 Found 151 hits with Last Name = 'shah' and Initial = 'sr'
Found 151 hits with Last Name = 'shah' and Initial = 'sr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420657
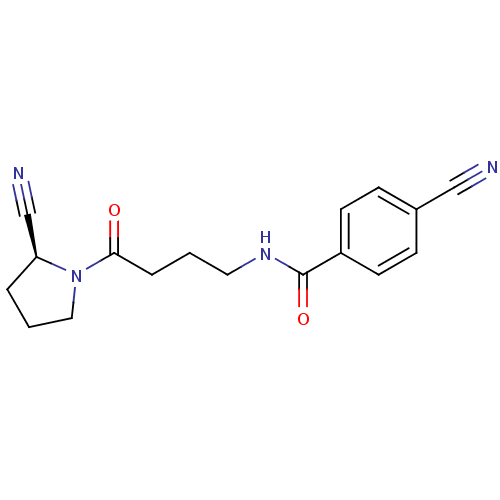
(CHEMBL2086613)Show SMILES O=C(CCCNC(=O)c1ccc(cc1)C#N)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H18N4O2/c18-11-13-5-7-14(8-6-13)17(23)20-9-1-4-16(22)21-10-2-3-15(21)12-19/h5-8,15H,1-4,9-10H2,(H,20,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124215
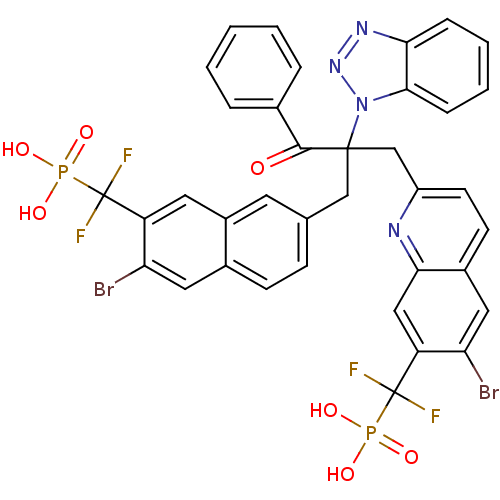
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C37H26Br2F4N4O7P2/c38-29-16-23-11-10-21(14-25(23)15-27(29)36(40,41)55(49,50)51)19-35(34(48)22-6-2-1-3-7-22,47-33-9-5-4-8-31(33)45-46-47)20-26-13-12-24-17-30(39)28(18-32(24)44-26)37(42,43)56(52,53)54/h1-18H,19-20H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11695

((2S)-1-{2-[(3-hydroxyadamantan-1-yl)amino]acetyl}p...)Show SMILES OC12CC3CC(C1)CC(C3)(C2)NCC(=O)N1CCC[C@H]1C#N |r,TLB:9:8:6:3.2.4,4:3:10:7.6.5,4:5:10:3.2.9,THB:9:3:6:10.7.8,11:8:6:3.2.4| Show InChI InChI=1S/C17H25N3O2/c18-9-14-2-1-3-20(14)15(21)10-19-16-5-12-4-13(6-16)8-17(22,7-12)11-16/h12-14,19,22H,1-8,10-11H2/t12?,13?,14-,16?,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420653
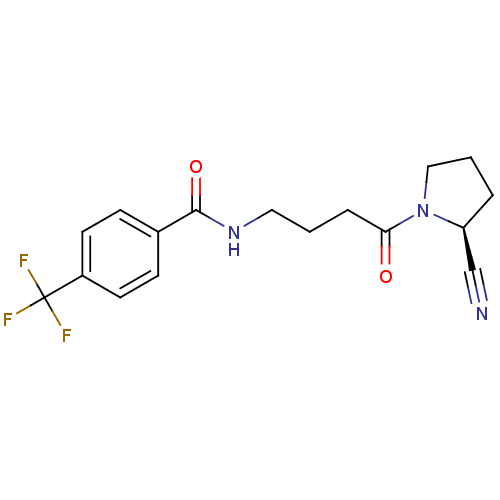
(CHEMBL2086614)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)NCCCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C17H18F3N3O2/c18-17(19,20)13-7-5-12(6-8-13)16(25)22-9-1-4-15(24)23-10-2-3-14(23)11-21/h5-8,14H,1-4,9-10H2,(H,22,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124216
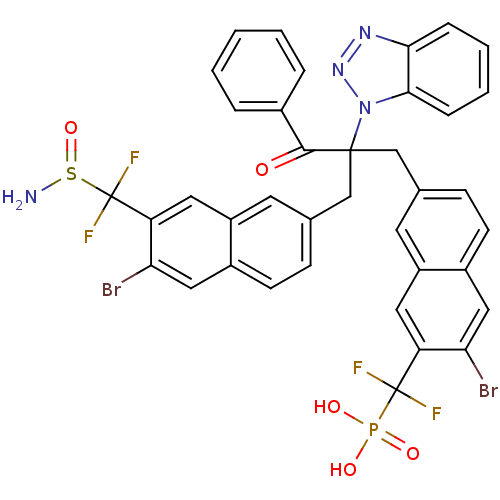
(({7-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N4O5PS/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)54(50,51)52)20-36(35(49)24-6-2-1-3-7-24,48-34-9-5-4-8-33(34)46-47-48)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)55(45)53/h1-19H,20-21,45H2,(H2,50,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124221
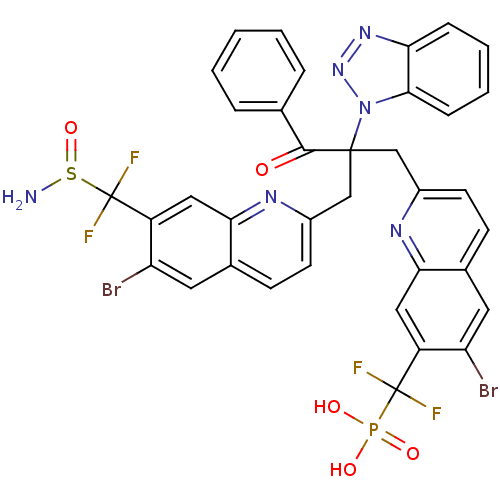
(({2-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C36H25Br2F4N6O5PS/c37-27-14-21-10-12-23(44-30(21)16-25(27)35(39,40)54(50,51)52)18-34(33(49)20-6-2-1-3-7-20,48-32-9-5-4-8-29(32)46-47-48)19-24-13-11-22-15-28(38)26(17-31(22)45-24)36(41,42)55(43)53/h1-17H,18-19,43H2,(H2,50,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362181
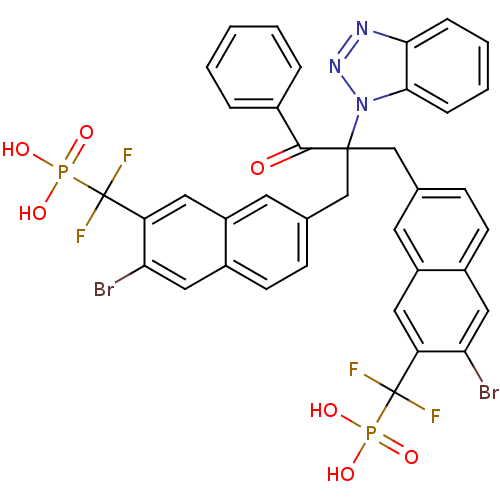
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N3O7P2/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)55(49,50)51)20-36(35(48)24-6-2-1-3-7-24,47-34-9-5-4-8-33(34)45-46-47)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)56(52,53)54/h1-19H,20-21H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362181

