 Found 1876 hits with Last Name = 'shannon' and Initial = 'm'
Found 1876 hits with Last Name = 'shannon' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21342
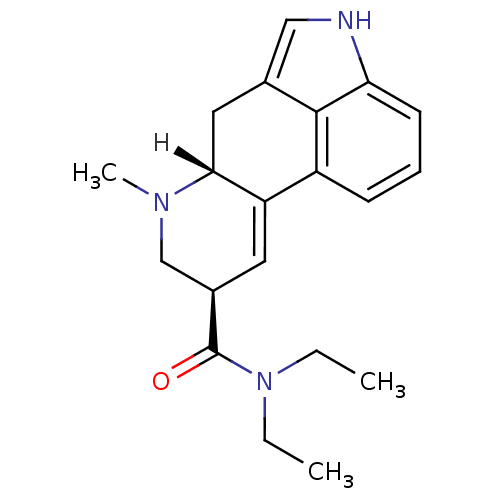
((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50016900
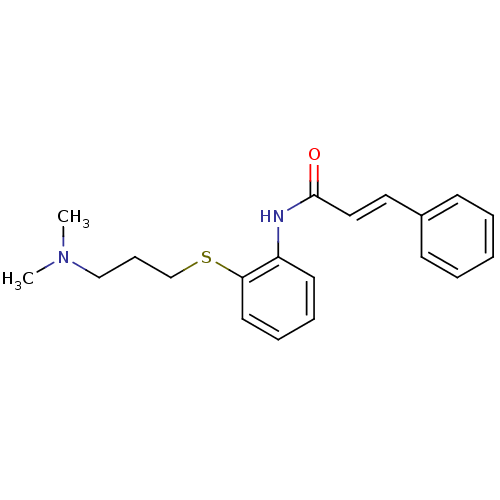
((E)-N-[2-(3-Dimethylamino-propylsulfanyl)-phenyl]-...)Show InChI InChI=1S/C20H24N2OS/c1-22(2)15-8-16-24-19-12-7-6-11-18(19)21-20(23)14-13-17-9-4-3-5-10-17/h3-7,9-14H,8,15-16H2,1-2H3,(H,21,23)/b14-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50016900

((E)-N-[2-(3-Dimethylamino-propylsulfanyl)-phenyl]-...)Show InChI InChI=1S/C20H24N2OS/c1-22(2)15-8-16-24-19-12-7-6-11-18(19)21-20(23)14-13-17-9-4-3-5-10-17/h3-7,9-14H,8,15-16H2,1-2H3,(H,21,23)/b14-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50016900

((E)-N-[2-(3-Dimethylamino-propylsulfanyl)-phenyl]-...)Show InChI InChI=1S/C20H24N2OS/c1-22(2)15-8-16-24-19-12-7-6-11-18(19)21-20(23)14-13-17-9-4-3-5-10-17/h3-7,9-14H,8,15-16H2,1-2H3,(H,21,23)/b14-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024206

(3-[2-(dimethylamino)ethyl]-1H-indol-5-ol | 3-[2-(d...)Show InChI InChI=1S/C12H16N2O/c1-14(2)6-5-9-8-13-12-4-3-10(15)7-11(9)12/h3-4,7-8,13,15H,5-6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM82087
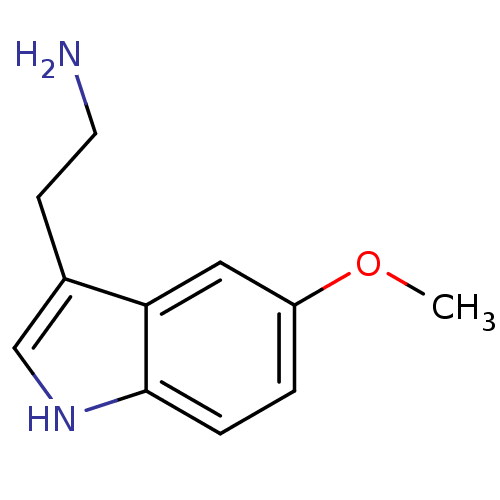
(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50014407
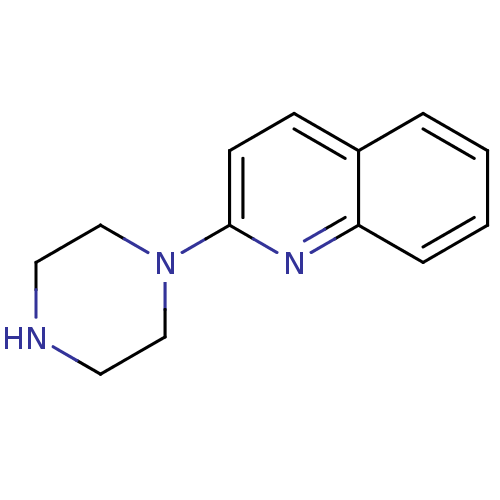
(2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...)Show InChI InChI=1S/C13H15N3/c1-2-4-12-11(3-1)5-6-13(15-12)16-9-7-14-8-10-16/h1-6,14H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024206

