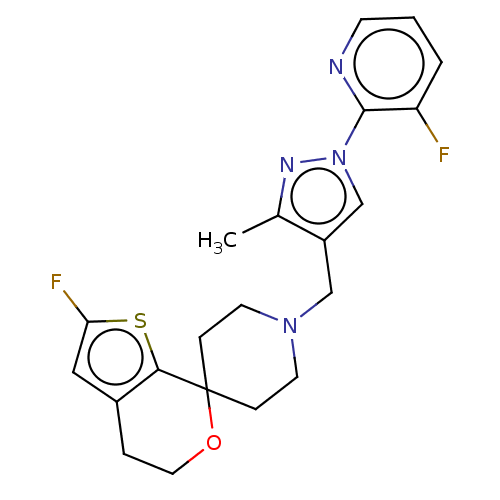
(CHEMBL3236473)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCCc1cc(F)sc21)-c1ncccc1F Show InChI InChI=1S/C21H22F2N4OS.C4H6O6/c1-14-16(13-27(25-14)20-17(22)3-2-7-24-20)12-26-8-5-21(6-9-26)19-15(4-10-28-21)11-18(23)29-19;5-1(3(7)8)2(6)4(9)10/h2-3,7,11,13H,4-6,8-10,12H2,1H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50493150
(CHEMBL2419140)Show InChI InChI=1S/C17H10ClN3S2/c18-13-8-6-12(7-9-13)11-20-16-15(10-19)23-17(22)21(16)14-4-2-1-3-5-14/h1-9,11H/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004166

(CHEMBL3236482)Show SMILES OC(C(O)C(O)=O)C(O)=O.CN(C)Cc1cccc(F)c1-n1cc(CN2CCC3(CC2)OCC(F)(F)c2cc(Cl)sc32)c(C)n1 Show InChI InChI=1S/C25H28ClF3N4OS.C4H6O6/c1-16-18(14-33(30-16)22-17(12-31(2)3)5-4-6-20(22)27)13-32-9-7-24(8-10-32)23-19(11-21(26)35-23)25(28,29)15-34-24;5-1(3(7)8)2(6)4(9)10/h4-6,11,14H,7-10,12-13,15H2,1-3H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949
(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004144
(CHEMBL3236480)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCC(F)(F)c1cc(Cl)sc21)-c1c(F)cccc1F Show InChI InChI=1S/C22H20ClF4N3OS.C4H6O6/c1-13-14(11-30(28-13)19-16(24)3-2-4-17(19)25)10-29-7-5-21(6-8-29)20-15(9-18(23)32-20)22(26,27)12-31-21;5-1(3(7)8)2(6)4(9)10/h2-4,9,11H,5-8,10,12H2,1H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004161
(CHEMBL3236481)Show SMILES OC(C(O)C(O)=O)C(O)=O.Cc1nn(cc1CN1CCC2(CC1)OCC(F)(F)c1cc(Cl)sc21)-c1ncccc1F Show InChI InChI=1S/C21H20ClF3N4OS.C4H6O6/c1-13-14(11-29(27-13)19-16(23)3-2-6-26-19)10-28-7-4-20(5-8-28)18-15(9-17(22)31-18)21(24,25)12-30-20;5-1(3(7)8)2(6)4(9)10/h2-3,6,9,11H,4-5,7-8,10,12H2,1H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004177
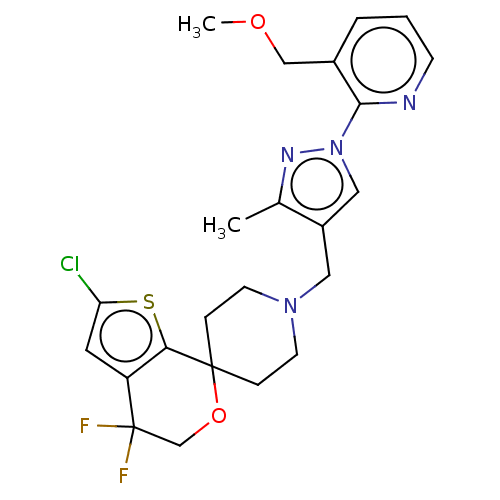
(CHEMBL3236488)Show SMILES OC(C(O)C(O)=O)C(O)=O.COCc1cccnc1-n1cc(CN2CCC3(CC2)OCC(F)(F)c2cc(Cl)sc32)c(C)n1 Show InChI InChI=1S/C23H25ClF2N4O2S.C4H6O6/c1-15-17(12-30(28-15)21-16(13-31-2)4-3-7-27-21)11-29-8-5-22(6-9-29)20-18(10-19(24)33-20)23(25,26)14-32-22;5-1(3(7)8)2(6)4(9)10/h3-4,7,10,12H,5-6,8-9,11,13-14H2,1-2H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530711
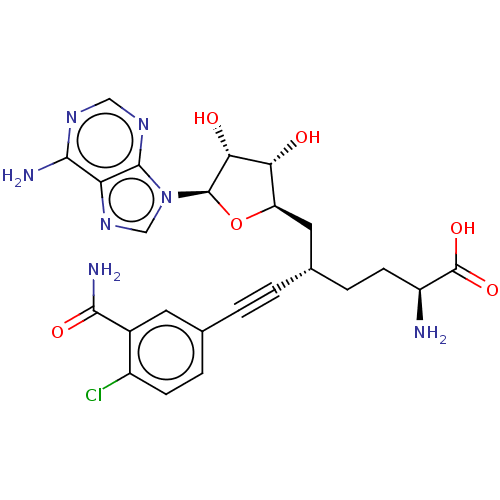
(CHEMBL4553052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(Cl)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26ClN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530711

