

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793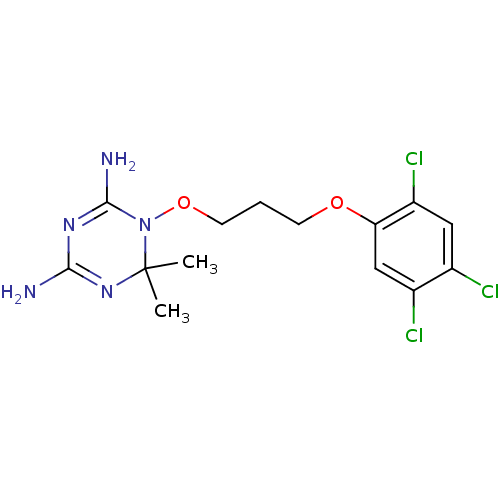 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0110 | -62.5 | 0.570 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0200 | -61.1 | 2.30 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase [N51I,C59R,S108N,I164L] (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18793 (6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.0370 | -59.5 | 18 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004193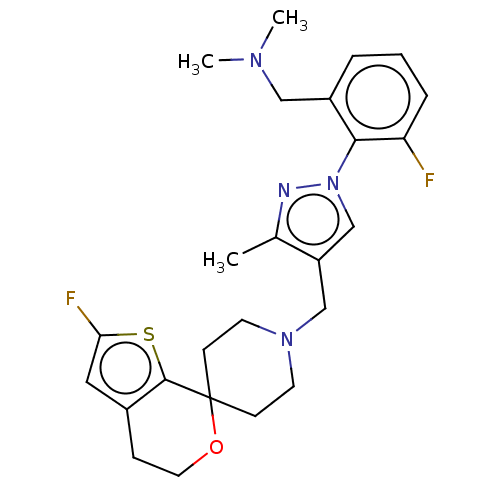 (CHEMBL3236476) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0648 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004180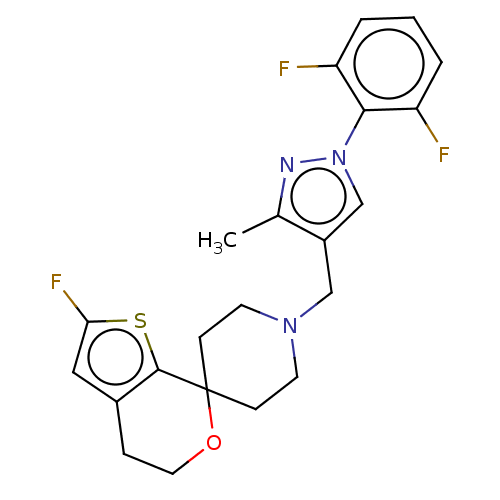 (CHEMBL3236474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0711 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004195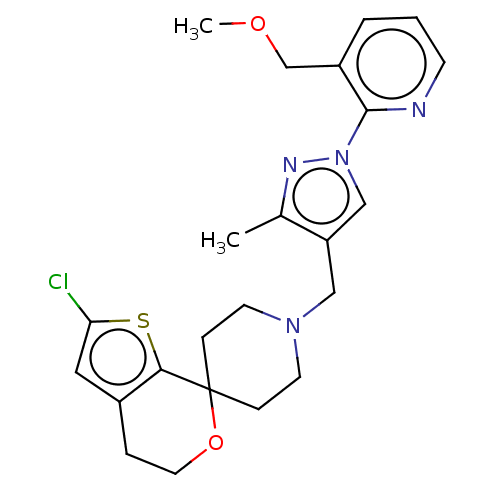 (CHEMBL3236478) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0908 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004175 (CHEMBL3236486) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0916 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004196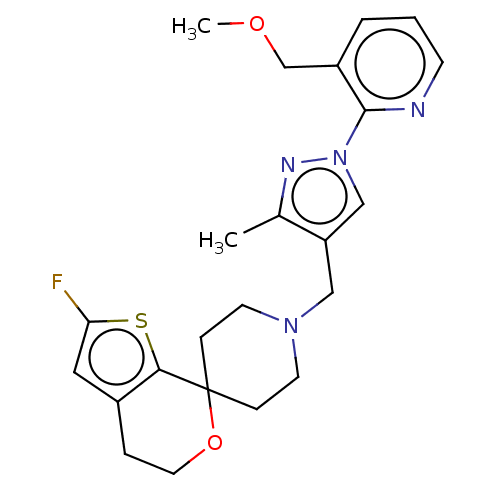 (CHEMBL3236479) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0939 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004181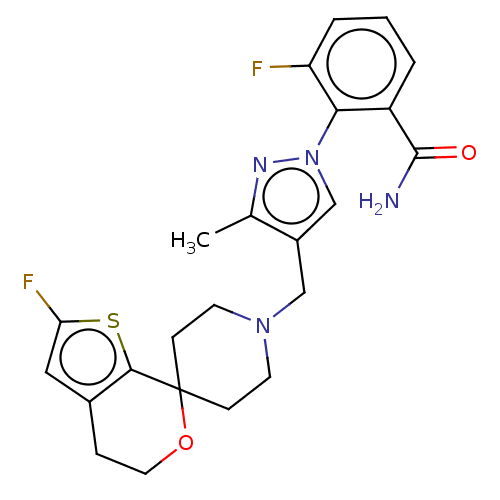 (CHEMBL3236475) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0954 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004174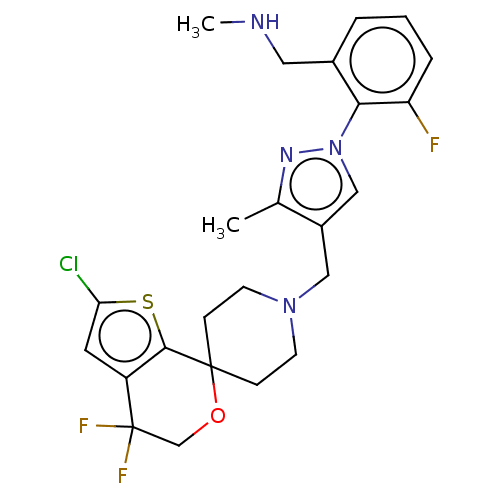 (CHEMBL3236485) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004176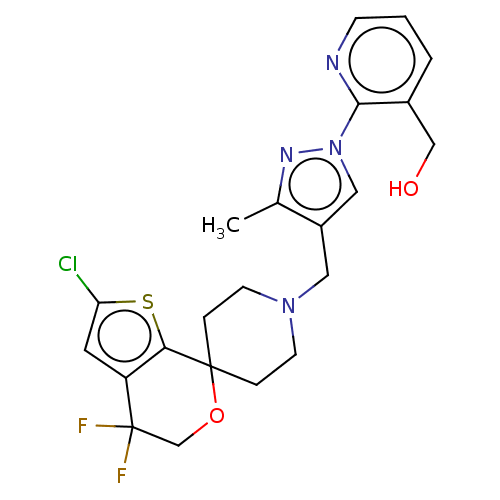 (CHEMBL3236487) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004173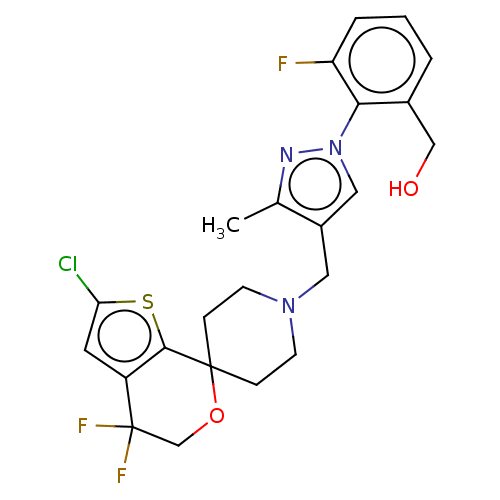 (CHEMBL3236484) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004194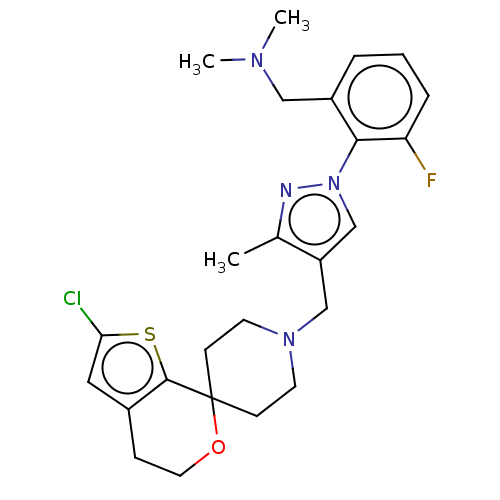 (CHEMBL3236477) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004179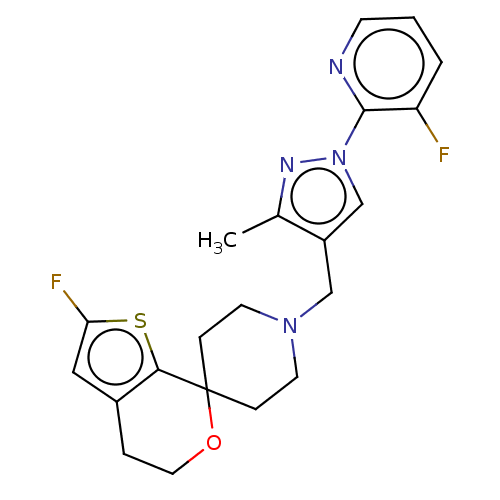 (CHEMBL3236473) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004166 (CHEMBL3236482) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004144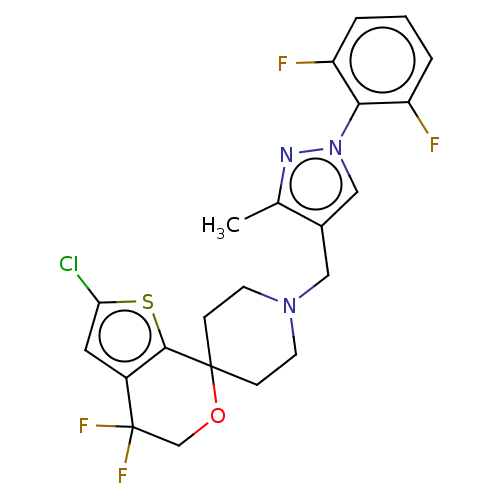 (CHEMBL3236480) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004161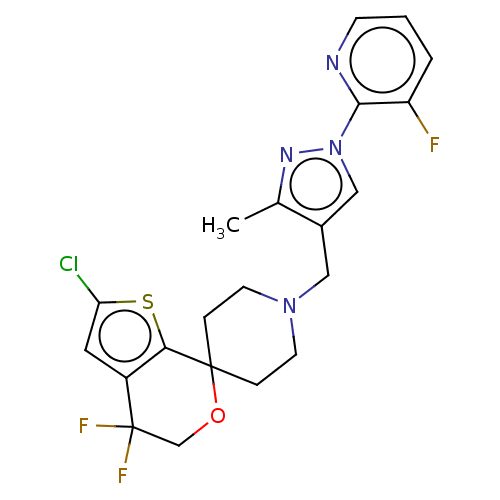 (CHEMBL3236481) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004177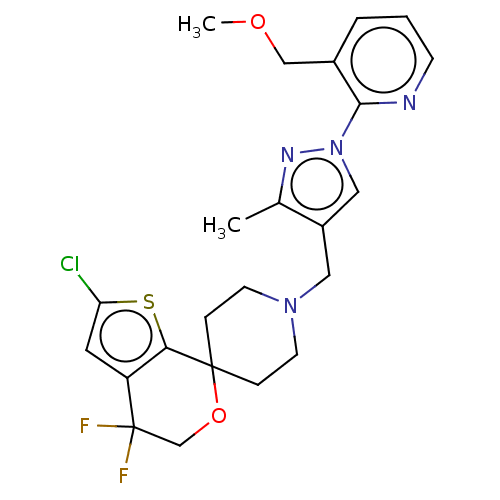 (CHEMBL3236488) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -55.4 | 80 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178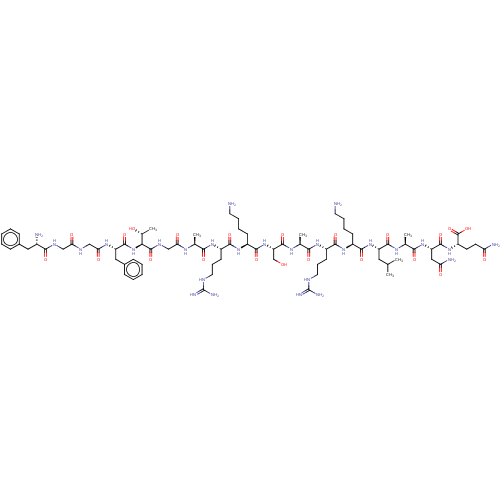 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792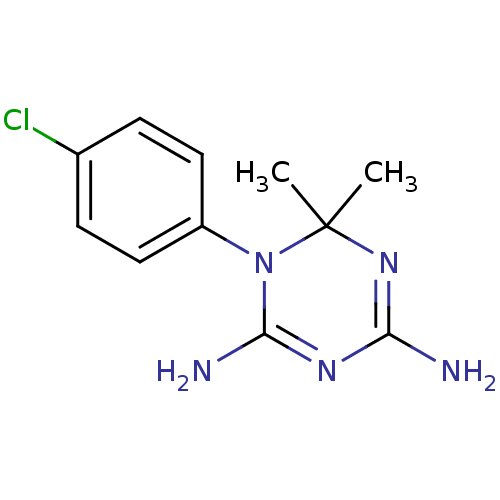 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | -54.4 | 37 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004171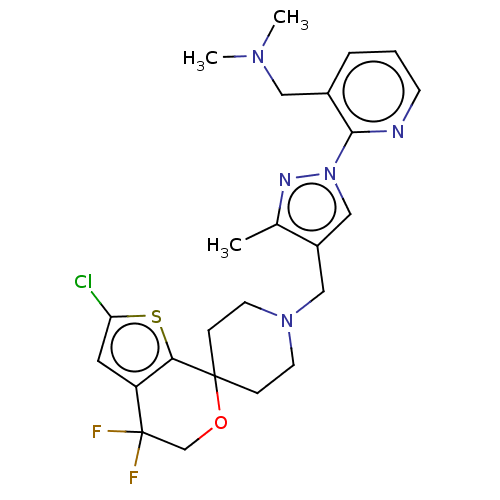 (CHEMBL3236483) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244204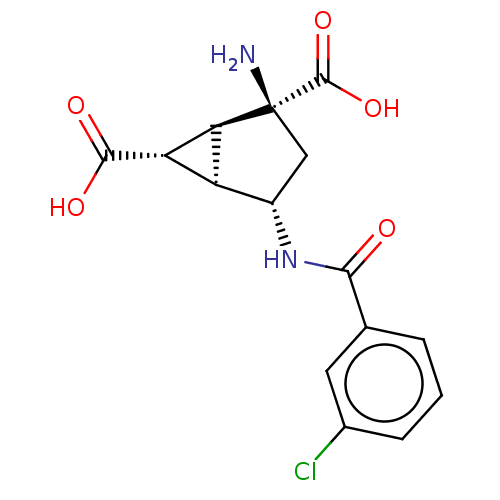 (CHEMBL4071962) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244219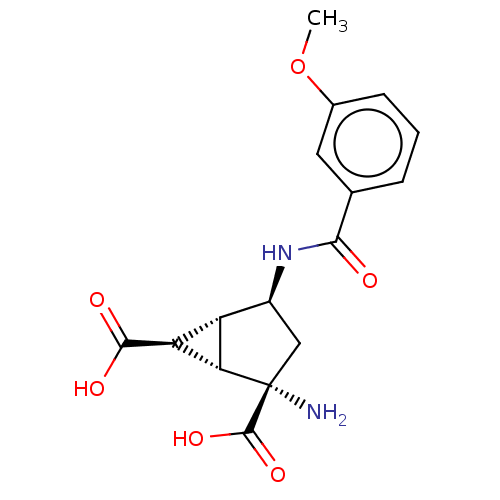 (CHEMBL4081453) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.927 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor from bovine hippocampus, used [3H]8-OH-DPAT as radioligand | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244218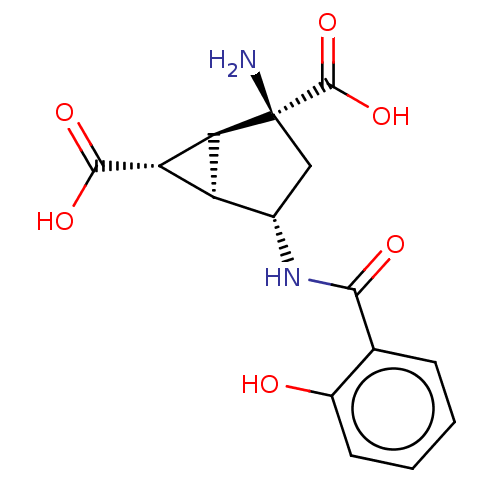 (CHEMBL4068189) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM8061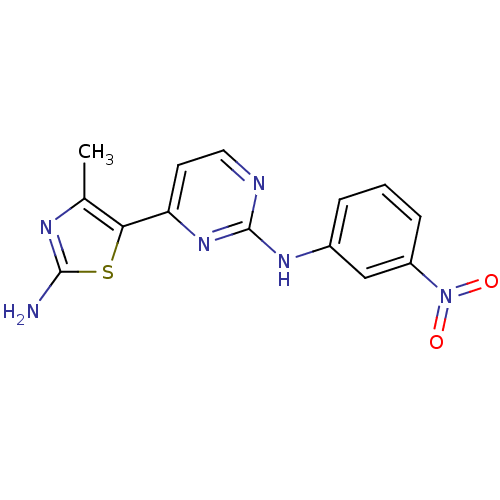 (2-Anilino-4-(thiazol-5-yl)pyrimidine deriv. 32 | 4...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2 | -50.