(3-[2-(dimethylamino)ethyl]-1H-indol-5-ol | 3-[2-(d...)Show InChI InChI=1S/C12H16N2O/c1-14(2)6-5-9-8-13-12-4-3-10(15)7-11(9)12/h3-4,7-8,13,15H,5-6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM82087

(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50014407

(2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...)Show InChI InChI=1S/C13H15N3/c1-2-4-12-11(3-1)5-6-13(15-12)16-9-7-14-8-10-16/h1-6,14H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024206

(3-[2-(dimethylamino)ethyl]-1H-indol-5-ol | 3-[2-(d...)Show InChI InChI=1S/C12H16N2O/c1-14(2)6-5-9-8-13-12-4-3-10(15)7-11(9)12/h3-4,7-8,13,15H,5-6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024210
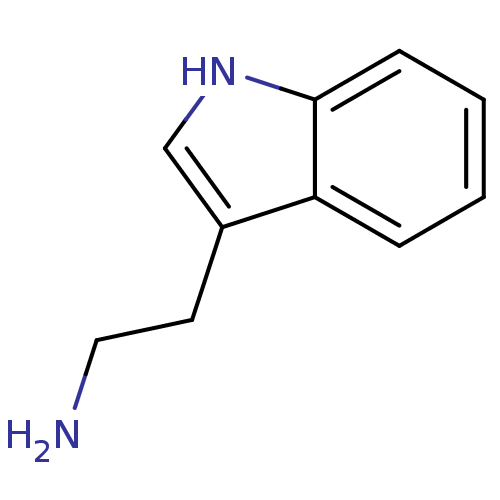
(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 538 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50014407

(2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...)Show InChI InChI=1S/C13H15N3/c1-2-4-12-11(3-1)5-6-13(15-12)16-9-7-14-8-10-16/h1-6,14H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM82087

(2-(5-methoxy-1H-indol-3-yl)ethanamine | 5-MT | 5-M...)Show InChI InChI=1S/C11H14N2O/c1-14-9-2-3-11-10(6-9)8(4-5-12)7-13-11/h2-3,6-7,13H,4-5,12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 647 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024210

(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024210

(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Life Sci 33: 2011-6 (1983)
Article DOI: 10.1016/0024-3205(83)90740-3
BindingDB Entry DOI: 10.7270/Q2DR2T0M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418177
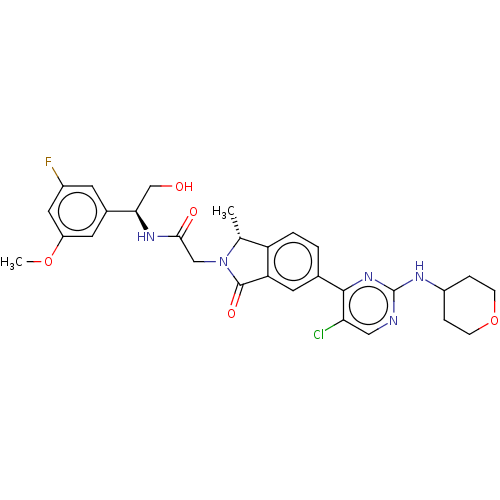
(2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)CN1[C@H](C)c2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H31ClFN5O5/c1-16-22-4-3-17(27-24(30)13-32-29(35-27)33-20-5-7-41-8-6-20)11-23(22)28(39)36(16)14-26(38)34-25(15-37)18-9-19(31)12-21(10-18)40-2/h3-4,9-13,16,20,25,37H,5-8,14-15H2,1-2H3,(H,34,38)(H,32,33,35)/t16-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418177

