 Found 50 hits with Last Name = 'shanker' and Initial = 'k'
Found 50 hits with Last Name = 'shanker' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin D
(Homo sapiens (Human)) | BDBM912

((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CC(C)C)C(C)C)C(C)C)[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C34H63N5O9/c1-17(2)12-23(37-33(47)31(21(9)10)39-34(48)30(20(7)8)38-27(42)14-19(5)6)25(40)15-28(43)35-22(11)32(46)36-24(13-18(3)4)26(41)16-29(44)45/h17-26,30-31,40-41H,12-16H2,1-11H3,(H,35,43)(H,36,46)(H,37,47)(H,38,42)(H,39,48)(H,44,45)/t22-,23-,24-,25-,26-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189981
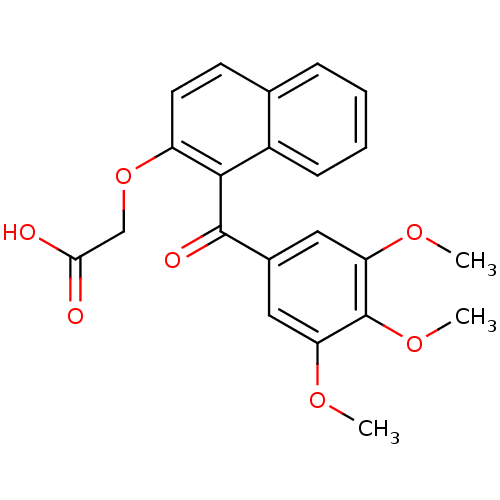
(2-[1-(3,4,5-trimethoxybenzoyl)naphthalen-2-yloxy]e...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)c1c(OCC(O)=O)ccc2ccccc12 Show InChI InChI=1S/C22H20O7/c1-26-17-10-14(11-18(27-2)22(17)28-3)21(25)20-15-7-5-4-6-13(15)8-9-16(20)29-12-19(23)24/h4-11H,12H2,1-3H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189983
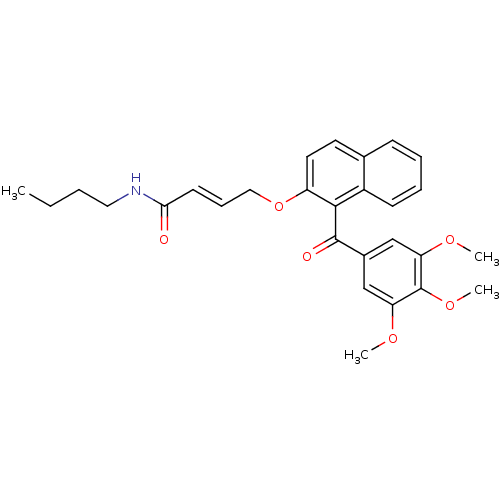
(CHEMBL214974 | N-butyl-4-(1-(3,4,5-trimethoxybenzo...)Show SMILES CCCCNC(=O)\C=C\COc1ccc2ccccc2c1C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C28H31NO6/c1-5-6-15-29-25(30)12-9-16-35-22-14-13-19-10-7-8-11-21(19)26(22)27(31)20-17-23(32-2)28(34-4)24(18-20)33-3/h7-14,17-18H,5-6,15-16H2,1-4H3,(H,29,30)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528362

(CHEMBL4466390)Show SMILES [H][C@@]12[C@H](O)C(=O)Oc3c(O)c(O)cc(c13)C(=O)O[C@H]1[C@H](OC(=O)c3cc(O)c(O)c(O)c3)O[C@@H]3COC(=O)c4ccccc4-c4c(O)c(O)c(O)cc4C(=O)O[C@H]1[C@@H]3OC(=O)[C@H]2CC(O)=O |r| Show InChI InChI=1S/C41H30O24/c42-17-5-11(6-18(43)26(17)48)35(53)65-41-34-33-31(21(60-41)10-59-36(54)13-4-2-1-3-12(13)23-14(37(55)63-33)7-19(44)27(49)29(23)51)61-39(57)16(9-22(46)47)24-25-15(38(56)64-34)8-20(45)28(50)32(25)62-40(58)30(24)52/h1-8,16,21,24,30-31,33-34,41-45,48-52H,9-10H2,(H,46,47)/t16-,21+,24-,30-,31+,33-,34+,41-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50528361
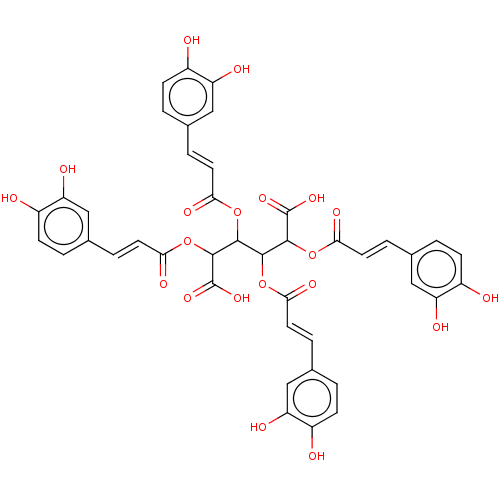
(CHEMBL4457983)Show SMILES OC(=O)C(OC(=O)\C=C\c1ccc(O)c(O)c1)C(OC(=O)\C=C\c1ccc(O)c(O)c1)C(OC(=O)\C=C\c1ccc(O)c(O)c1)C(OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O Show InChI InChI=1S/C42H34O20/c43-25-9-1-21(17-29(25)47)5-13-33(51)59-37(39(41(55)56)61-35(53)15-7-23-3-11-27(45)31(49)19-23)38(60-34(52)14-6-22-2-10-26(44)30(48)18-22)40(42(57)58)62-36(54)16-8-24-4-12-28(46)32(50)20-24/h1-20,37-40,43-50H,(H,55,56)(H,57,58)/b13-5+,14-6+,15-7+,16-8+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50528362

(CHEMBL4466390)Show SMILES [H][C@@]12[C@H](O)C(=O)Oc3c(O)c(O)cc(c13)C(=O)O[C@H]1[C@H](OC(=O)c3cc(O)c(O)c(O)c3)O[C@@H]3COC(=O)c4ccccc4-c4c(O)c(O)c(O)cc4C(=O)O[C@H]1[C@@H]3OC(=O)[C@H]2CC(O)=O |r| Show InChI InChI=1S/C41H30O24/c42-17-5-11(6-18(43)26(17)48)35(53)65-41-34-33-31(21(60-41)10-59-36(54)13-4-2-1-3-12(13)23-14(37(55)63-33)7-19(44)27(49)29(23)51)61-39(57)16(9-22(46)47)24-25-15(38(56)64-34)8-20(45)28(50)32(25)62-40(58)30(24)52/h1-8,16,21,24,30-31,33-34,41-45,48-52H,9-10H2,(H,46,47)/t16-,21+,24-,30-,31+,33-,34+,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of 5-lox (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528358