(CHEMBL4553052)Show SMILES N[C@@H](CC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1ccc(Cl)c(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C24H26ClN7O6/c25-14-5-3-11(7-13(14)21(28)35)1-2-12(4-6-15(26)24(36)37)8-16-18(33)19(34)23(38-16)32-10-31-17-20(27)29-9-30-22(17)32/h3,5,7,9-10,12,15-16,18-19,23,33-34H,4,6,8,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t12-,15-,16+,18+,19+,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512

(5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...)Show InChI InChI=1S/C12H13ClN4/c1-2-9-10(11(14)17-12(15)16-9)7-3-5-8(13)6-4-7/h3-6H,2H2,1H3,(H4,14,15,16,17) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -55.4 | 80 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178
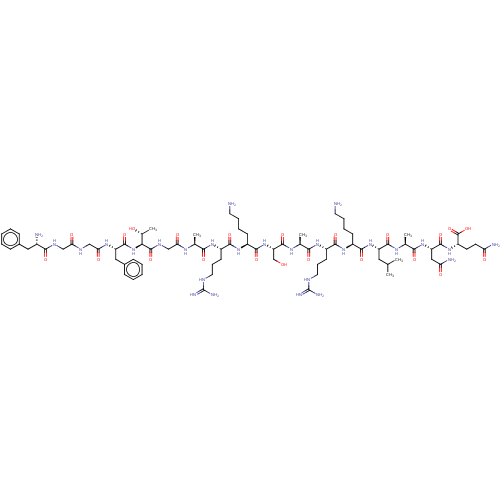
(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530731
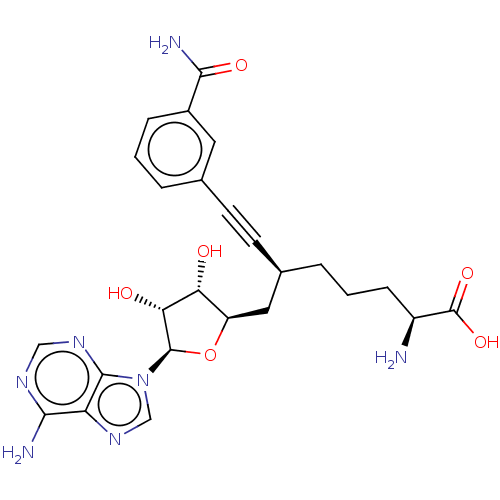
(CHEMBL4580446)Show SMILES N[C@@H](CCC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C25H29N7O6/c26-16(25(36)37)6-2-4-14(8-7-13-3-1-5-15(9-13)22(28)35)10-17-19(33)20(34)24(38-17)32-12-31-18-21(27)29-11-30-23(18)32/h1,3,5,9,11-12,14,16-17,19-20,24,33-34H,2,4,6,10,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16+,17-,19-,20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Nicotinamide N-methyltransferase
(Homo sapiens (Human)) | BDBM50530731