(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C38H27Br2F4N3O7P2/c39-31-18-25-12-10-22(14-27(25)16-29(31)37(41,42)55(49,50)51)20-36(35(48)24-6-2-1-3-7-24,47-34-9-5-4-8-33(34)45-46-47)21-23-11-13-26-19-32(40)30(17-28(26)15-23)38(43,44)56(52,53)54/h1-19H,20-21H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124220
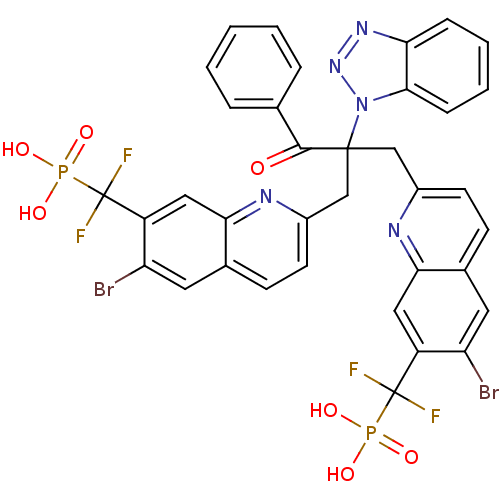
(({2-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C36H25Br2F4N5O7P2/c37-27-14-21-10-12-23(43-30(21)16-25(27)35(39,40)55(49,50)51)18-34(33(48)20-6-2-1-3-7-20,47-32-9-5-4-8-29(32)45-46-47)19-24-13-11-22-15-28(38)26(17-31(22)44-24)36(41,42)56(52,53)54/h1-17H,18-19H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124217
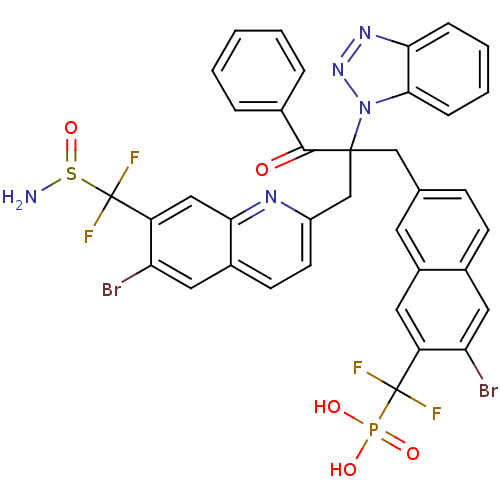
(({7-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4c3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C37H26Br2F4N5O5PS/c38-29-16-23-11-10-21(14-25(23)15-27(29)36(40,41)54(50,51)52)19-35(34(49)22-6-2-1-3-7-22,48-33-9-5-4-8-31(33)46-47-48)20-26-13-12-24-17-30(39)28(18-32(24)45-26)37(42,43)55(44)53/h1-18H,19-20,44H2,(H2,50,51,52) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11113

(6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...)Show InChI InChI=1S/C15H18N6O/c16-8-12-3-4-14(20-10-12)19-6-5-18-11-15(22)21-7-1-2-13(21)9-17/h3-4,10,13,18H,1-2,5-7,11H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004098
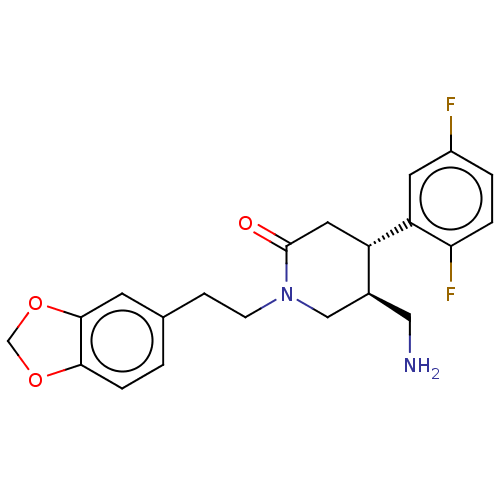
(CHEMBL3236121)Show SMILES NC[C@H]1CN(CCc2ccc3OCOc3c2)C(=O)C[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N2O3/c22-15-2-3-18(23)17(8-15)16-9-21(26)25(11-14(16)10-24)6-5-13-1-4-19-20(7-13)28-12-27-19/h1-4,7-8,14,16H,5-6,9-12,24H2/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362195
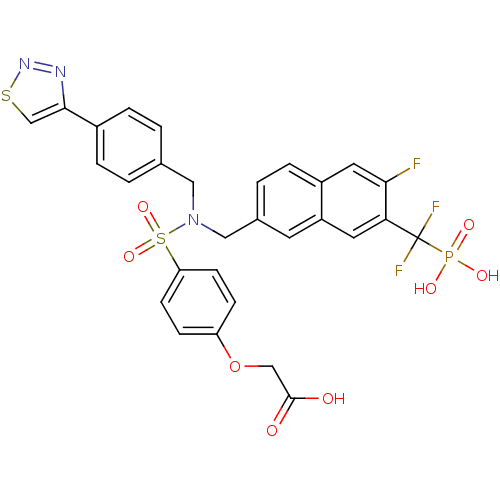
(CHEMBL1938828)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(F)c(cc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C29H23F3N3O8PS2/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)44(38,39)40)15-35(14-18-1-4-20(5-2-18)27-17-45-34-33-27)46(41,42)24-9-7-23(8-10-24)43-16-28(36)37/h1-13,17H,14-16H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420651
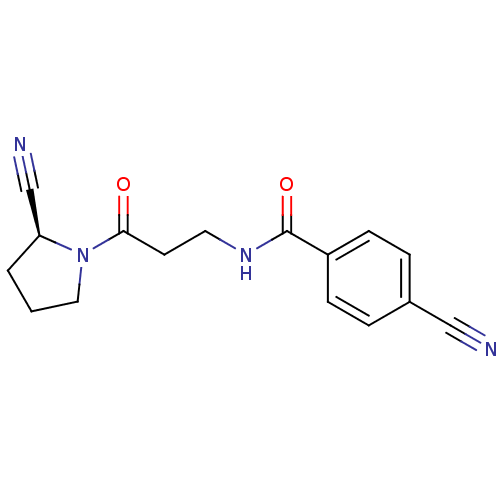
(CHEMBL2086611)Show SMILES O=C(CCNC(=O)c1ccc(cc1)C#N)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H16N4O2/c17-10-12-3-5-13(6-4-12)16(22)19-8-7-15(21)20-9-1-2-14(20)11-18/h3-6,14H,1-2,7-9H2,(H,19,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362196
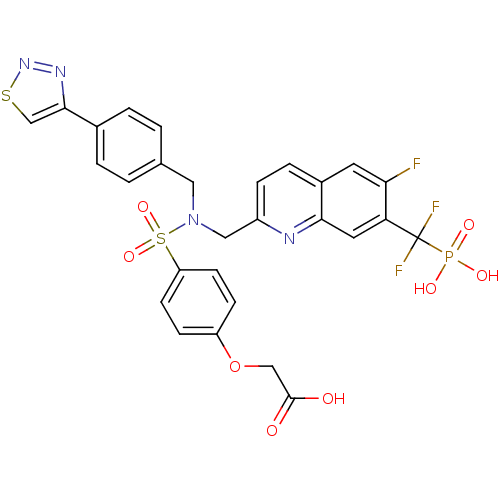