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Cyclacel Limited | Assay Description In vitro kinase assay using purified enzyme, was incubated at 30 °C with substrate, and test compounds in the presence of 100 uM ATP/ [gamma-32P... | J Med Chem 47: 1662-75 (2004) Article DOI: 10.1021/jm0309957 BindingDB Entry DOI: 10.7270/Q2H993DD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244206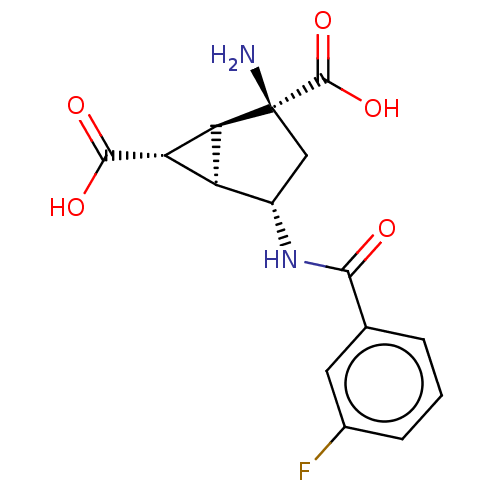 (CHEMBL4091203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244203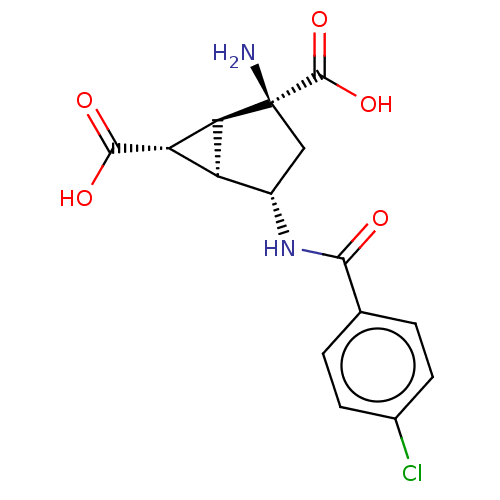 (CHEMBL4095567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244202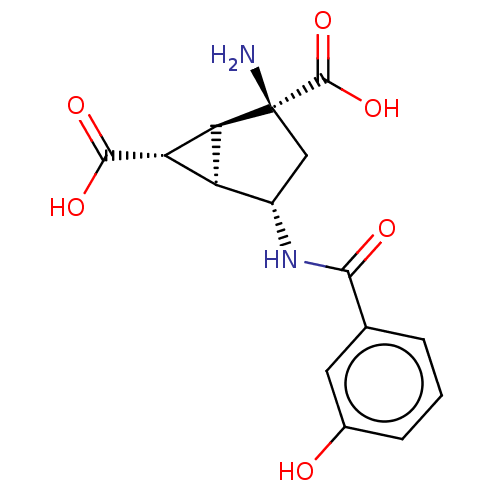 (CHEMBL4091735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570310 (CHEMBL4876861) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50233524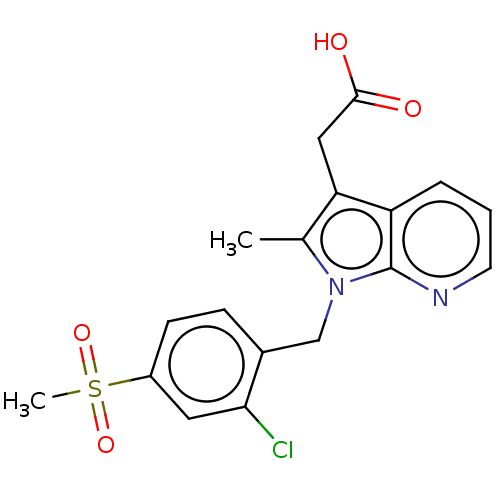 (CHEMBL3981414) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human DP2 receptor expressed in CHO cell membranes after 60 mins by scintillation proximity assay | ACS Med Chem Lett 8: 582-586 (2017) Article DOI: 10.1021/acsmedchemlett.7b00157 BindingDB Entry DOI: 10.7270/Q2TH8PXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570295 (CHEMBL4854124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50233520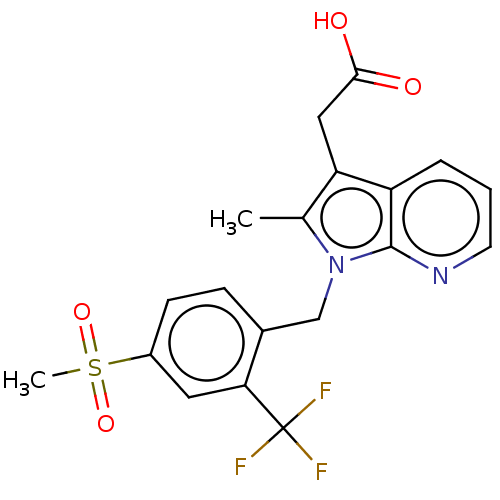 (Fevipiprant | NVP-QAW039 | QAW039) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human DP2 receptor expressed in CHO cell membranes after 60 mins by scintillation proximity assay | ACS Med Chem Lett 8: 582-586 (2017) Article DOI: 10.1021/acsmedchemlett.7b00157 BindingDB Entry DOI: 10.7270/Q2TH8PXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244221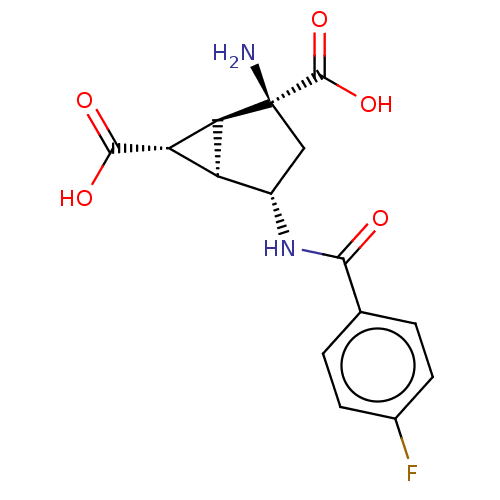 (CHEMBL4063336) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor from bovine hippocampus, used [3H]8-OH-DPAT as radioligand | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244188 (CHEMBL4061162) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570317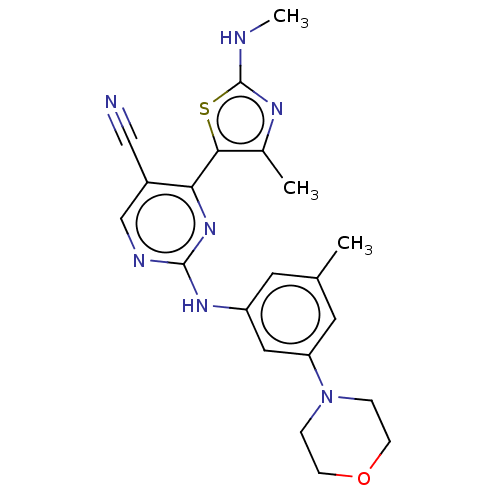 (CHEMBL4863915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570294 (CHEMBL4855334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244207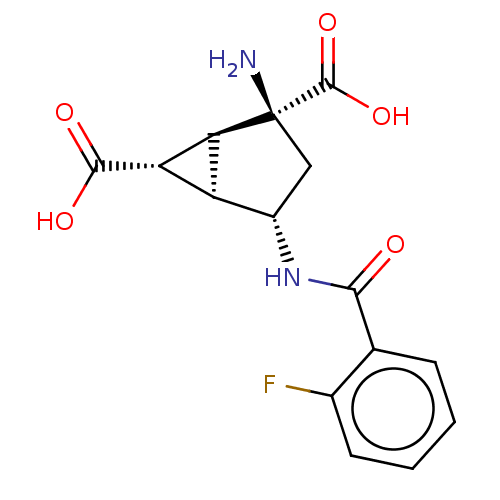 (CHEMBL4090293) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-LY459477 from recombinant human mGlu3 receptor expressed in HEK cell membranes after 90 mins by liquid scintillation counting | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570316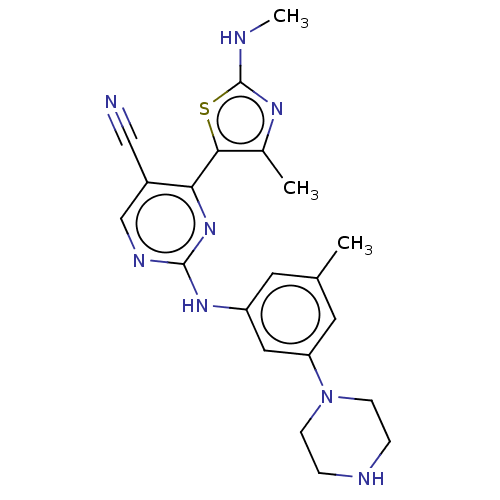 (CHEMBL4864851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570314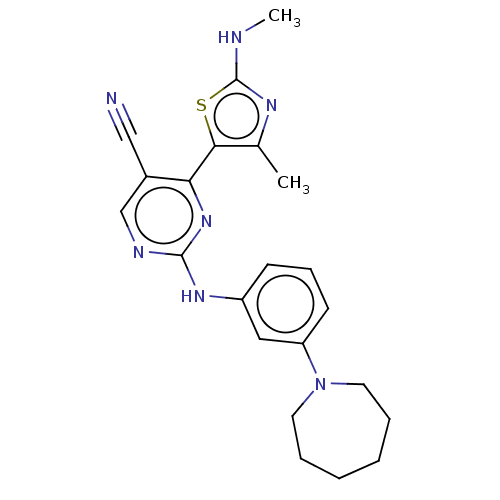 (CHEMBL4847048) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50233523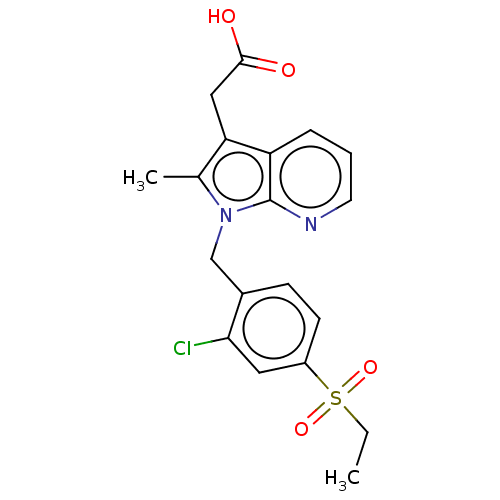 (CHEMBL3931906) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human DP2 receptor expressed in CHO cell membranes after 60 mins by scintillation proximity assay | ACS Med Chem Lett 8: 582-586 (2017) Article DOI: 10.1021/acsmedchemlett.7b00157 BindingDB Entry DOI: 10.7270/Q2TH8PXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM132734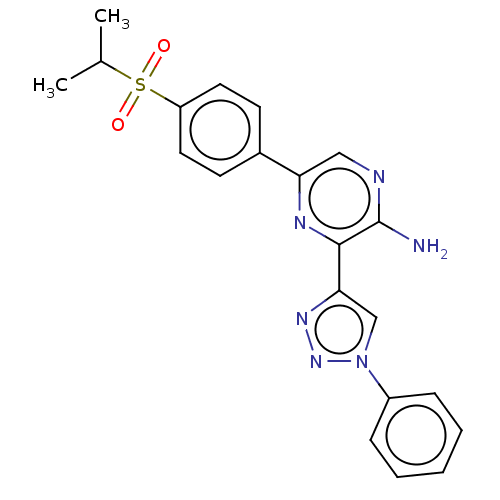 (US8846917, I-1) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | -46.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Vertex Pharmaceuticals Incorporated US Patent | Assay Description Compounds were screened for their ability to inhibit ATR kinase using a radioactive-phosphate incorporation assay. Assays were carried out in a mixtu... | US Patent US8846917 (2014) BindingDB Entry DOI: 10.7270/Q2CC0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.20 | -46.8 | 2.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mahidol University | Assay Description The concentration of inhibitor that inhibited 50% of the parasite growth (IC50) was determined from the