(CHEMBL4580446)Show SMILES N[C@@H](CCC[C@@H](C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C#Cc1cccc(c1)C(N)=O)C(O)=O |r| Show InChI InChI=1S/C25H29N7O6/c26-16(25(36)37)6-2-4-14(8-7-13-3-1-5-15(9-13)22(28)35)10-17-19(33)20(34)24(38-17)32-12-31-18-21(27)29-11-30-23(18)32/h1,3,5,9,11-12,14,16-17,19-20,24,33-34H,2,4,6,10,26H2,(H2,28,35)(H,36,37)(H2,27,29,30)/t14-,16+,17-,19-,20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged wild type human NNMT expressed in Escherichia coli NiCo21(DE3) assessed as reduction in 1-methylquinolinium leve... |
J Med Chem 62: 9837-9873 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01238
BindingDB Entry DOI: 10.7270/Q2D50RDJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50493156

(CHEMBL2419150)Show SMILES Brc1ccc(\C=N\c2c(sc(=S)n2CCc2ccccc2)C#N)cc1 Show InChI InChI=1S/C19H14BrN3S2/c20-16-8-6-15(7-9-16)13-22-18-17(12-21)25-19(24)23(18)11-10-14-4-2-1-3-5-14/h1-9,13H,10-11H2/b22-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPCPX from human A1 receptor expressed in HEK293 cell membranes after 60 mins by scintillation counting |
Bioorg Med Chem 21: 6077-83 (2013)
Article DOI: 10.1016/j.bmc.2013.07.005
BindingDB Entry DOI: 10.7270/Q2CC13NZ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2
(Homo sapiens (Human)) | BDBM50599949

(CHEMBL3274851) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128637
BindingDB Entry DOI: 10.7270/Q24B35B0 |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792
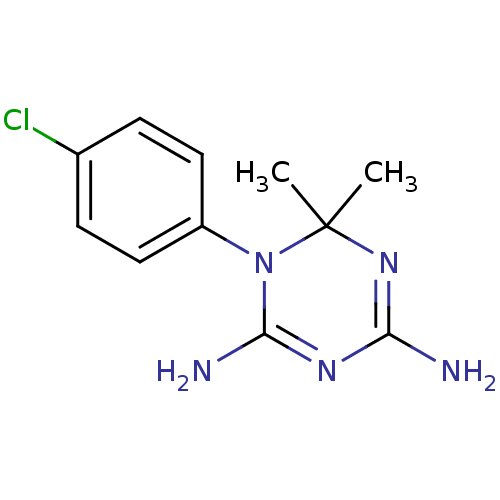
(1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...)Show InChI InChI=1S/C11H14ClN5/c1-11(2)16-9(13)15-10(14)17(11)8-5-3-7(12)4-6-8/h3-6H,1-2H3,(H4,13,14,15,16) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -54.4 | 37 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University
| Assay Description
The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... |
Nat Struct Biol 10: 257-65 (2003)
Article DOI: 10.1038/nsb921
BindingDB Entry DOI: 10.7270/Q2HH6HBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50244020
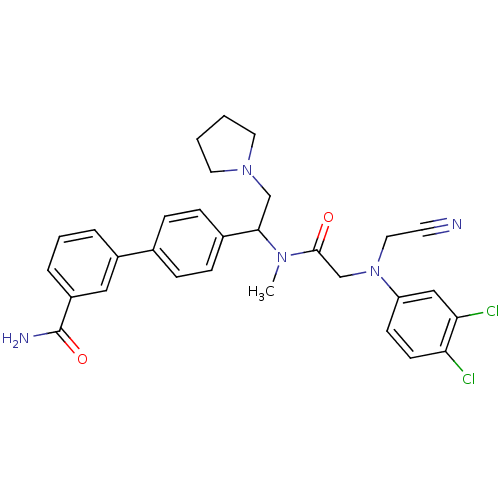
(4'-[1-({2-[Cyanomethyl-(3,4-dichloro-phenyl)-amino...)Show SMILES CN(C(CN1CCCC1)c1ccc(cc1)-c1cccc(c1)C(N)=O)C(=O)CN(CC#N)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C30H31Cl2N5O2/c1-35(29(38)20-37(16-13-33)25-11-12-26(31)27(32)18-25)28(19-36-14-2-3-15-36)22-9-7-21(8-10-22)23-5-4-6-24(17-23)30(34)39/h4-12,17-18,28H,2-3,14-16,19-20H2,1H3,(H2,34,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to human urotensin2 receptor |
Bioorg Med Chem Lett 18: 3716-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.058
BindingDB Entry DOI: 10.7270/Q2RN37NJ |
More data for this
Ligand-Target Pair | |
Neuroendocrine convertase 2
(Homo sapiens (Human)) | BDBM50533413