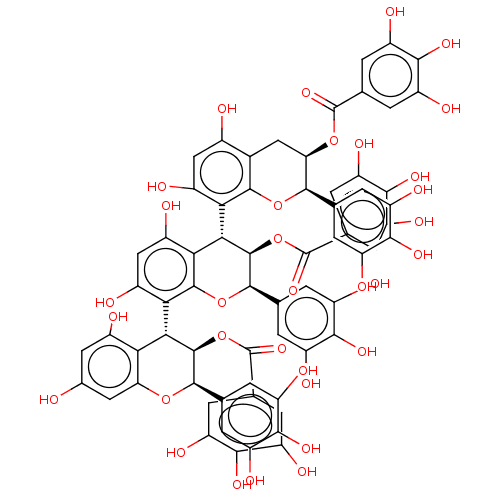
(CHEMBL4588699)Show SMILES Oc1cc(O)c2[C@@H]([C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1)c1c(O)cc(O)c2[C@@H]([C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc12)c1cc(O)c(O)c(O)c1)c1c(O)cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc12)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C66H50O33/c67-24-13-27(69)45-43(14-24)94-58(19-3-33(75)52(86)34(76)4-19)62(98-65(92)22-9-39(81)55(89)40(82)10-22)49(45)47-29(71)17-30(72)48-50(63(99-66(93)23-11-41(83)56(90)42(84)12-23)59(97-61(47)48)20-5-35(77)53(87)36(78)6-20)46-28(70)16-26(68)25-15-44(95-64(91)21-7-37(79)54(88)38(80)8-21)57(96-60(25)46)18-1-31(73)51(85)32(74)2-18/h1-14,16-17,44,49-50,57-59,62-63,67-90H,15H2/t44-,49-,50+,57-,58-,59-,62-,63-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189976
(CHEMBL212492 | N-(4-methoxyphenyl)-4-(1-(3,4,5-tri...)Show SMILES COc1ccc(NC(=O)\C=C\COc2ccc3ccccc3c2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1 Show InChI InChI=1S/C31H29NO7/c1-35-23-14-12-22(13-15-23)32-28(33)10-7-17-39-25-16-11-20-8-5-6-9-24(20)29(25)30(34)21-18-26(36-2)31(38-4)27(19-21)37-3/h5-16,18-19H,17H2,1-4H3,(H,32,33)/b10-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50528375
(CHEMBL4579886)Show SMILES OC1C(CC(O)(CC1OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O)OC(=O)\C=C\c1ccc(O)c(O)c1 Show InChI InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(30)36-19-11-25(35,24(33)34)12-20(23(19)32)37-22(31)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-29,32,35H,11-12H2,(H,33,34)/b7-3+,8-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50528355
(CHEMBL4532635)Show SMILES OC1CC(O)(CC(OC(=O)\C=C\c2ccc(O)c(O)c2)C1OC(=O)\C=C\c1ccc(O)c(O)c1)C(O)=O Show InChI InChI=1S/C25H24O12/c26-15-5-1-13(9-17(15)28)3-7-21(31)36-20-12-25(35,24(33)34)11-19(30)23(20)37-22(32)8-4-14-2-6-16(27)18(29)10-14/h1-10,19-20,23,26-30,35H,11-12H2,(H,33,34)/b7-3+,8-4+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50528363
(CHEMBL4472181)Show SMILES C\C=C(/C)C(=O)O[C@H]1[C@H](OC(C)=O)[C@]23C[C@@]1(O)O[C@H]1C[C@@]4(C)[C@@H](CC=C4[C@@](C)(C21)C(=O)C[C@H]3C(C)C)c1ccoc1 |r,c:25| Show InChI InChI=1S/C33H42O8/c1-8-18(4)29(36)40-28-27(39-19(5)34)32-16-33(28,37)41-23-14-30(6)21(20-11-12-38-15-20)9-10-24(30)31(7,26(23)32)25(35)13-22(32)17(2)3/h8,10-12,15,17,21-23,26-28,37H,9,13-14,16H2,1-7H3/b18-8+/t21-,22-,23-,26?,27-,28-,30-,31+,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of iNOS (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Interleukin-1 beta
(Homo sapiens (Human)) | BDBM50528363