sigmoidal curve obtained by plotting the perc... | Nat Struct Biol 10: 257-65 (2003) Article DOI: 10.1038/nsb921 BindingDB Entry DOI: 10.7270/Q2HH6HBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aryl hydrocarbon receptor (Rattus norvegicus) | BDBM50240990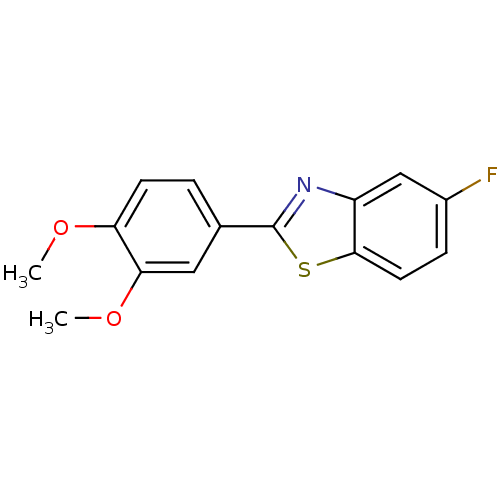 (2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole | 5-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cardiff University Curated by ChEMBL | Assay Description Displacement of [3H]tetrachlorodibenzo-p-dioxin from aryl hydrocarbon receptor in CRL:WI rat liver cytosol | J Med Chem 51: 5135-9 (2008) Article DOI: 10.1021/jm800418z BindingDB Entry DOI: 10.7270/Q23X86F6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 3 (Homo sapiens (Human)) | BDBM50244210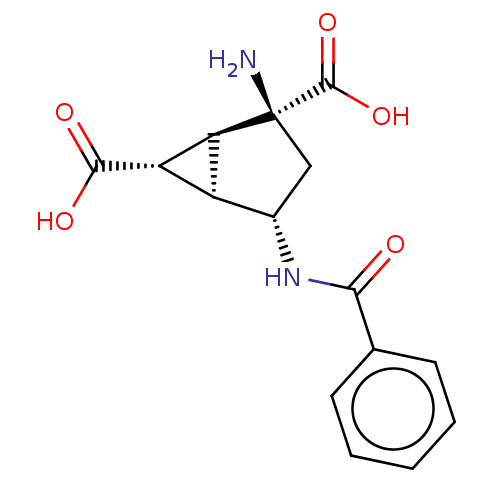 (CHEMBL4095995) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for antagonistic activity against D2 receptor from rat striatum, used [3H]raclopride as radioligand | J Med Chem 61: 2303-2328 (2018) Article DOI: 10.1021/acs.jmedchem.7b01481 BindingDB Entry DOI: 10.7270/Q2W95CM9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50233522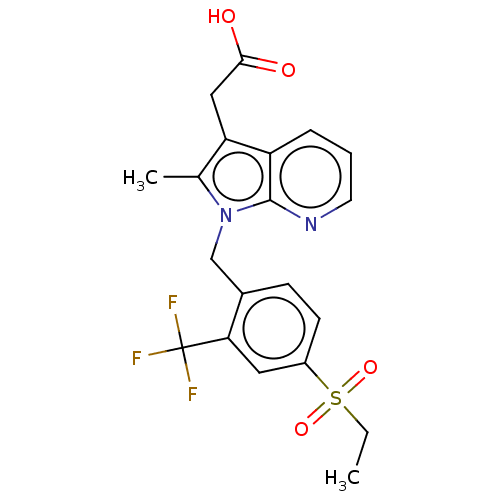 (CHEMBL3932668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human DP2 receptor expressed in CHO cell membranes after 60 mins by scintillation proximity assay | ACS Med Chem Lett 8: 582-586 (2017) Article DOI: 10.1021/acsmedchemlett.7b00157 BindingDB Entry DOI: 10.7270/Q2TH8PXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570301 (CHEMBL4868287) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570308 (CHEMBL4863865) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50509915 (CHEMBL4441579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McMaster University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP19A1 using DBF as substrate measured after 1 hr by fluorescence assay | Bioorg Med Chem Lett 29: 1395-1398 (2019) Article DOI: 10.1016/j.bmcl.2019.03.033 BindingDB Entry DOI: 10.7270/Q2F1932J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM50570309 (CHEMBL4868954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human full length CDK9/Cyclin T1 using KTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIADWC as substrate incubated for 40 mins in presen... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113244 BindingDB Entry DOI: 10.7270/Q2RX9GV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4473 total ) | Next | Last >> |