(CHEMBL1938829)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(F)c(cc2n1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C28H22F3N4O8PS2/c29-24-11-19-5-6-20(32-25(19)12-23(24)28(30,31)44(38,39)40)14-35(13-17-1-3-18(4-2-17)26-16-45-34-33-26)46(41,42)22-9-7-21(8-10-22)43-15-27(36)37/h1-12,16H,13-15H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420654
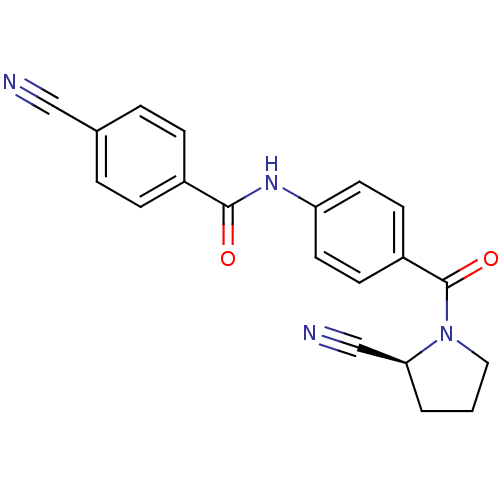
(CHEMBL2086615)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC[C@H]1C#N)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H16N4O2/c21-12-14-3-5-15(6-4-14)19(25)23-17-9-7-16(8-10-17)20(26)24-11-1-2-18(24)13-22/h3-10,18H,1-2,11H2,(H,23,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420652
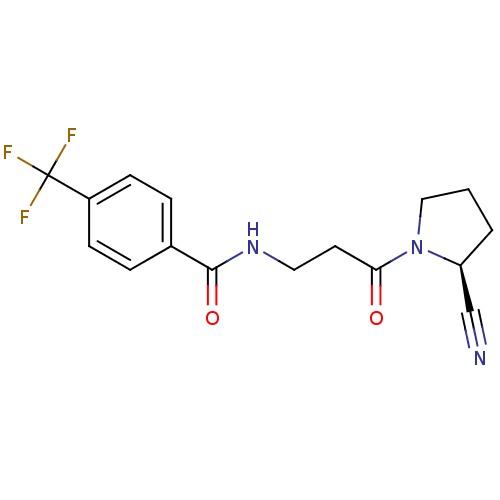
(CHEMBL2086612)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)NCCC(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C16H16F3N3O2/c17-16(18,19)12-5-3-11(4-6-12)15(24)21-8-7-14(23)22-9-1-2-13(22)10-20/h3-6,13H,1-2,7-9H2,(H,21,24)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420655
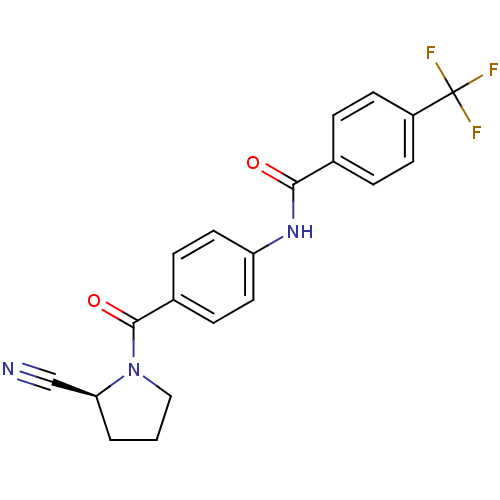
(CHEMBL2086616)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)Nc1ccc(cc1)C(=O)N1CCC[C@H]1C#N |r| Show InChI InChI=1S/C20H16F3N3O2/c21-20(22,23)15-7-3-13(4-8-15)18(27)25-16-9-5-14(6-10-16)19(28)26-11-1-2-17(26)12-24/h3-10,17H,1-2,11H2,(H,25,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362191
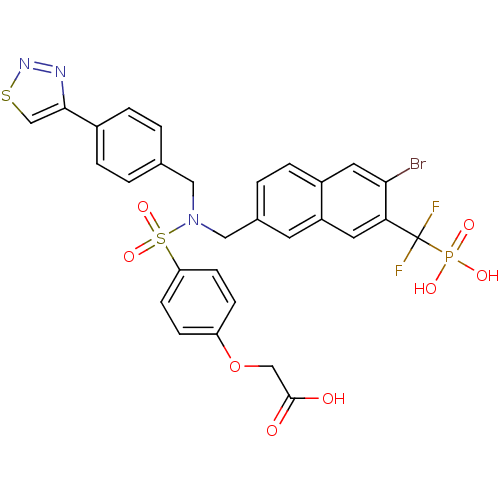
(CHEMBL1938824)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(Br)c(cc2c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C29H23BrF2N3O8PS2/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)44(38,39)40)15-35(14-18-1-4-20(5-2-18)27-17-45-34-33-27)46(41,42)24-9-7-23(8-10-24)43-16-28(36)37/h1-13,17H,14-16H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM11162

((1R)-3-oxo-3-[3-(trifluoroethyl)-5,6-dihydro[1,2,4...)Show SMILES N[C@@H](CC(=O)N1CCn2c(C1)nnc2C(F)(F)F)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C16H15F6N5O/c17-10-6-12(19)11(18)4-8(10)3-9(23)5-14(28)26-1-2-27-13(7-26)24-25-15(27)16(20,21)22/h4,6,9H,1-3,5,7,23H2/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362192
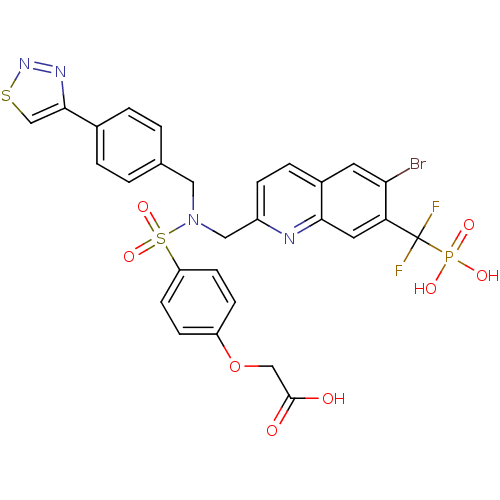
(CHEMBL1938825)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc2cc(Br)c(cc2n1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C28H22BrF2N4O8PS2/c29-24-11-19-5-6-20(32-25(19)12-23(24)28(30,31)44(38,39)40)14-35(13-17-1-3-18(4-2-17)26-16-45-34-33-26)46(41,42)22-9-7-21(8-10-22)43-15-27(36)37/h1-12,16H,13-15H2,(H,36,37)(H2,38,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004103
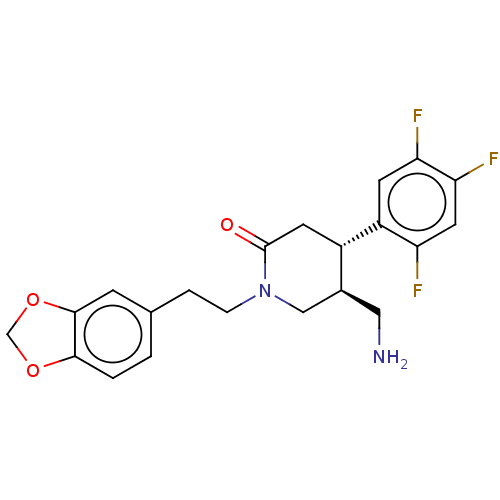