(2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)CN1[C@H](C)c2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H31ClFN5O5/c1-16-22-4-3-17(27-24(30)13-32-29(35-27)33-20-5-7-41-8-6-20)11-23(22)28(39)36(16)14-26(38)34-25(15-37)18-9-19(31)12-21(10-18)40-2/h3-4,9-13,16,20,25,37H,5-8,14-15H2,1-2H3,(H,34,38)(H,32,33,35)/t16-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418281

((2R)-2-[6-(5-chloro-2-{[(2S)-1- hydroxypropan-2-yl...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(N[C@@H](C)CO)ncc1Cl |r| Show InChI InChI=1S/C27H29ClFN5O5/c1-14(12-35)31-27-30-10-22(28)24(33-27)16-4-5-17-11-34(26(38)21(17)8-16)15(2)25(37)32-23(13-36)18-6-19(29)9-20(7-18)39-3/h4-10,14-15,23,35-36H,11-13H2,1-3H3,(H,32,37)(H,30,31,33)/t14-,15+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418281

((2R)-2-[6-(5-chloro-2-{[(2S)-1- hydroxypropan-2-yl...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(N[C@@H](C)CO)ncc1Cl |r| Show InChI InChI=1S/C27H29ClFN5O5/c1-14(12-35)31-27-30-10-22(28)24(33-27)16-4-5-17-11-34(26(38)21(17)8-16)15(2)25(37)32-23(13-36)18-6-19(29)9-20(7-18)39-3/h4-10,14-15,23,35-36H,11-13H2,1-3H3,(H,32,37)(H,30,31,33)/t14-,15+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418188

(2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES CO[C@H]1N(CC(=O)N[C@H](CO)c2cc(F)cc(OC)c2)C(=O)c2cc(ccc12)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H31ClFN5O6/c1-40-20-10-17(9-18(31)12-20)24(15-37)34-25(38)14-36-27(39)22-11-16(3-4-21(22)28(36)41-2)26-23(30)13-32-29(35-26)33-19-5-7-42-8-6-19/h3-4,9-13,19,24,28,37H,5-8,14-15H2,1-2H3,(H,34,38)(H,32,33,35)/t24-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM417954

(US10457669, Example 615)Show SMILES COc1cccc(n1)[C@@H](CO)NC(=O)CN1[C@@H](C)c2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C28H31ClN6O5/c1-16-19-7-6-17(26-21(29)13-30-28(34-26)31-18-8-10-40-11-9-18)12-20(19)27(38)35(16)14-24(37)32-23(15-36)22-4-3-5-25(33-22)39-2/h3-7,12-13,16,18,23,36H,8-11,14-15H2,1-2H3,(H,32,37)(H,30,31,34)/t16-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418188

(2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES CO[C@H]1N(CC(=O)N[C@H](CO)c2cc(F)cc(OC)c2)C(=O)c2cc(ccc12)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H31ClFN5O6/c1-40-20-10-17(9-18(31)12-20)24(15-37)34-25(38)14-36-27(39)22-11-16(3-4-21(22)28(36)41-2)26-23(30)13-32-29(35-26)33-19-5-7-42-8-6-19/h3-4,9-13,19,24,28,37H,5-8,14-15H2,1-2H3,(H,34,38)(H,32,33,35)/t24-,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM417999
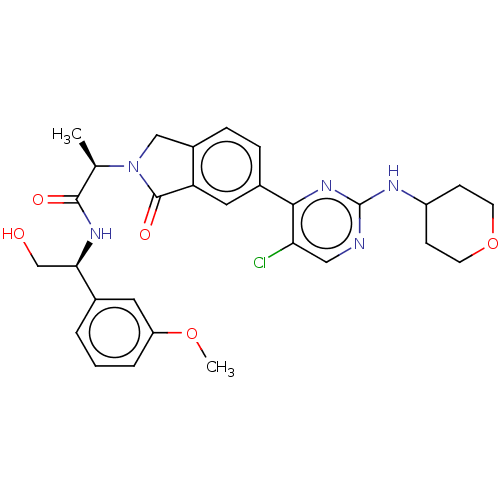
(US10457669, Example 675 | US11001575, Example 675)Show SMILES COc1cccc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H32ClN5O5/c1-17(27(37)33-25(16-36)18-4-3-5-22(12-18)39-2)35-15-20-7-6-19(13-23(20)28(35)38)26-24(30)14-31-29(34-26)32-21-8-10-40-11-9-21/h3-7,12-14,17,21,25,36H,8-11,15-16H2,1-2H3,(H,33,37)(H,31,32,34)/t17-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM417999