(CHEMBL4472181)Show SMILES C\C=C(/C)C(=O)O[C@H]1[C@H](OC(C)=O)[C@]23C[C@@]1(O)O[C@H]1C[C@@]4(C)[C@@H](CC=C4[C@@](C)(C21)C(=O)C[C@H]3C(C)C)c1ccoc1 |r,c:25| Show InChI InChI=1S/C33H42O8/c1-8-18(4)29(36)40-28-27(39-19(5)34)32-16-33(28,37)41-23-14-30(6)21(20-11-12-38-15-20)9-10-24(30)31(7,26(23)32)25(35)13-22(32)17(2)3/h8,10-12,15,17,21-23,26-28,37H,9,13-14,16H2,1-7H3/b18-8+/t21-,22-,23-,26?,27-,28-,30-,31+,32-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of IL1-beta (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189980

(CHEMBL213373 | N-octyl-2-(1-(3,4,5-trimethoxybenzo...)Show SMILES CCCCCCCCNC(=O)COc1ccc2ccccc2c1C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C30H37NO6/c1-5-6-7-8-9-12-17-31-27(32)20-37-24-16-15-21-13-10-11-14-23(21)28(24)29(33)22-18-25(34-2)30(36-4)26(19-22)35-3/h10-11,13-16,18-19H,5-9,12,17,20H2,1-4H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528376
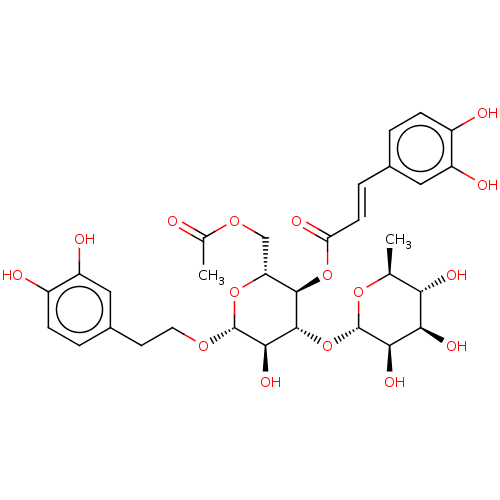
(CHEMBL4473793)Show SMILES [H][C@@]1(O[C@@H]2[C@@H](O)[C@H](OCCc3ccc(O)c(O)c3)O[C@H](COC(C)=O)[C@H]2OC(=O)\C=C\c2ccc(O)c(O)c2)O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C31H38O16/c1-14-24(38)25(39)26(40)31(44-14)47-29-27(41)30(42-10-9-17-4-7-19(34)21(36)12-17)45-22(13-43-15(2)32)28(29)46-23(37)8-5-16-3-6-18(33)20(35)11-16/h3-8,11-12,14,22,24-31,33-36,38-41H,9-10,13H2,1-2H3/b8-5+/t14-,22+,24-,25+,26+,27+,28+,29+,30+,31-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50241867
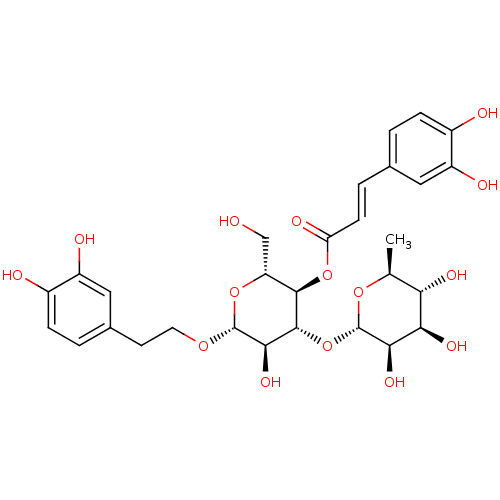
(((2R,3R,4R,5R,6R)-6-(3,4-dihydroxyphenethoxy)-5-hy...)Show SMILES C[C@@H]1O[C@@H](O[C@@H]2[C@@H](O)[C@H](OCCc3ccc(O)c(O)c3)O[C@H](CO)[C@H]2OC(=O)\C=C\c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C29H36O15/c1-13-22(36)23(37)24(38)29(41-13)44-27-25(39)28(40-9-8-15-3-6-17(32)19(34)11-15)42-20(12-30)26(27)43-21(35)7-4-14-2-5-16(31)18(33)10-14/h2-7,10-11,13,20,22-34,36-39H,8-9,12H2,1H3/b7-4+/t13-,20+,22-,23+,24+,25+,26+,27+,28+,29-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189985

(4-[1-(3,4,5-trimethoxybenzoyl)naphthalen-2-yloxy]-...)Show SMILES COc1cc(NC(=O)\C=C\COc2ccc3ccccc3c2C(=O)c2cc(OC)c(OC)c(OC)c2)cc(OC)c1OC Show InChI InChI=1S/C33H33NO9/c1-37-25-16-21(17-26(38-2)32(25)41-5)31(36)30-23-11-8-7-10-20(23)13-14-24(30)43-15-9-12-29(35)34-22-18-27(39-3)33(42-6)28(19-22)40-4/h7-14,16-19H,15H2,1-6H3,(H,34,35)/b12-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50528362

(CHEMBL4466390)Show SMILES [H][C@@]12[C@H](O)C(=O)Oc3c(O)c(O)cc(c13)C(=O)O[C@H]1[C@H](OC(=O)c3cc(O)c(O)c(O)c3)O[C@@H]3COC(=O)c4ccccc4-c4c(O)c(O)c(O)cc4C(=O)O[C@H]1[C@@H]3OC(=O)[C@H]2CC(O)=O |r| Show InChI InChI=1S/C41H30O24/c42-17-5-11(6-18(43)26(17)48)35(53)65-41-34-33-31(21(60-41)10-59-36(54)13-4-2-1-3-12(13)23-14(37(55)63-33)7-19(44)27(49)29(23)51)61-39(57)16(9-22(46)47)24-25-15(38(56)64-34)8-20(45)28(50)32(25)62-40(58)30(24)52/h1-8,16,21,24,30-31,33-34,41-45,48-52H,9-10H2,(H,46,47)/t16-,21+,24-,30-,31+,33-,34+,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX1 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528357
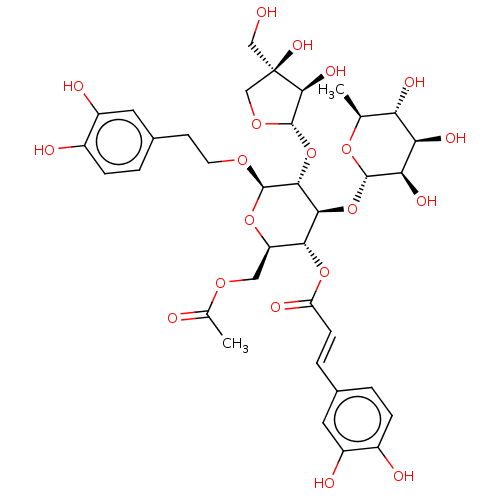