(CHEMBL3126388)Show SMILES CCCCCCCCCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)CCl |r| Show InChI InChI=1S/C34H66ClN11O5/c1-4-5-6-7-8-9-10-18-28(48)43-25(17-14-21-42-34(39)40)31(50)46-29(23(2)3)32(51)45-26(15-11-12-19-36)30(49)44-24(27(47)22-35)16-13-20-41-33(37)38/h23-26,29H,4-22,36H2,1-3H3,(H,43,48)(H,44,49)(H,45,51)(H,46,50)(H4,37,38,41)(H4,39,40,42)/t24-,25-,26-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal truncated human SPC2 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry |
J Med Chem 59: 7719-37 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01516
BindingDB Entry DOI: 10.7270/Q2M048X4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50130293

(7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...)Show SMILES Clc1cccc(N2CCN(CCCCOc3ccc4CCC(=O)Nc4c3)CC2)c1Cl Show InChI InChI=1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 28: 1400-11 (2003)
Article DOI: 10.1038/sj.npp.1300203
BindingDB Entry DOI: 10.7270/Q2639N9T |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004171
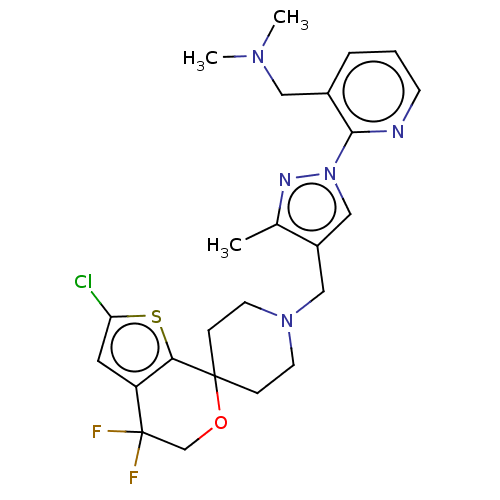
(CHEMBL3236483)Show SMILES OC(C(O)C(O)=O)C(O)=O.CN(C)Cc1cccnc1-n1cc(CN2CCC3(CC2)OCC(F)(F)c2cc(Cl)sc32)c(C)n1 Show InChI InChI=1S/C24H28ClF2N5OS.C4H6O6/c1-16-18(14-32(29-16)22-17(12-30(2)3)5-4-8-28-22)13-31-9-6-23(7-10-31)21-19(11-20(25)34-21)24(26,27)15-33-23;5-1(3(7)8)2(6)4(9)10/h4-5,8,11,14H,6-7,9-10,12-13,15H2,1-3H3;1-2,5-6H,(H,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting |
J Med Chem 57: 3418-29 (2014)
Article DOI: 10.1021/jm500117r
BindingDB Entry DOI: 10.7270/Q26Q1ZS0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data