(CHEMBL3236126)Show SMILES NC[C@H]1CN(CCc2ccc3OCOc3c2)C(=O)C[C@@H]1c1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C21H21F3N2O3/c22-16-8-18(24)17(23)6-15(16)14-7-21(27)26(10-13(14)9-25)4-3-12-1-2-19-20(5-12)29-11-28-19/h1-2,5-6,8,13-14H,3-4,7,9-11,25H2/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124211
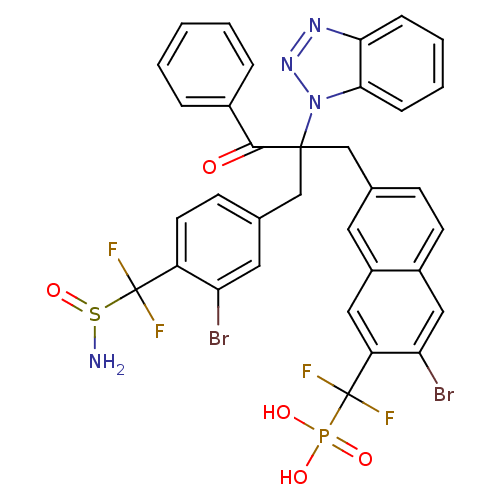
(({7-[2-({4-[(aminosulfinyl)difluoromethyl]-3-bromo...)Show SMILES NS(=O)C(F)(F)c1ccc(CC(Cc2ccc3cc(Br)c(cc3c2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)n2nnc3ccccc23)cc1Br Show InChI InChI=1S/C34H25Br2F4N4O5PS/c35-27-15-21(11-13-25(27)34(39,40)51(41)49)19-32(31(45)22-6-2-1-3-7-22,44-30-9-5-4-8-29(30)42-43-44)18-20-10-12-23-17-28(36)26(16-24(23)14-20)33(37,38)50(46,47)48/h1-17H,18-19,41H2,(H2,46,47,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420640
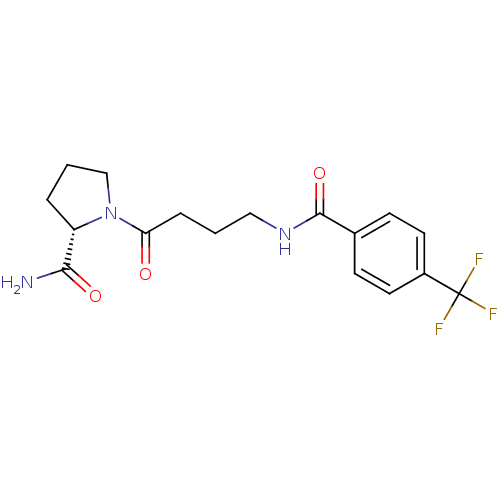
(CHEMBL2086600)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCCNC(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C17H20F3N3O3/c18-17(19,20)12-7-5-11(6-8-12)16(26)22-9-1-4-14(24)23-10-2-3-13(23)15(21)25/h5-8,13H,1-4,9-10H2,(H2,21,25)(H,22,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124209
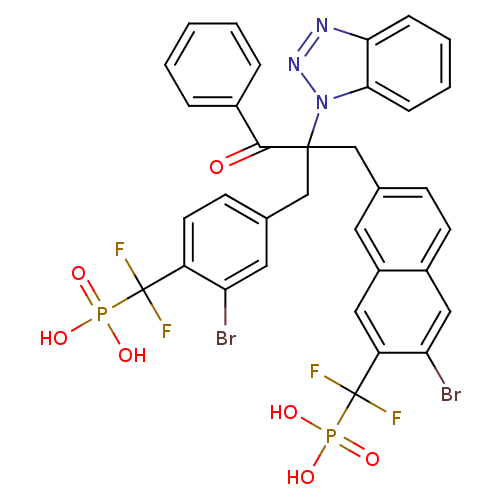
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({3-bromo-4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc3cc(Br)c(cc3c2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)n2nnc3ccccc23)cc1Br Show InChI InChI=1S/C34H25Br2F4N3O7P2/c35-27-15-21(11-13-25(27)33(37,38)51(45,46)47)19-32(31(44)22-6-2-1-3-7-22,43-30-9-5-4-8-29(30)41-42-43)18-20-10-12-23-17-28(36)26(16-24(23)14-20)34(39,40)52(48,49)50/h1-17H,18-19H2,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
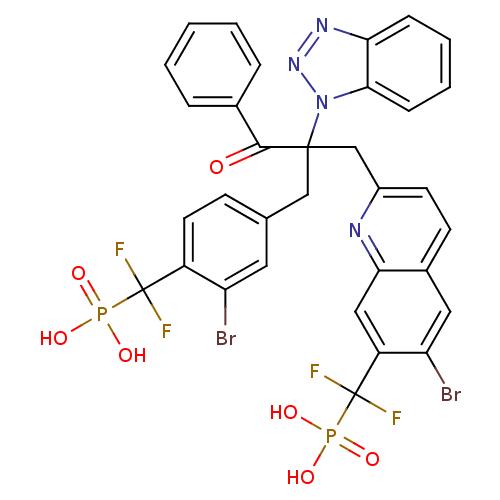
(Homo sapiens (Human)) | BDBM124218
(({2-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({3-bromo-4-...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc3cc(Br)c(cc3n2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)n2nnc3ccccc23)cc1Br Show InChI InChI=1S/C33H24Br2F4N4O7P2/c34-25-14-19(10-13-23(25)32(36,37)51(45,46)47)17-31(30(44)20-6-2-1-3-7-20,43-29-9-5-4-8-27(29)41-42-43)18-22-12-11-21-15-26(35)24(16-28(21)40-22)33(38,39)52(48,49)50/h1-16H,17-18H2,(H2,45,46,47)(H2,48,49,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
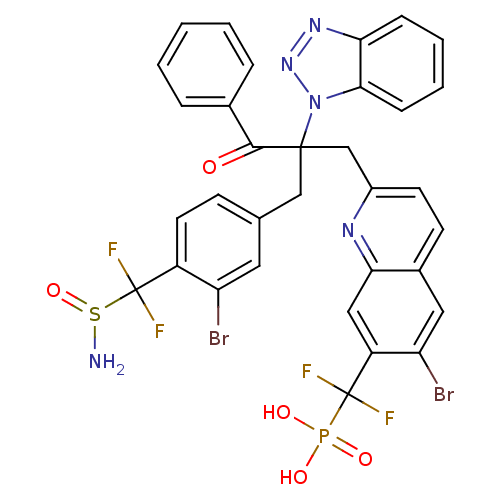
(Homo sapiens (Human)) | BDBM124219