(CHEMBL4530118)Show SMILES [H][C@@]1(O[C@H]2[C@H](OCCc3ccc(O)c(O)c3)O[C@H](COC(C)=O)[C@@H](OC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H]2O[C@]2([H])O[C@@H](C)[C@H](O)[C@@H](O)[C@H]2O)OC[C@](O)(CO)[C@H]1O |r| Show InChI InChI=1S/C36H46O20/c1-16-26(44)27(45)28(46)33(52-16)55-30-29(54-25(43)8-5-18-3-6-20(39)22(41)11-18)24(13-50-17(2)38)53-34(49-10-9-19-4-7-21(40)23(42)12-19)31(30)56-35-32(47)36(48,14-37)15-51-35/h3-8,11-12,16,24,26-35,37,39-42,44-48H,9-10,13-15H2,1-2H3/b8-5+/t16-,24+,26-,27+,28+,29+,30-,31+,32-,33-,34+,35-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528356
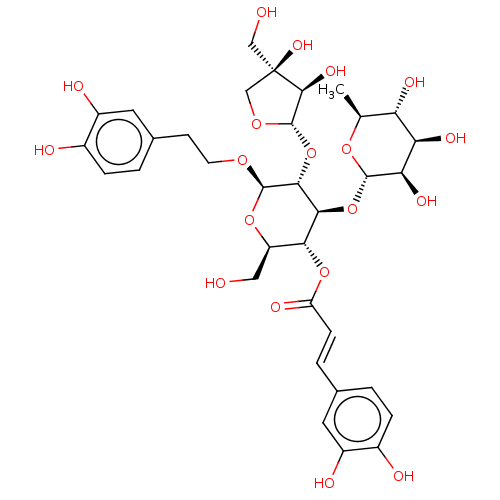
(CHEMBL4467432)Show SMILES [H][C@@]1(O[C@H]2[C@H](OCCc3ccc(O)c(O)c3)O[C@H](CO)[C@@H](OC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H]2O[C@]2([H])O[C@@H](C)[C@H](O)[C@@H](O)[C@H]2O)OC[C@](O)(CO)[C@H]1O |r| Show InChI InChI=1S/C34H44O19/c1-15-24(42)25(43)26(44)31(49-15)52-28-27(51-23(41)7-4-16-2-5-18(37)20(39)10-16)22(12-35)50-32(47-9-8-17-3-6-19(38)21(40)11-17)29(28)53-33-30(45)34(46,13-36)14-48-33/h2-7,10-11,15,22,24-33,35-40,42-46H,8-9,12-14H2,1H3/b7-4+/t15-,22+,24-,25+,26+,27+,28-,29+,30-,31-,32+,33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528370
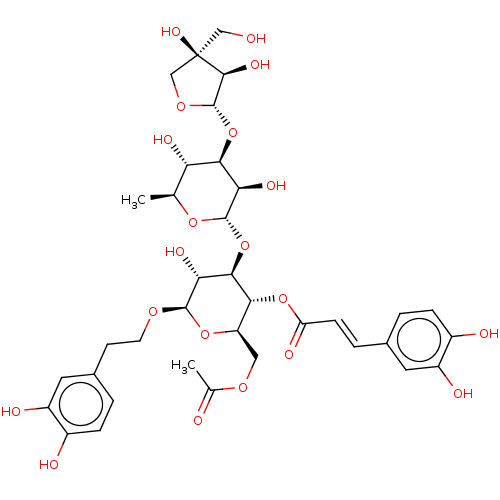
(CHEMBL4452548)Show SMILES [H][C@@]1(O[C@@H]2[C@@H](O)[C@H](C)O[C@@]([H])(O[C@@H]3[C@@H](O)[C@H](OCCc4ccc(O)c(O)c4)O[C@H](COC(C)=O)[C@H]3OC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H]2O)OC[C@](O)(CO)[C@H]1O |r| Show InChI InChI=1S/C36H46O20/c1-16-26(44)30(55-35-32(47)36(48,14-37)15-51-35)27(45)34(52-16)56-31-28(46)33(49-10-9-19-4-7-21(40)23(42)12-19)53-24(13-50-17(2)38)29(31)54-25(43)8-5-18-3-6-20(39)22(41)11-18/h3-8,11-12,16,24,26-35,37,39-42,44-48H,9-10,13-15H2,1-2H3/b8-5+/t16-,24+,26-,27+,28+,29+,30+,31+,32-,33+,34-,35-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528372
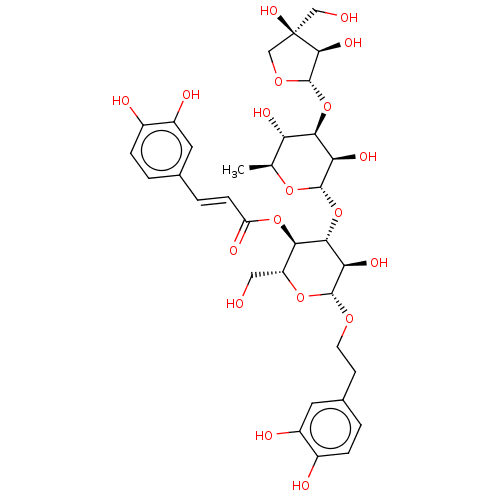
(CHEMBL4455571)Show SMILES [H][C@@]1(O[C@@H]2[C@@H](O)[C@H](C)O[C@@]([H])(O[C@@H]3[C@@H](O)[C@H](OCCc4ccc(O)c(O)c4)O[C@H](CO)[C@H]3OC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H]2O)OC[C@](O)(CO)[C@H]1O |r| Show InChI InChI=1S/C34H44O19/c1-15-24(42)28(52-33-30(45)34(46,13-36)14-48-33)25(43)32(49-15)53-29-26(44)31(47-9-8-17-3-6-19(38)21(40)11-17)50-22(12-35)27(29)51-23(41)7-4-16-2-5-18(37)20(39)10-16/h2-7,10-11,15,22,24-33,35-40,42-46H,8-9,12-14H2,1H3/b7-4+/t15-,22+,24-,25+,26+,27+,28+,29+,30-,31+,32-,33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528368
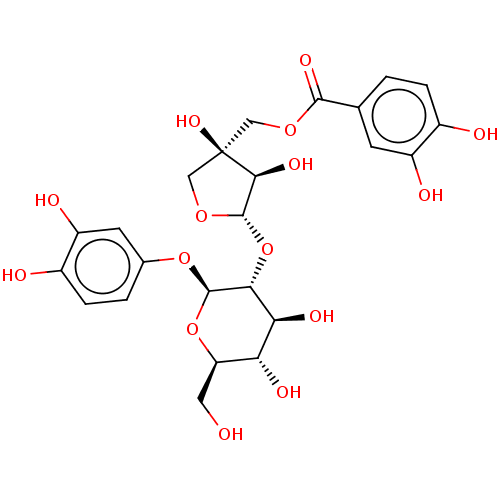
(CHEMBL4465765)Show SMILES [H][C@@]1(O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2Oc2ccc(O)c(O)c2)OC[C@](O)(COC(=O)c2ccc(O)c(O)c2)[C@H]1O |r| Show InChI InChI=1S/C24H28O15/c25-7-16-17(30)18(31)19(22(38-16)37-11-2-4-13(27)15(29)6-11)39-23-20(32)24(34,9-36-23)8-35-21(33)10-1-3-12(26)14(28)5-10/h1-6,16-20,22-23,25-32,34H,7-9H2/t16-,17-,18+,19-,20+,22-,23+,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528374
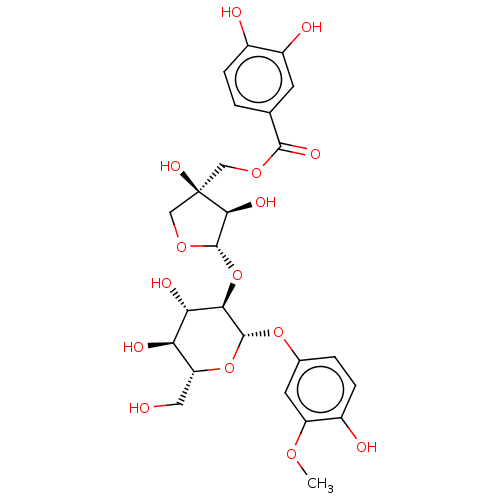