(US10457669, Example 675 | US11001575, Example 675)Show SMILES COc1cccc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C29H32ClN5O5/c1-17(27(37)33-25(16-36)18-4-3-5-22(12-18)39-2)35-15-20-7-6-19(13-23(20)28(35)38)26-24(30)14-31-29(34-26)32-21-8-10-40-11-9-21/h3-7,12-14,17,21,25,36H,8-11,15-16H2,1-2H3,(H,33,37)(H,31,32,34)/t17-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM417953
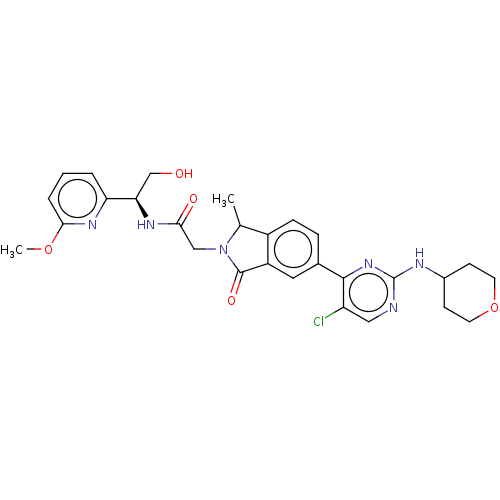
(US10457669, Example 614 | US11001575, Example 616)Show SMILES COc1cccc(n1)[C@@H](CO)NC(=O)CN1C(C)c2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl |r| Show InChI InChI=1S/C28H31ClN6O5/c1-16-19-7-6-17(26-21(29)13-30-28(34-26)31-18-8-10-40-11-9-18)12-20(19)27(38)35(16)14-24(37)32-23(15-36)22-4-3-5-25(33-22)39-2/h3-7,12-13,16,18,23,36H,8-11,14-15H2,1-2H3,(H,32,37)(H,30,31,34)/t16?,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418230

((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1nc(ccc1Cl)N1CCN(C)CC1 |r| Show InChI InChI=1S/C32H38Cl2N8O4/c1-19(30(44)37-26(18-43)29-24(33)5-6-27(38-29)41-11-9-40(2)10-12-41)42-17-21-4-3-20(15-23(21)31(42)45)28-25(34)16-35-32(39-28)36-22-7-13-46-14-8-22/h3-6,15-16,19,22,26,43H,7-14,17-18H2,1-2H3,(H,37,44)(H,35,36,39)/t19-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418223

((2R)-2-(3-{5-chloro-2-[(2-methyl-2H- 1,2,3-triazol...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ncc(cc2C1=O)-c1nc(Nc2cnn(C)n2)ncc1Cl |r| Show InChI InChI=1S/C26H25ClFN9O4/c1-13(24(39)32-21(12-38)14-4-16(28)7-17(5-14)41-3)37-11-20-18(25(37)40)6-15(8-29-20)23-19(27)9-30-26(34-23)33-22-10-31-36(2)35-22/h4-10,13,21,38H,11-12H2,1-3H3,(H,32,39)(H,30,33,34,35)/t13-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418223

((2R)-2-(3-{5-chloro-2-[(2-methyl-2H- 1,2,3-triazol...)Show SMILES COc1cc(F)cc(c1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ncc(cc2C1=O)-c1nc(Nc2cnn(C)n2)ncc1Cl |r| Show InChI InChI=1S/C26H25ClFN9O4/c1-13(24(39)32-21(12-38)14-4-16(28)7-17(5-14)41-3)37-11-20-18(25(37)40)6-15(8-29-20)23-19(27)9-30-26(34-23)33-22-10-31-36(2)35-22/h4-10,13,21,38H,11-12H2,1-3H3,(H,32,39)(H,30,33,34,35)/t13-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418230

((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1nc(ccc1Cl)N1CCN(C)CC1 |r| Show InChI InChI=1S/C32H38Cl2N8O4/c1-19(30(44)37-26(18-43)29-24(33)5-6-27(38-29)41-11-9-40(2)10-12-41)42-17-21-4-3-20(15-23(21)31(42)45)28-25(34)16-35-32(39-28)36-22-7-13-46-14-8-22/h3-6,15-16,19,22,26,43H,7-14,17-18H2,1-2H3,(H,37,44)(H,35,36,39)/t19-,26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418266

