 Found 1122 hits with Last Name = 'shearer' and Initial = 'bg'
Found 1122 hits with Last Name = 'shearer' and Initial = 'bg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086433
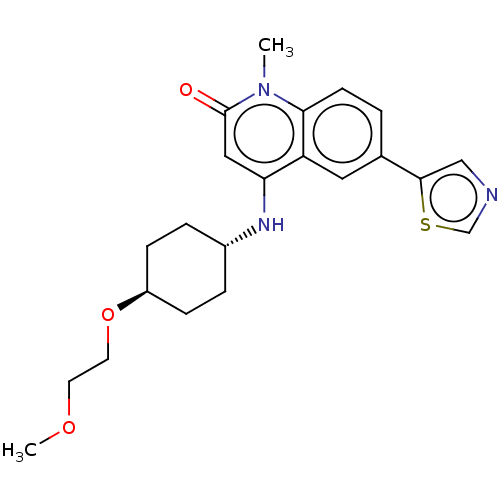
(CHEMBL3426034)Show SMILES COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2ccc(cc12)-c1cncs1 |r,wU:5.4,wD:8.11,(9.09,10.61,;8.02,10,;8.02,8.46,;6.68,7.69,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.01,6.15,;1.33,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.4,.91,;-6.43,2.07,;-5.65,3.39,;-4.14,3.06,)| Show InChI InChI=1S/C22H27N3O3S/c1-25-20-8-3-15(21-13-23-14-29-21)11-18(20)19(12-22(25)26)24-16-4-6-17(7-5-16)28-10-9-27-2/h3,8,11-14,16-17,24H,4-7,9-10H2,1-2H3/t16-,17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086438

(CHEMBL3426039)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1 |r,wU:2.1,wD:5.8,(6.69,7.38,;6.68,6.15,;5.35,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.02,6.16,;1.34,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.39,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.41,.92,;-6.43,2.07,;-5.65,3.4,;-4.15,3.07,)| Show InChI InChI=1S/C21H25N3O2S/c1-13-8-14(19-11-22-12-27-19)9-17-18(10-20(25)24(2)21(13)17)23-15-4-6-16(26-3)7-5-15/h8-12,15-16,23H,4-7H2,1-3H3/t15-,16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086434
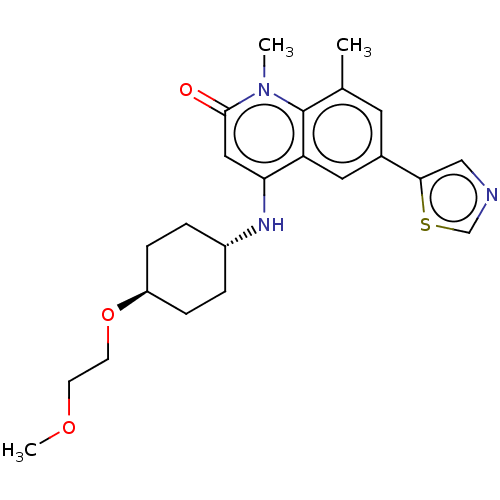
(CHEMBL3426035)Show SMILES COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1 |r,wU:5.4,wD:8.11,(9.09,10.61,;8.02,10,;8.02,8.46,;6.68,7.69,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.01,6.15,;1.33,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.4,.91,;-6.43,2.07,;-5.65,3.39,;-4.14,3.06,)| Show InChI InChI=1S/C23H29N3O3S/c1-15-10-16(21-13-24-14-30-21)11-19-20(12-22(27)26(2)23(15)19)25-17-4-6-18(7-5-17)29-9-8-28-3/h10-14,17-18,25H,4-9H2,1-3H3/t17-,18- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065807
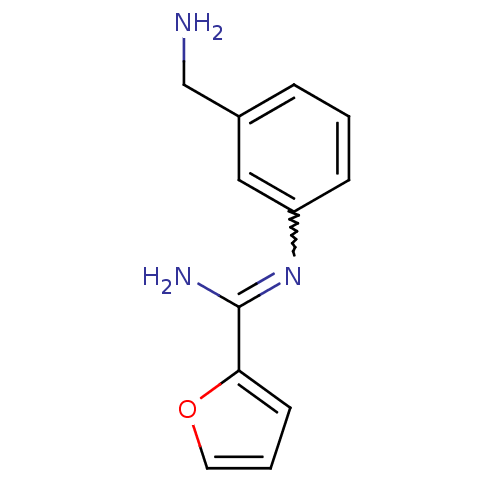
(CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065843
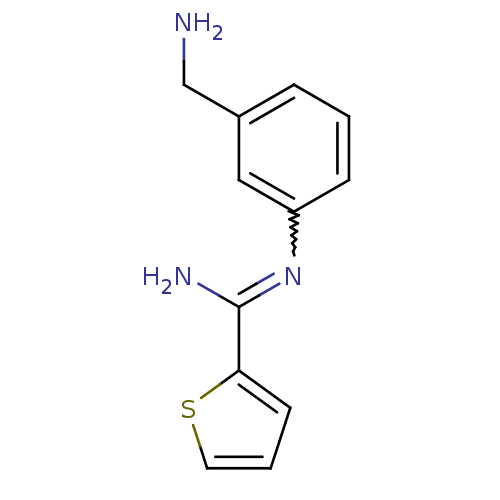
(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065823
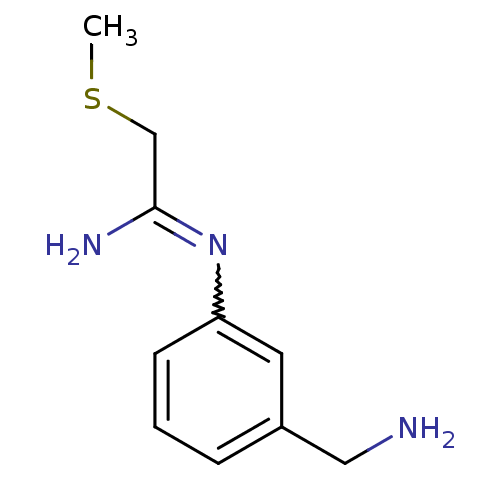
(CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...)Show InChI InChI=1S/C10H15N3S/c1-14-7-10(12)13-9-4-2-3-8(5-9)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065813