(CHEMBL4556386)Show SMILES [H][C@@]1(O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2Oc2ccc(O)c(OC)c2)OC[C@](O)(COC(=O)c2ccc(O)c(O)c2)[C@H]1O |r| Show InChI InChI=1S/C25H30O15/c1-35-16-7-12(3-5-14(16)28)38-23-20(19(31)18(30)17(8-26)39-23)40-24-21(32)25(34,10-37-24)9-36-22(33)11-2-4-13(27)15(29)6-11/h2-7,17-21,23-24,26-32,34H,8-10H2,1H3/t17-,18-,19+,20-,21+,23-,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528353
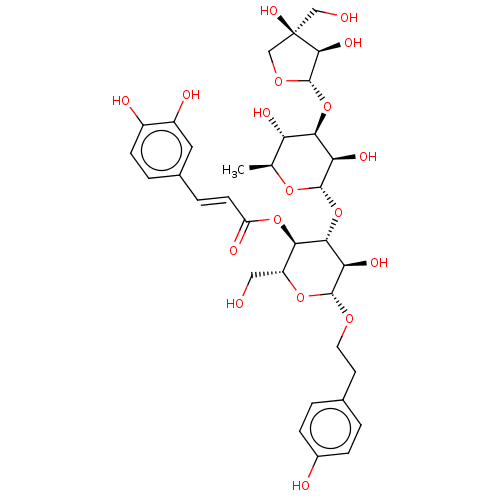
(CHEMBL4576547)Show SMILES [H][C@@]1(O[C@@H]2[C@@H](O)[C@H](C)O[C@@]([H])(O[C@@H]3[C@@H](O)[C@H](OCCc4ccc(O)cc4)O[C@H](CO)[C@H]3OC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H]2O)OC[C@](O)(CO)[C@H]1O |r| Show InChI InChI=1S/C34H44O18/c1-16-24(41)28(51-33-30(44)34(45,14-36)15-47-33)25(42)32(48-16)52-29-26(43)31(46-11-10-17-2-6-19(37)7-3-17)49-22(13-35)27(29)50-23(40)9-5-18-4-8-20(38)21(39)12-18/h2-9,12,16,22,24-33,35-39,41-45H,10-11,13-15H2,1H3/b9-5+/t16-,22+,24-,25+,26+,27+,28+,29+,30-,31+,32-,33-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ovine COX2 using arachidonic acid as substrate preincubated for 15 mins followed by arachidonic acid addition measured afte... |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit A/B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP)
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase supercoiling activity using relaxed pBR322 as substrate after 30 mins by agarose gel electrophore... |
Bioorg Med Chem 26: 4551-4559 (2018)
Article DOI: 10.1016/j.bmc.2018.07.049
BindingDB Entry DOI: 10.7270/Q2XD14C3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463548
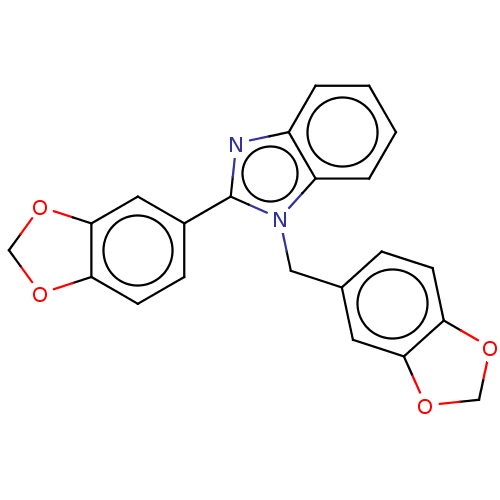
(CHEMBL4239753)Show SMILES C(c1ccc2OCOc2c1)n1c(nc2ccccc12)-c1ccc2OCOc2c1 Show InChI InChI=1S/C22H16N2O4/c1-2-4-17-16(3-1)23-22(15-6-8-19-21(10-15)28-13-26-19)24(17)11-14-5-7-18-20(9-14)27-12-25-18/h1-10H,11-13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP)
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase supercoiling activity using relaxed pBR322 as substrate after 30 mins by agarose gel electrophore... |
Bioorg Med Chem 26: 4551-4559 (2018)
Article DOI: 10.1016/j.bmc.2018.07.049
BindingDB Entry DOI: 10.7270/Q2XD14C3 |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189984
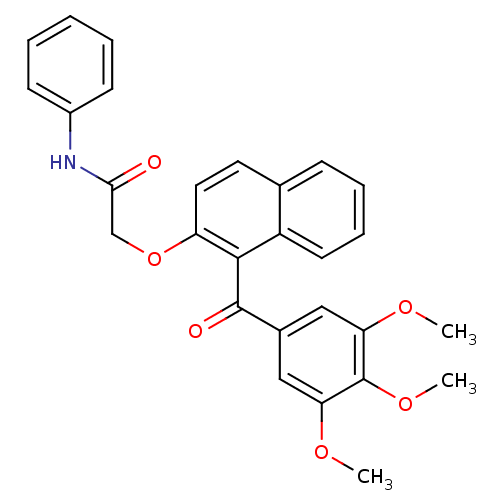
(CHEMBL378015 | N-phenyl-2-(1-(3,4,5-trimethoxybenz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)c1c(OCC(=O)Nc2ccccc2)ccc2ccccc12 Show InChI InChI=1S/C28H25NO6/c1-32-23-15-19(16-24(33-2)28(23)34-3)27(31)26-21-12-8-7-9-18(21)13-14-22(26)35-17-25(30)29-20-10-5-4-6-11-20/h4-16H,17H2,1-3H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463549
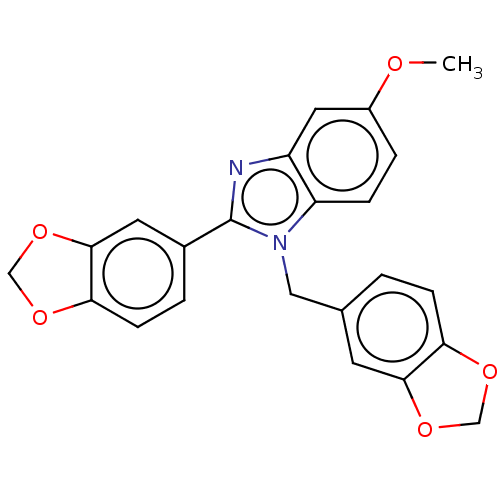
(CHEMBL4237298)Show SMILES COc1ccc2n(Cc3ccc4OCOc4c3)c(nc2c1)-c1ccc2OCOc2c1 Show InChI InChI=1S/C23H18N2O5/c1-26-16-4-5-18-17(10-16)24-23(15-3-7-20-22(9-15)30-13-28-20)25(18)11-14-2-6-19-21(8-14)29-12-27-19/h2-10H,11-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP)
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase supercoiling activity using relaxed pBR322 as substrate after 30 mins by agarose gel electrophore... |
Bioorg Med Chem 26: 4551-4559 (2018)