(({2-[2-({4-[(aminosulfinyl)difluoromethyl]-3-bromo...)Show SMILES NS(=O)C(F)(F)c1ccc(CC(Cc2ccc3cc(Br)c(cc3n2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)n2nnc3ccccc23)cc1Br Show InChI InChI=1S/C33H24Br2F4N5O5PS/c34-25-14-19(10-13-23(25)33(38,39)51(40)49)17-31(30(45)20-6-2-1-3-7-20,44-29-9-5-4-8-27(29)42-43-44)18-22-12-11-21-15-26(35)24(16-28(21)41-22)32(36,37)50(46,47)48/h1-16H,17-18,40H2,(H2,46,47,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
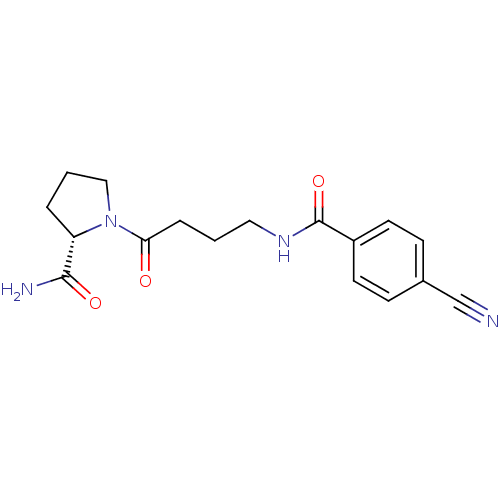
(Homo sapiens (Human)) | BDBM50420639
(CHEMBL2086599)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCCNC(=O)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C17H20N4O3/c18-11-12-5-7-13(8-6-12)17(24)20-9-1-4-15(22)21-10-2-3-14(21)16(19)23/h5-8,14H,1-4,9-10H2,(H2,19,23)(H,20,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420633
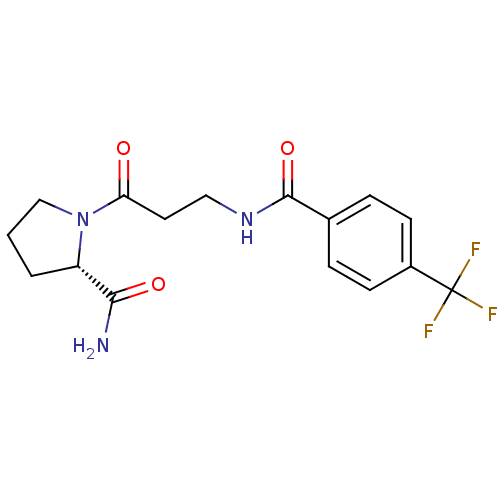
(CHEMBL2086592)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCNC(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C16H18F3N3O3/c17-16(18,19)11-5-3-10(4-6-11)15(25)21-8-7-13(23)22-9-1-2-12(22)14(20)24/h3-6,12H,1-2,7-9H2,(H2,20,24)(H,21,25)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420648
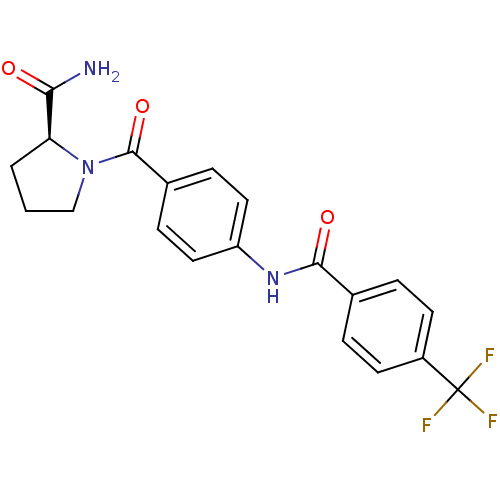
(CHEMBL2086608)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc(NC(=O)c2ccc(cc2)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C20H18F3N3O3/c21-20(22,23)14-7-3-12(4-8-14)18(28)25-15-9-5-13(6-10-15)19(29)26-11-1-2-16(26)17(24)27/h3-10,16H,1-2,11H2,(H2,24,27)(H,25,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420632
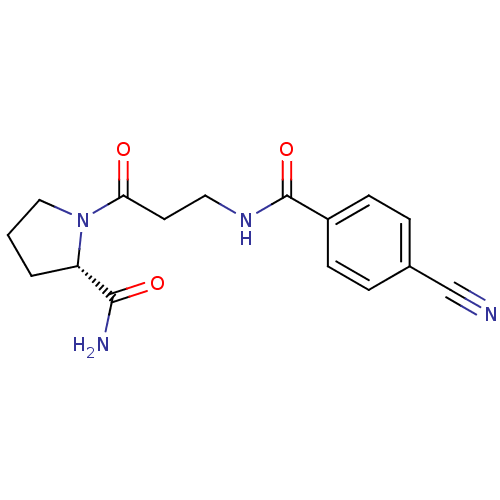
(CHEMBL2086591)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCNC(=O)c1ccc(cc1)C#N |r| Show InChI InChI=1S/C16H18N4O3/c17-10-11-3-5-12(6-4-11)16(23)19-8-7-14(21)20-9-1-2-13(20)15(18)22/h3-6,13H,1-2,7-9H2,(H2,18,22)(H,19,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362186
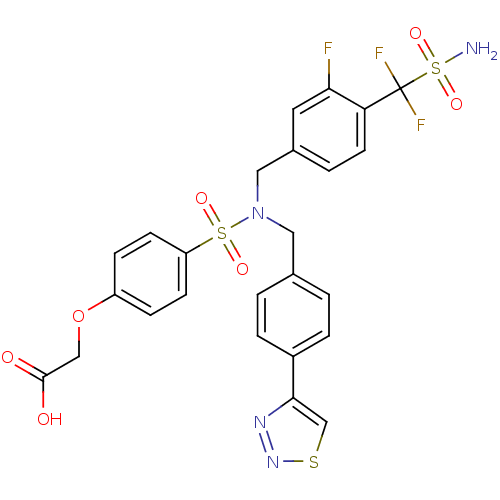
(CHEMBL1938819)Show SMILES NS(=O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1F Show InChI InChI=1S/C25H21F3N4O7S3/c26-22-11-17(3-10-21(22)25(27,28)42(29,37)38)13-32(12-16-1-4-18(5-2-16)23-15-40-31-30-23)41(35,36)20-8-6-19(7-9-20)39-14-24(33)34/h1-11,15H,12-14H2,(H,33,34)(H2,29,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420647
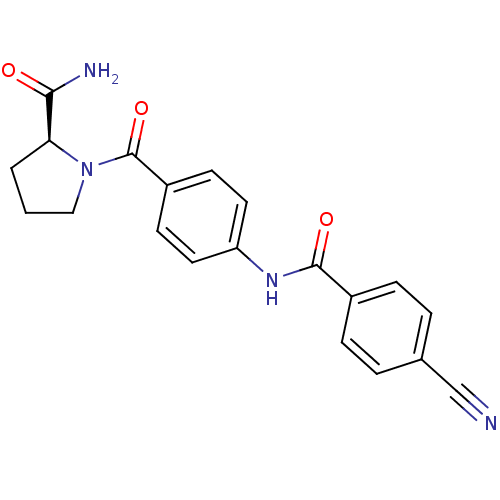
(CHEMBL2086607)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)c1ccc(NC(=O)c2ccc(cc2)C#N)cc1 |r| Show InChI InChI=1S/C20H18N4O3/c21-12-13-3-5-14(6-4-13)19(26)23-16-9-7-15(8-10-16)20(27)24-11-1-2-17(24)18(22)25/h3-10,17H,1-2,11H2,(H2,22,25)(H,23,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
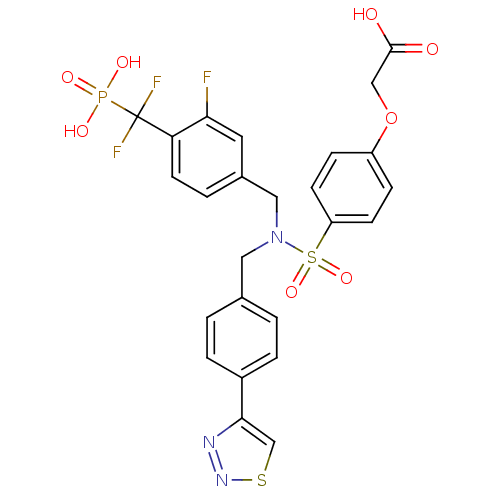
(Homo sapiens (Human)) | BDBM50362183
(CHEMBL1938816)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(F)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21F3N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