((2R)-2-(6-{5-chloro-2-[(1-methyl-1H- 1,2,3-triazol...)Show SMILES C[C@@H](NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(Nc2cnnn2C)ncc1Cl)c1cccc(n1)N1CCN(C)CC1 |r| Show InChI InChI=1S/C30H34ClN11O2/c1-18(24-6-5-7-25(35-24)41-12-10-39(3)11-13-41)34-28(43)19(2)42-17-21-9-8-20(14-22(21)29(42)44)27-23(31)15-32-30(37-27)36-26-16-33-38-40(26)4/h5-9,14-16,18-19H,10-13,17H2,1-4H3,(H,34,43)(H,32,36,37)/t18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418192
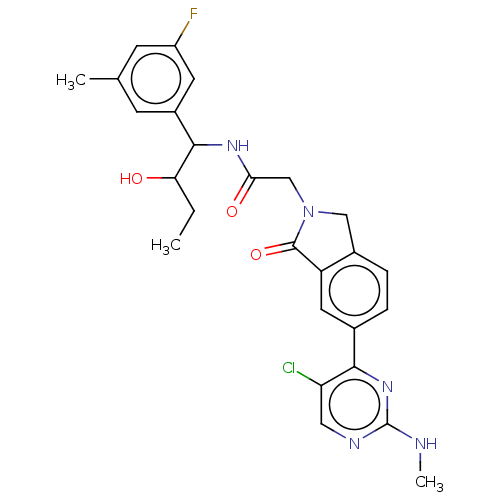
(2-{6-[5-chloro-2- (methylamino)pyrimidin-4-yl]-1-o...)Show SMILES CCC(O)C(NC(=O)CN1Cc2ccc(cc2C1=O)-c1nc(NC)ncc1Cl)c1cc(C)cc(F)c1 Show InChI InChI=1S/C26H27ClFN5O3/c1-4-21(34)24(17-7-14(2)8-18(28)9-17)31-22(35)13-33-12-16-6-5-15(10-19(16)25(33)36)23-20(27)11-30-26(29-3)32-23/h5-11,21,24,34H,4,12-13H2,1-3H3,(H,31,35)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418229

((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1cc(ncc1F)N1CCN(C)CC1 |r| Show InChI InChI=1S/C32H38ClFN8O4/c1-19(30(44)38-27(18-43)24-14-28(35-16-26(24)34)41-9-7-40(2)8-10-41)42-17-21-4-3-20(13-23(21)31(42)45)29-25(33)15-36-32(39-29)37-22-5-11-46-12-6-22/h3-4,13-16,19,22,27,43H,5-12,17-18H2,1-2H3,(H,38,44)(H,36,37,39)/t19-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418266

((2R)-2-(6-{5-chloro-2-[(1-methyl-1H- 1,2,3-triazol...)Show SMILES C[C@@H](NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(Nc2cnnn2C)ncc1Cl)c1cccc(n1)N1CCN(C)CC1 |r| Show InChI InChI=1S/C30H34ClN11O2/c1-18(24-6-5-7-25(35-24)41-12-10-39(3)11-13-41)34-28(43)19(2)42-17-21-9-8-20(14-22(21)29(42)44)27-23(31)15-32-30(37-27)36-26-16-33-38-40(26)4/h5-9,14-16,18-19H,10-13,17H2,1-4H3,(H,34,43)(H,32,36,37)/t18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418192

(2-{6-[5-chloro-2- (methylamino)pyrimidin-4-yl]-1-o...)Show SMILES CCC(O)C(NC(=O)CN1Cc2ccc(cc2C1=O)-c1nc(NC)ncc1Cl)c1cc(C)cc(F)c1 Show InChI InChI=1S/C26H27ClFN5O3/c1-4-21(34)24(17-7-14(2)8-18(28)9-17)31-22(35)13-33-12-16-6-5-15(10-19(16)25(33)36)23-20(27)11-30-26(29-3)32-23/h5-11,21,24,34H,4,12-13H2,1-3H3,(H,31,35)(H,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418229