Article DOI: 10.1016/j.bmc.2018.07.049
BindingDB Entry DOI: 10.7270/Q2XD14C3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM22360
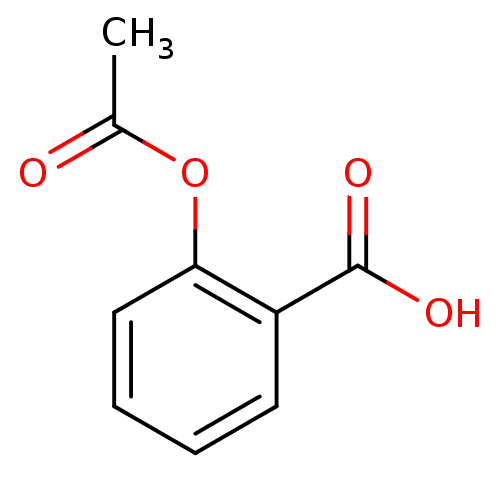
(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50528367
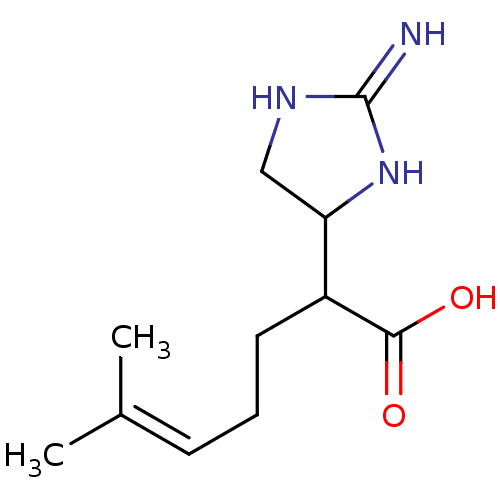
(CHEMBL4444825)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6](-[#6]-1-[#6]-[#7]-[#6](=[#7])-[#7]-1)-[#6](-[#8])=O Show InChI InChI=1S/C11H19N3O2/c1-7(2)4-3-5-8(10(15)16)9-6-13-11(12)14-9/h4,8-9H,3,5-6H2,1-2H3,(H,15,16)(H3,12,13,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463550
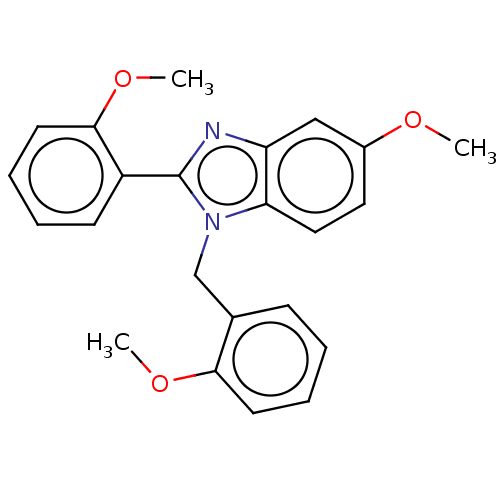
(CHEMBL4251156)Show InChI InChI=1S/C23H22N2O3/c1-26-17-12-13-20-19(14-17)24-23(18-9-5-7-11-22(18)28-3)25(20)15-16-8-4-6-10-21(16)27-2/h4-14H,15H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.58E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP)
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase supercoiling activity using relaxed pBR322 as substrate after 30 mins by agarose gel electrophore... |
Bioorg Med Chem 26: 4551-4559 (2018)
Article DOI: 10.1016/j.bmc.2018.07.049
BindingDB Entry DOI: 10.7270/Q2XD14C3 |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50463551
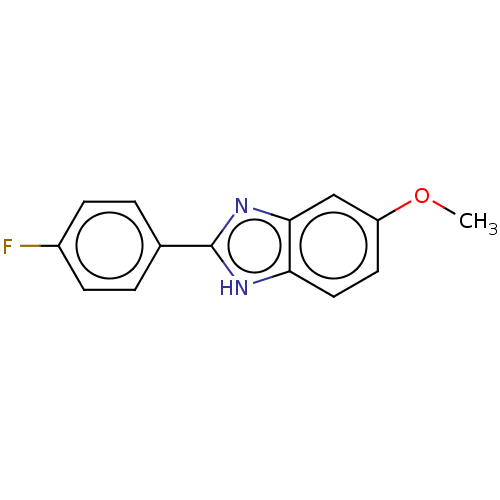
(CHEMBL4243541)Show InChI InChI=1S/C14H11FN2O/c1-18-11-6-7-12-13(8-11)17-14(16-12)9-2-4-10(15)5-3-9/h2-8H,1H3,(H,16,17) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP)
Curated by ChEMBL
| Assay Description
Inhibition of Mycobacterium tuberculosis DNA gyrase supercoiling activity using relaxed pBR322 as substrate after 30 mins by agarose gel electrophore... |
Bioorg Med Chem 26: 4551-4559 (2018)
Article DOI: 10.1016/j.bmc.2018.07.049
BindingDB Entry DOI: 10.7270/Q2XD14C3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528371
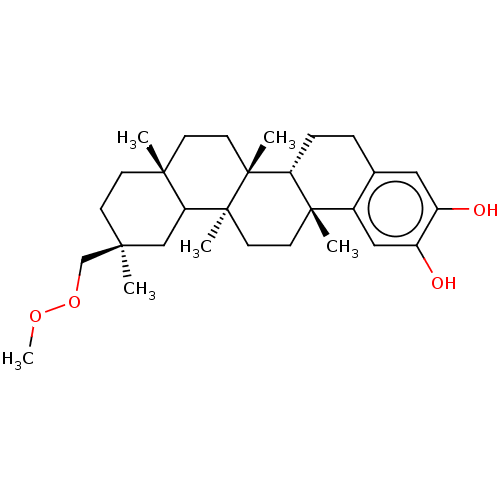
(CHEMBL4443307)Show SMILES [H][C@@]12CCc3cc(O)c(O)cc3[C@]1(C)CC[C@@]1(C)C3C[C@@](C)(COOC)CC[C@]3(C)CC[C@]21C |r| Show InChI InChI=1S/C29H44O4/c1-25(18-33-32-6)9-10-26(2)11-13-28(4)23-8-7-19-15-21(30)22(31)16-20(19)27(23,3)12-14-29(28,5)24(26)17-25/h15-16,23-24,30-31H,7-14,17-18H2,1-6H3/t23-,24?,25+,26-,27+,28-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528366
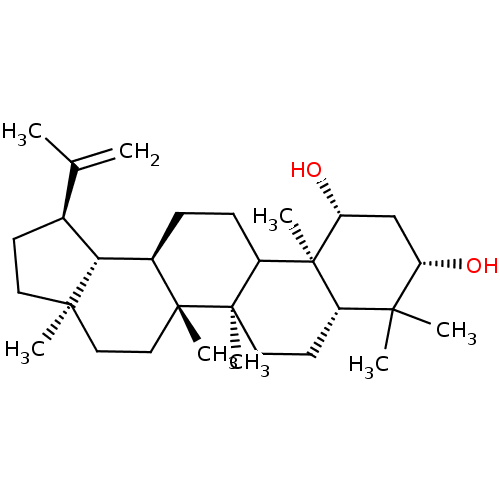
(CHEMBL4475300)Show SMILES [H][C@]12[C@@H](CC[C@]1(C)CC[C@]1(C)[C@]2([H])CCC2[C@@]1(C)CC[C@@]1([H])C(C)(C)[C@@H](O)C[C@@H](O)[C@]21C)C(C)=C |r| Show InChI InChI=1S/C30H50O2/c1-18(2)19-11-13-27(5)15-16-28(6)20(25(19)27)9-10-22-29(28,7)14-12-21-26(3,4)23(31)17-24(32)30(21,22)8/h19-25,31-32H,1,9-17H2,2-8H3/t19-,20+,21-,22?,23-,24+,25+,27+,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528364
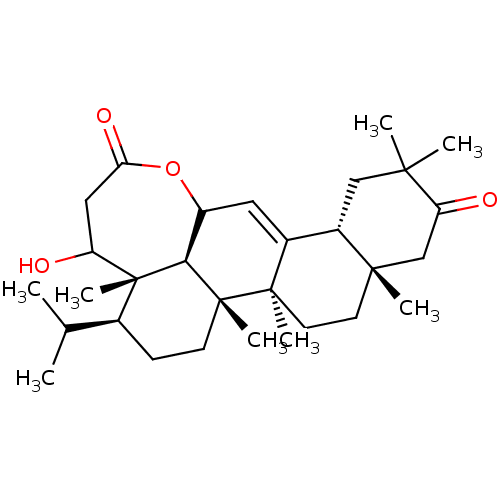
(CHEMBL4453592)Show SMILES [H][C@@]12CC(C)(C)C(=O)C[C@]1(C)CC[C@]1(C)C2=CC2OC(=O)CC(O)[C@@]3(C)[C@@H](CC[C@]1(C)[C@]23[H])C(C)C |r,t:17| Show InChI InChI=1S/C30H46O4/c1-17(2)18-9-10-29(7)25-21(34-24(33)14-22(31)30(18,25)8)13-19-20-15-26(3,4)23(32)16-27(20,5)11-12-28(19,29)6/h13,17-18,20-22,25,31H,9-12,14-16H2,1-8H3/t18-,20-,21?,22?,25-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528365