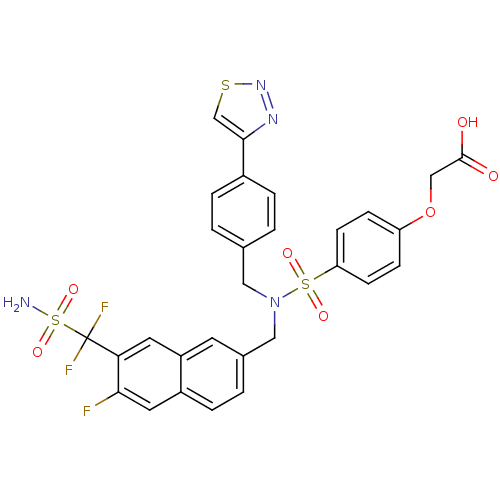
(Homo sapiens (Human)) | BDBM50362197
(CHEMBL1938830)Show SMILES NS(=O)(=O)C(F)(F)c1cc2cc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1F Show InChI InChI=1S/C29H23F3N4O7S3/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)46(33,41)42)15-36(14-18-1-4-20(5-2-18)27-17-44-35-34-27)45(39,40)24-9-7-23(8-10-24)43-16-28(37)38/h1-13,17H,14-16H2,(H,37,38)(H2,33,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
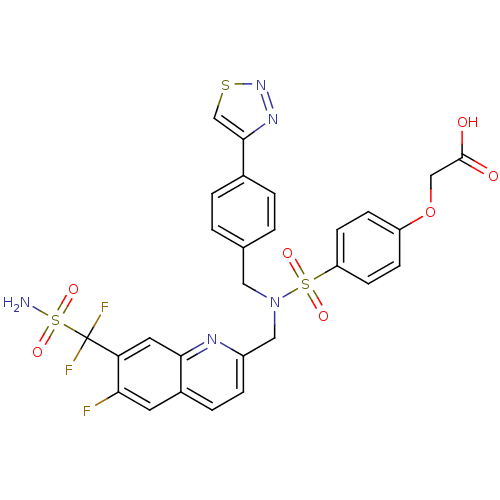
(Homo sapiens (Human)) | BDBM50362198
(CHEMBL1938831)Show SMILES NS(=O)(=O)C(F)(F)c1cc2nc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1F Show InChI InChI=1S/C28H22F3N5O7S3/c29-24-11-19-5-6-20(33-25(19)12-23(24)28(30,31)46(32,41)42)14-36(13-17-1-3-18(4-2-17)26-16-44-35-34-26)45(39,40)22-9-7-21(8-10-22)43-15-27(37)38/h1-12,16H,13-15H2,(H,37,38)(H2,32,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362193
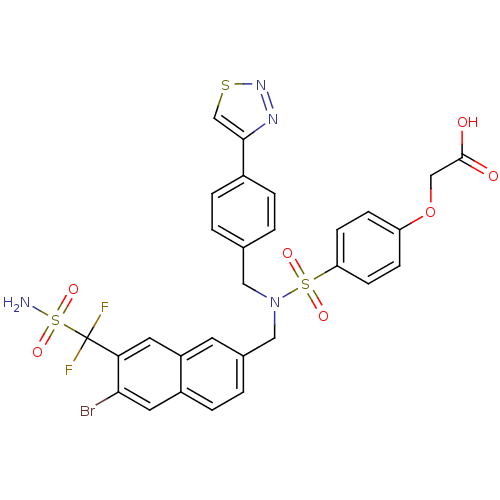
(CHEMBL1938826)Show SMILES NS(=O)(=O)C(F)(F)c1cc2cc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1Br Show InChI InChI=1S/C29H23BrF2N4O7S3/c30-26-13-21-6-3-19(11-22(21)12-25(26)29(31,32)46(33,41)42)15-36(14-18-1-4-20(5-2-18)27-17-44-35-34-27)45(39,40)24-9-7-23(8-10-24)43-16-28(37)38/h1-13,17H,14-16H2,(H,37,38)(H2,33,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362194
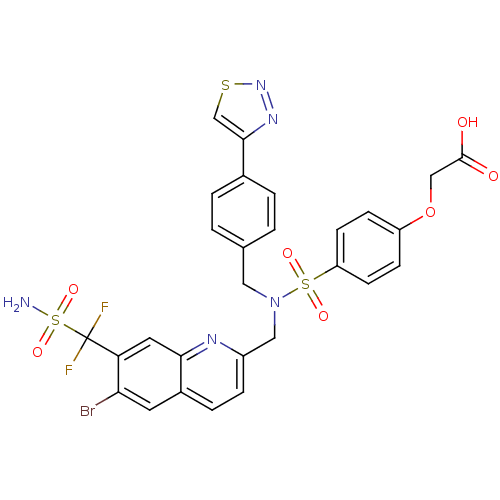
(CHEMBL1938827)Show SMILES NS(=O)(=O)C(F)(F)c1cc2nc(CN(Cc3ccc(cc3)-c3csnn3)S(=O)(=O)c3ccc(OCC(O)=O)cc3)ccc2cc1Br Show InChI InChI=1S/C28H22BrF2N5O7S3/c29-24-11-19-5-6-20(33-25(19)12-23(24)28(30,31)46(32,41)42)14-36(13-17-1-3-18(4-2-17)26-16-44-35-34-26)45(39,40)22-9-7-21(8-10-22)43-15-27(37)38/h1-12,16H,13-15H2,(H,37,38)(H2,32,41,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004130
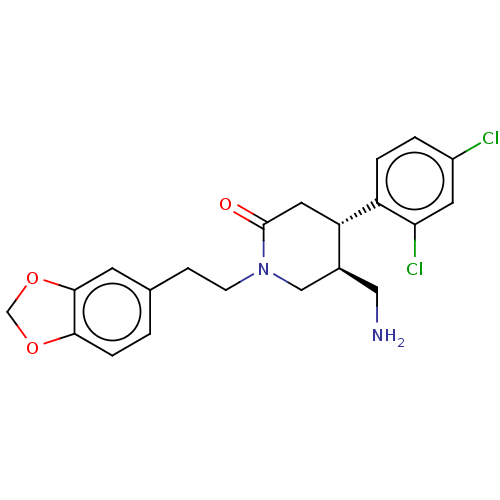
(CHEMBL3236131)Show SMILES NC[C@H]1CN(CCc2ccc3OCOc3c2)C(=O)C[C@@H]1c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C21H22Cl2N2O3/c22-15-2-3-16(18(23)8-15)17-9-21(26)25(11-14(17)10-24)6-5-13-1-4-19-20(7-13)28-12-27-19/h1-4,7-8,14,17H,5-6,9-12,24H2/t14-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124208
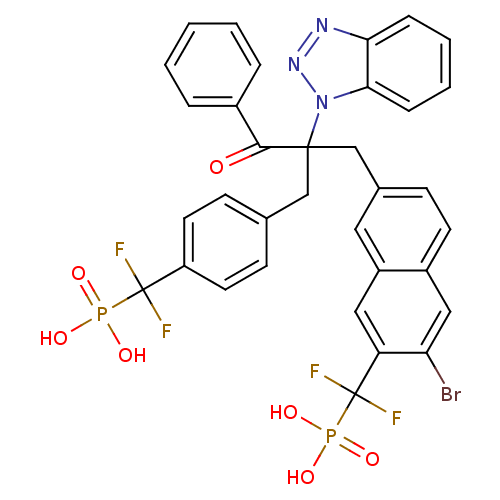
(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({4-[difluor...)Show SMILES OP(O)(=O)C(F)(F)c1ccc(CC(Cc2ccc3cc(Br)c(cc3c2)C(F)(F)P(O)(O)=O)(C(=O)c2ccccc2)n2nnc3ccccc23)cc1 Show InChI InChI=1S/C34H26BrF4N3O7P2/c35-28-18-24-13-10-22(16-25(24)17-27(28)34(38,39)51(47,48)49)20-32(31(43)23-6-2-1-3-7-23,42-30-9-5-4-8-29(30)40-41-42)19-21-11-14-26(15-12-21)33(36,37)50(44,45)46/h1-18H,19-20H2,(H2,44,45,46)(H2,47,48,49) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50362185