((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...)Show SMILES C[C@@H](N1Cc2ccc(cc2C1=O)-c1nc(NC2CCOCC2)ncc1Cl)C(=O)N[C@H](CO)c1cc(ncc1F)N1CCN(C)CC1 |r| Show InChI InChI=1S/C32H38ClFN8O4/c1-19(30(44)38-27(18-43)24-14-28(35-16-26(24)34)41-9-7-40(2)8-10-41)42-17-21-4-3-20(13-23(21)31(42)45)29-25(33)15-36-32(39-29)37-22-5-11-46-12-6-22/h3-4,13-16,19,22,27,43H,5-12,17-18H2,1-2H3,(H,38,44)(H,36,37,39)/t19-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418307
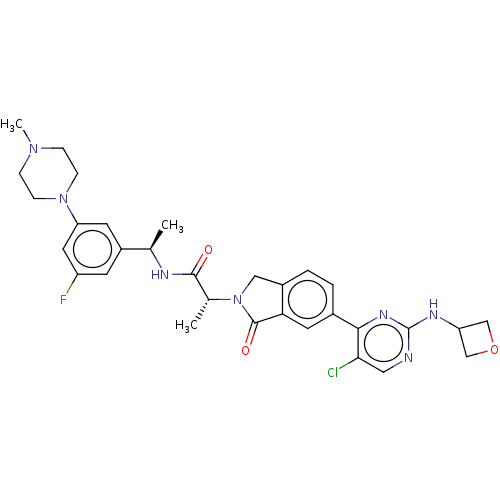
((2R)-2-(6-}5-chloro-2-[(oxetan-3- yl)amino]pyrimid...)Show SMILES C[C@@H](NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(NC2COC2)ncc1Cl)c1cc(F)cc(c1)N1CCN(C)CC1 |r| Show InChI InChI=1S/C31H35ClFN7O3/c1-18(22-10-23(33)13-25(11-22)39-8-6-38(3)7-9-39)35-29(41)19(2)40-15-21-5-4-20(12-26(21)30(40)42)28-27(32)14-34-31(37-28)36-24-16-43-17-24/h4-5,10-14,18-19,24H,6-9,15-17H2,1-3H3,(H,35,41)(H,34,36,37)/t18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418305

((2R)-2-(6-{5-chloro-2-[(2- methylpyrimidin-4-yl)am...)Show SMILES CCNc1cccc(n1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(Nc2ccnc(C)n2)ncc1Cl |r| Show InChI InChI=1S/C29H30ClN9O3/c1-4-31-24-7-5-6-22(35-24)23(15-40)36-27(41)16(2)39-14-19-9-8-18(12-20(19)28(39)42)26-21(30)13-33-29(38-26)37-25-10-11-32-17(3)34-25/h5-13,16,23,40H,4,14-15H2,1-3H3,(H,31,35)(H,36,41)(H,32,33,34,37,38)/t16-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US10457669 (2019)
BindingDB Entry DOI: 10.7270/Q2B27XNR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418307

((2R)-2-(6-}5-chloro-2-[(oxetan-3- yl)amino]pyrimid...)Show SMILES C[C@@H](NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(NC2COC2)ncc1Cl)c1cc(F)cc(c1)N1CCN(C)CC1 |r| Show InChI InChI=1S/C31H35ClFN7O3/c1-18(22-10-23(33)13-25(11-22)39-8-6-38(3)7-9-39)35-29(41)19(2)40-15-21-5-4-20(12-26(21)30(40)42)28-27(32)14-34-31(37-28)36-24-16-43-17-24/h4-5,10-14,18-19,24H,6-9,15-17H2,1-3H3,(H,35,41)(H,34,36,37)/t18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM418305

((2R)-2-(6-{5-chloro-2-[(2- methylpyrimidin-4-yl)am...)Show SMILES CCNc1cccc(n1)[C@@H](CO)NC(=O)[C@@H](C)N1Cc2ccc(cc2C1=O)-c1nc(Nc2ccnc(C)n2)ncc1Cl |r| Show InChI InChI=1S/C29H30ClN9O3/c1-4-31-24-7-5-6-22(35-24)23(15-40)36-27(41)16(2)39-14-19-9-8-18(12-20(19)28(39)42)26-21(30)13-33-29(38-26)37-25-10-11-32-17(3)34-25/h5-13,16,23,40H,4,14-15H2,1-3H3,(H,31,35)(H,36,41)(H,32,33,34,37,38)/t16-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OTSUKA PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
Activity of ERK2 enzyme (Life Technologies) was determined using a time-resolved fluorescence format measuring the phosphorylation of a truncated ver... |
US Patent US11001575 (2021)
BindingDB Entry DOI: 10.7270/Q2TB1B01 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data