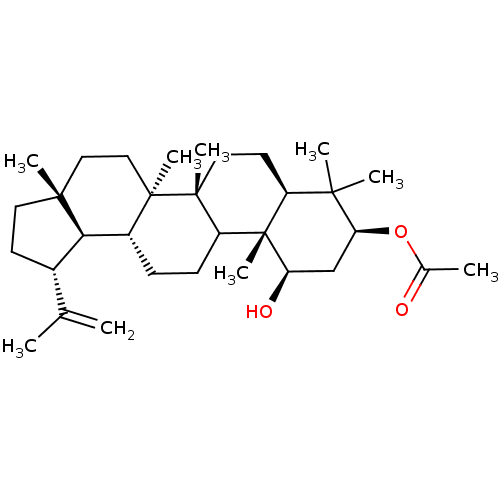
(CHEMBL4450052)Show SMILES [H][C@]12[C@@H](CC[C@]1(C)CC[C@]1(C)[C@]2([H])CCC2[C@@]1(C)CC[C@@]1([H])C(C)(C)[C@H](C[C@@H](O)[C@]21C)OC(C)=O)C(C)=C |r| Show InChI InChI=1S/C32H52O3/c1-19(2)21-12-14-29(6)16-17-30(7)22(27(21)29)10-11-24-31(30,8)15-13-23-28(4,5)26(35-20(3)33)18-25(34)32(23,24)9/h21-27,34H,1,10-18H2,2-9H3/t21-,22+,23-,24?,25+,26-,27+,29+,30+,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528354
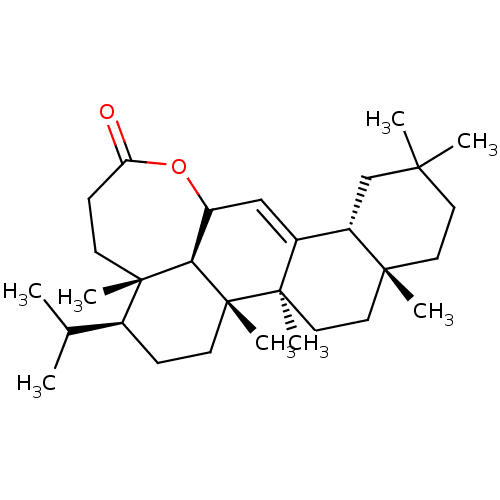
(CHEMBL4444718)Show SMILES [H][C@@]12CC(C)(C)CC[C@]1(C)CC[C@]1(C)C2=CC2OC(=O)CC[C@@]3(C)[C@@H](CC[C@]1(C)[C@]23[H])C(C)C |r,t:16| Show InChI InChI=1S/C30H48O2/c1-19(2)20-9-12-30(8)25-23(32-24(31)10-11-28(20,25)6)17-21-22-18-26(3,4)13-14-27(22,5)15-16-29(21,30)7/h17,19-20,22-23,25H,9-16,18H2,1-8H3/t20-,22-,23?,25+,27+,28-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50528373
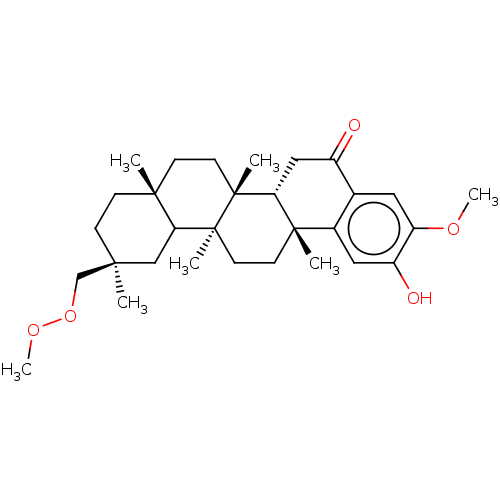
(CHEMBL4552843)Show SMILES [H][C@@]12CC(=O)c3cc(OC)c(O)cc3[C@]1(C)CC[C@@]1(C)C3C[C@@](C)(COOC)CC[C@]3(C)CC[C@]21C |r| Show InChI InChI=1S/C30H44O5/c1-26(18-35-34-7)8-9-27(2)10-12-29(4)24-16-21(31)19-14-23(33-6)22(32)15-20(19)28(24,3)11-13-30(29,5)25(27)17-26/h14-15,24-25,32H,8-13,16-18H2,1-7H3/t24-,25?,26+,27-,28+,29-,30+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM22360

(2-(acetyloxy)benzoate | 2-(acetyloxy)benzoic acid ...)Show InChI InChI=1S/C9H8O4/c1-6(10)13-8-5-3-2-4-7(8)9(11)12/h2-5H,1H3,(H,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528367

(CHEMBL4444825)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]-[#6](-[#6]-1-[#6]-[#7]-[#6](=[#7])-[#7]-1)-[#6](-[#8])=O Show InChI InChI=1S/C11H19N3O2/c1-7(2)4-3-5-8(10(15)16)9-6-13-11(12)14-9/h4,8-9H,3,5-6H2,1-2H3,(H,15,16)(H3,12,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189978
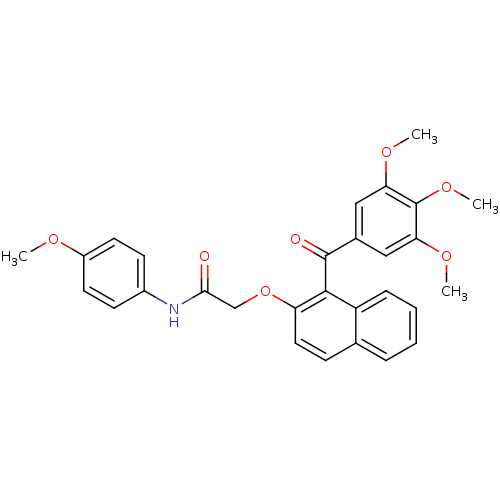
(CHEMBL377142 | N-(4-methoxyphenyl)-2-(1-(3,4,5-tri...)Show SMILES COc1ccc(NC(=O)COc2ccc3ccccc3c2C(=O)c2cc(OC)c(OC)c(OC)c2)cc1 Show InChI InChI=1S/C29H27NO7/c1-33-21-12-10-20(11-13-21)30-26(31)17-37-23-14-9-18-7-5-6-8-22(18)27(23)28(32)19-15-24(34-2)29(36-4)25(16-19)35-3/h5-16H,17H2,1-4H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189977
(4-(1-(3,4,5-trimethoxybenzoyl)naphthalen-2-yloxy)b...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)c1c(OC\C=C\C(O)=O)ccc2ccccc12 Show InChI InChI=1S/C24H22O7/c1-28-19-13-16(14-20(29-2)24(19)30-3)23(27)22-17-8-5-4-7-15(17)10-11-18(22)31-12-6-9-21(25)26/h4-11,13-14H,12H2,1-3H3,(H,25,26)/b9-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50189982

(2-[1-(3,4,5-trimethoxybenzoyl)naphthalene-2-yloxy]...)Show SMILES CCOC(=O)COc1ccc2ccccc2c1C(=O)c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C24H24O7/c1-5-30-21(25)14-31-18-11-10-15-8-6-7-9-17(15)22(18)23(26)16-12-19(27-2)24(29-4)20(13-16)28-3/h6-13H,5,14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
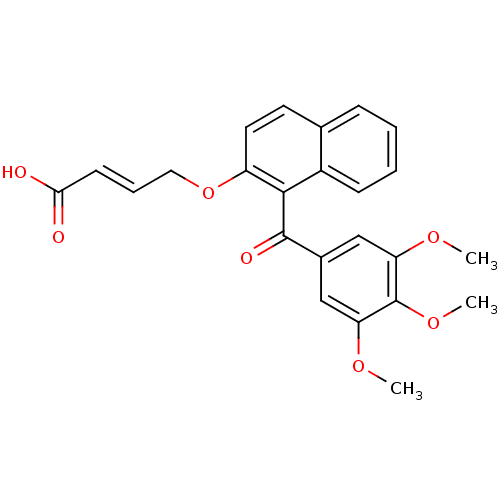
Cathepsin D