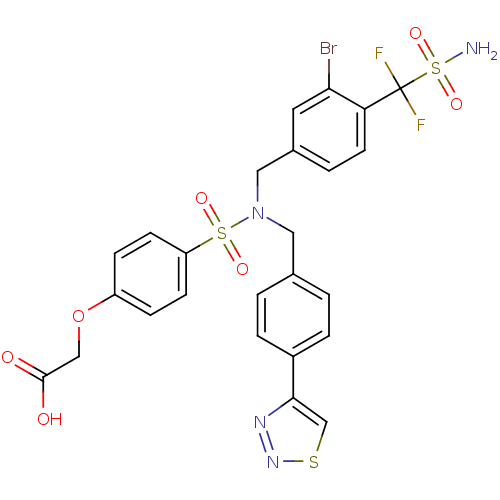
(CHEMBL1938818)Show SMILES NS(=O)(=O)C(F)(F)c1ccc(CN(Cc2ccc(cc2)-c2csnn2)S(=O)(=O)c2ccc(OCC(O)=O)cc2)cc1Br Show InChI InChI=1S/C25H21BrF2N4O7S3/c26-22-11-17(3-10-21(22)25(27,28)42(29,37)38)13-32(12-16-1-4-18(5-2-16)23-15-40-31-30-23)41(35,36)20-8-6-19(7-9-20)39-14-24(33)34/h1-11,15H,12-14H2,(H,33,34)(H2,29,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50171096
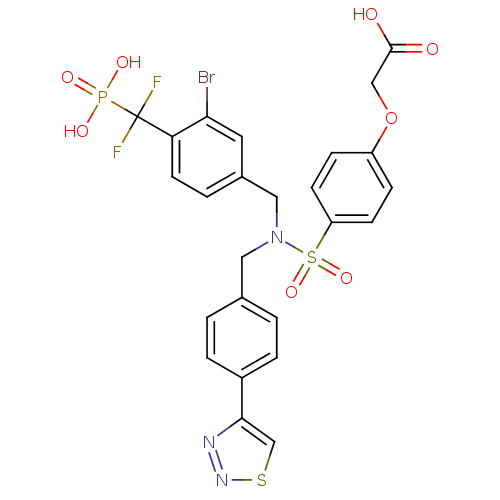
(CHEMBL371929 | {4-[[3-Bromo-4-(difluoro-phosphono-...)Show SMILES OC(=O)COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(cc1)-c1csnn1)Cc1ccc(c(Br)c1)C(F)(F)P(O)(O)=O Show InChI InChI=1S/C25H21BrF2N3O8PS2/c26-22-11-17(3-10-21(22)25(27,28)40(34,35)36)13-31(12-16-1-4-18(5-2-16)23-15-41-30-29-23)42(37,38)20-8-6-19(7-9-20)39-14-24(32)33/h1-11,15H,12-14H2,(H,32,33)(H2,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assay |
Bioorg Med Chem Lett 22: 1111-7 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.122
BindingDB Entry DOI: 10.7270/Q2ZS2X05 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420638
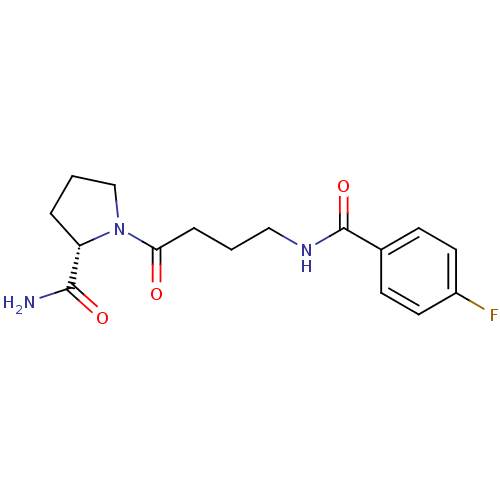
(CHEMBL2086598)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCCNC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C16H20FN3O3/c17-12-7-5-11(6-8-12)16(23)19-9-1-4-14(21)20-10-2-3-13(20)15(18)22/h5-8,13H,1-4,9-10H2,(H2,18,22)(H,19,23)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004097
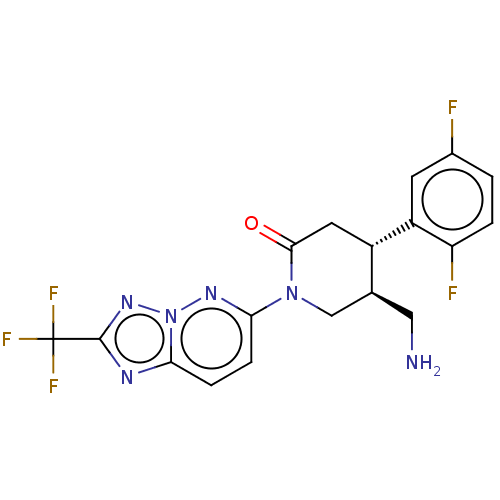
(CHEMBL3236120)Show SMILES NC[C@H]1CN(C(=O)C[C@@H]1c1cc(F)ccc1F)c1ccc2nc(nn2n1)C(F)(F)F |r| Show InChI InChI=1S/C18H15F5N6O/c19-10-1-2-13(20)12(5-10)11-6-16(30)28(8-9(11)7-24)15-4-3-14-25-17(18(21,22)23)27-29(14)26-15/h1-5,9,11H,6-8,24H2/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004094
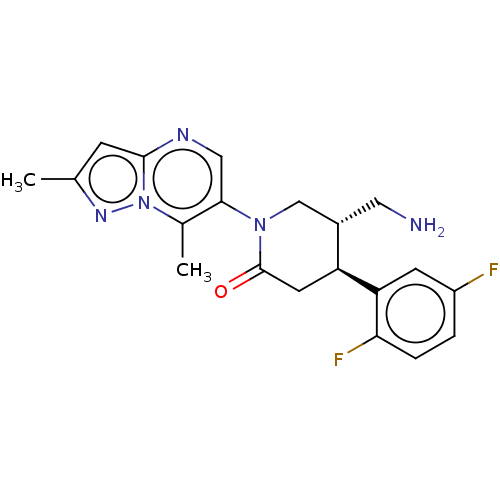
(CHEMBL3236117)Show SMILES Cc1cc2ncc(N3C[C@H](CN)[C@H](CC3=O)c3cc(F)ccc3F)c(C)n2n1 |r| Show InChI InChI=1S/C20H21F2N5O/c1-11-5-19-24-9-18(12(2)27(19)25-11)26-10-13(8-23)15(7-20(26)28)16-6-14(21)3-4-17(16)22/h3-6,9,13,15H,7-8,10,23H2,1-2H3/t13-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004096

(CHEMBL3236119)Show SMILES NC[C@H]1CN(C(=O)C[C@@H]1c1cc(F)ccc1F)c1ccc2nnc(n2n1)C(F)(F)F |r| Show InChI InChI=1S/C18H15F5N6O/c19-10-1-2-13(20)12(5-10)11-6-16(30)28(8-9(11)7-24)15-4-3-14-25-26-17(18(21,22)23)29(14)27-15/h1-5,9,11H,6-8,24H2/t9-,11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124221