(Homo sapiens (Human)) | BDBM50189979
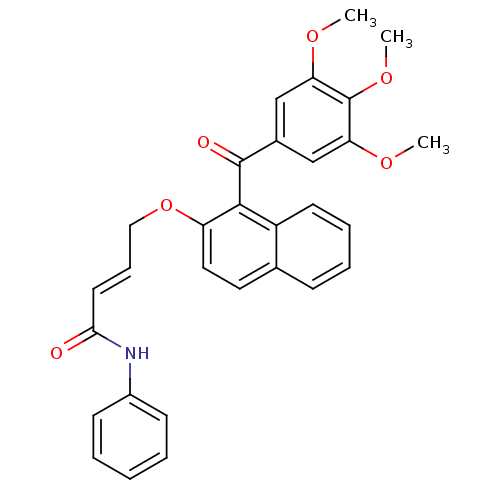
(CHEMBL378329 | N-phenyl-4-(1-(3,4,5-trimethoxybenz...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)c1c(OC\C=C\C(=O)Nc2ccccc2)ccc2ccccc12 Show InChI InChI=1S/C30H27NO6/c1-34-25-18-21(19-26(35-2)30(25)36-3)29(33)28-23-13-8-7-10-20(23)15-16-24(28)37-17-9-14-27(32)31-22-11-5-4-6-12-22/h4-16,18-19H,17H2,1-3H3,(H,31,32)/b14-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Medicinal and Aromatic Plants
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin D |
Bioorg Med Chem Lett 16: 4603-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.010
BindingDB Entry DOI: 10.7270/Q2FB53QN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528369
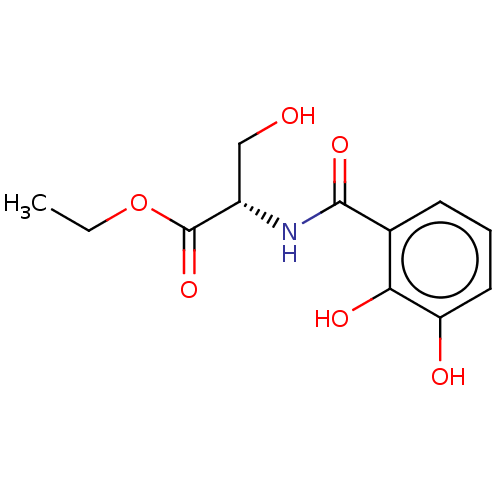
(CHEMBL4472111)Show InChI InChI=1S/C12H15NO6/c1-2-19-12(18)8(6-14)13-11(17)7-4-3-5-9(15)10(7)16/h3-5,8,14-16H,2,6H2,1H3,(H,13,17)/t8-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 using arachidonic acid as substrate by enzyme immunoassay |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50528360
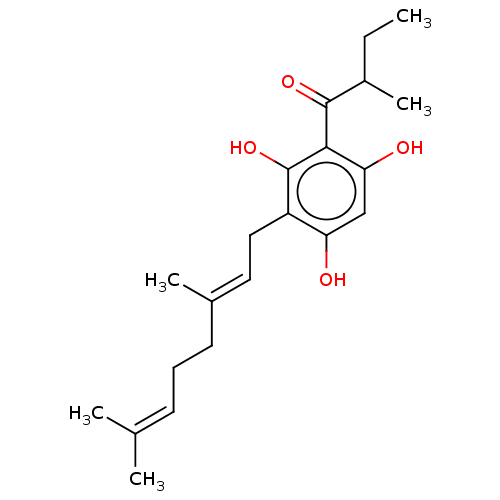
(CHEMBL3331100)Show SMILES [#6]-[#6]-[#6](-[#6])-[#6](=O)-c1c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])c1-[#8] Show InChI InChI=1S/C21H30O4/c1-6-15(5)20(24)19-18(23)12-17(22)16(21(19)25)11-10-14(4)9-7-8-13(2)3/h8,10,12,15,22-23,25H,6-7,9,11H2,1-5H3/b14-10+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 by EIA |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50528359
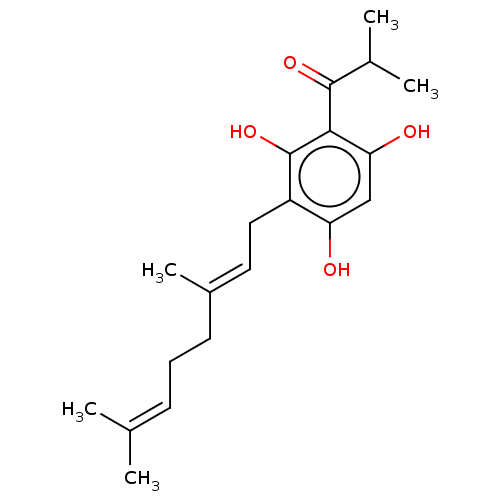
(CHEMBL524326)Show SMILES [#6]-[#6](-[#6])-[#6](=O)-c1c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8] Show InChI InChI=1S/C20H28O4/c1-12(2)7-6-8-14(5)9-10-15-16(21)11-17(22)18(20(15)24)19(23)13(3)4/h7,9,11,13,21-22,24H,6,8,10H2,1-5H3/b14-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 by EIA |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50528360

(CHEMBL3331100)Show SMILES [#6]-[#6]-[#6](-[#6])-[#6](=O)-c1c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])c1-[#8] Show InChI InChI=1S/C21H30O4/c1-6-15(5)20(24)19-18(23)12-17(22)16(21(19)25)11-10-14(4)9-7-8-13(2)3/h8,10,12,15,22-23,25H,6-7,9,11H2,1-5H3/b14-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.62E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 (unknown origin) by EIA |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50528359

(CHEMBL524326)Show SMILES [#6]-[#6](-[#6])-[#6](=O)-c1c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8] Show InChI InChI=1S/C20H28O4/c1-12(2)7-6-8-14(5)9-10-15-16(21)11-17(22)18(20(15)24)19(23)13(3)4/h7,9,11,13,21-22,24H,6,8,10H2,1-5H3/b14-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.99E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Delhi
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by EIA |
Eur J Med Chem 179: 272-309 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.034
BindingDB Entry DOI: 10.7270/Q2FX7DXP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data