(({2-[2-({7-[(aminosulfinyl)difluoromethyl]-6-bromo...)Show SMILES NS(=O)C(F)(F)c1cc2nc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C36H25Br2F4N6O5PS/c37-27-14-21-10-12-23(44-30(21)16-25(27)35(39,40)54(50,51)52)18-34(33(49)20-6-2-1-3-7-20,48-32-9-5-4-8-29(32)46-47-48)19-24-13-11-22-15-28(38)26(17-31(22)45-24)36(41,42)55(43)53/h1-17H,18-19,43H2,(H2,50,51,52) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50004093
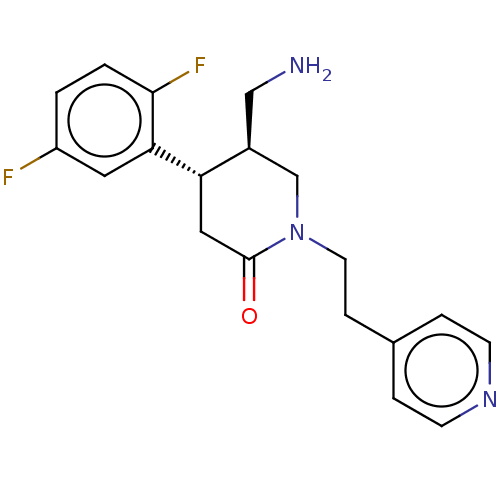
(CHEMBL3236116)Show SMILES NC[C@H]1CN(CCc2ccncc2)C(=O)C[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C19H21F2N3O/c20-15-1-2-18(21)17(9-15)16-10-19(25)24(12-14(16)11-22)8-5-13-3-6-23-7-4-13/h1-4,6-7,9,14,16H,5,8,10-12,22H2/t14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
M.S. University of Baroda
Curated by ChEMBL
| Assay Description
Inhibition of human DPP-4 expressed in baculovirus expression system using H-Gly-Pro-AMC as substrate after 30 mins by fluorometric analysis |
Bioorg Med Chem Lett 24: 1918-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.009
BindingDB Entry DOI: 10.7270/Q2G73G7F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124215

(({7-[2-(1H-1,2,3-benzotriazol-1-yl)-2-({6-bromo-7-...)Show SMILES OP(O)(=O)C(F)(F)c1cc2cc(CC(Cc3ccc4cc(Br)c(cc4n3)C(F)(F)P(O)(O)=O)(C(=O)c3ccccc3)n3nnc4ccccc34)ccc2cc1Br Show InChI InChI=1S/C37H26Br2F4N4O7P2/c38-29-16-23-11-10-21(14-25(23)15-27(29)36(40,41)55(49,50)51)19-35(34(48)22-6-2-1-3-7-22,47-33-9-5-4-8-31(33)45-46-47)20-26-13-12-24-17-30(39)28(18-32(24)44-26)37(42,43)56(52,53)54/h1-18H,19-20H2,(H2,49,50,51)(H2,52,53,54) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
| Assay Description
The in vitro enzyme inhibitory activity was evaluated by using the pNPP assay. Para-nitrophenylphosphate (pNPP) and test compounds were added to assa... |
ChemMedChem 6: 1011-6 (2011)
Article DOI: 10.1002/cmdc.201100077
BindingDB Entry DOI: 10.7270/Q2XW4HGZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50420631
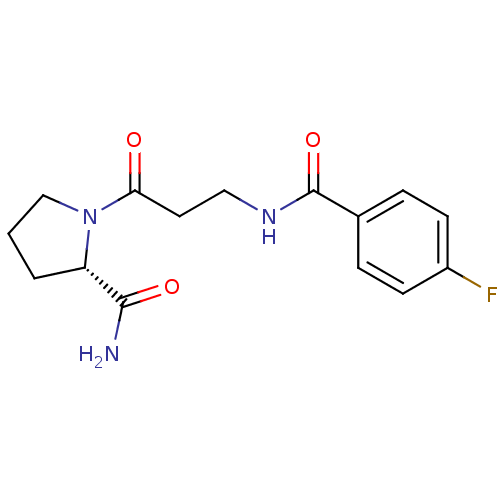
(CHEMBL2086590)Show SMILES NC(=O)[C@@H]1CCCN1C(=O)CCNC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C15H18FN3O3/c16-11-5-3-10(4-6-11)15(22)18-8-7-13(20)19-9-1-2-12(19)14(17)21/h3-6,12H,1-2,7-9H2,(H2,17,21)(H,18,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Zydus Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human DPP4 expressed in baculovirus expression system using Gly-Pro-AMC substrate incubated or 30 mins by fluorescence based assay |
Bioorg Med Chem Lett 22: 3516-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.078
BindingDB Entry DOI: 10.7270/Q2QZ2C77 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data