(CHEMBL555584 | N-(3-Aminomethyl-phenyl)-2-fluoro-a...)Show InChI InChI=1S/C9H12FN3/c10-5-9(12)13-8-3-1-2-7(4-8)6-11/h1-4H,5-6,11H2,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086425
(CHEMBL3426030)Show InChI InChI=1S/C18H19N3O2S/c1-21-16-3-2-12(17-10-19-11-24-17)8-14(16)15(9-18(21)22)20-13-4-6-23-7-5-13/h2-3,8-11,13,20H,4-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065823

(CHEMBL555794 | N-(3-Aminomethyl-phenyl)-2-methylsu...)Show InChI InChI=1S/C10H15N3S/c1-14-7-10(12)13-9-4-2-3-8(5-9)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50225106

((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...)Show SMILES N[C@@H](CCCNC(N)=N[N+]([O-])=O)C(O)=O |r,w:8.8| Show InChI InChI=1S/C6H13N5O4/c7-4(5(12)13)2-1-3-9-6(8)10-11(14)15/h4H,1-3,7H2,(H,12,13)(H3,8,9,10)/t4-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human endothelial nitric oxide synthase (eNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065833
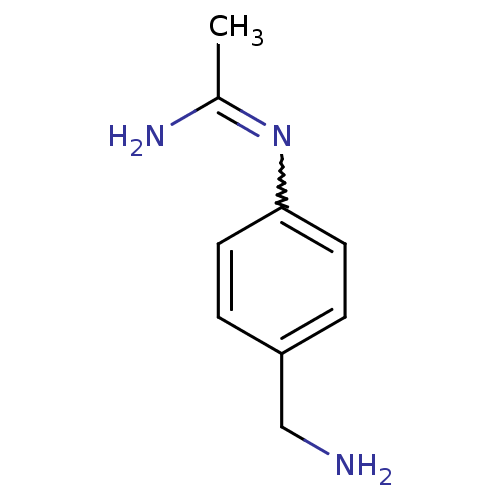
(CHEMBL542432 | N-(4-Aminomethyl-phenyl)-acetamidin...)Show InChI InChI=1S/C9H13N3/c1-7(11)12-9-4-2-8(6-10)3-5-9/h2-5H,6,10H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065842
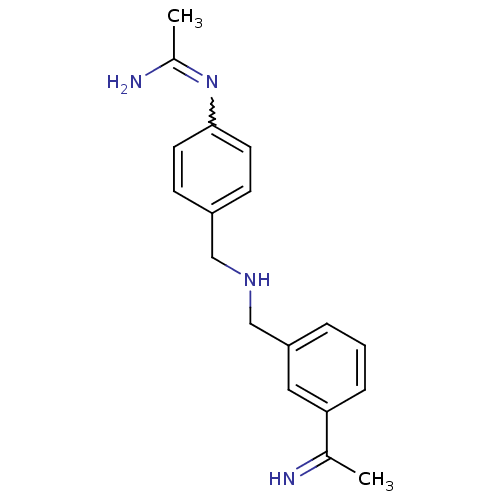
(CHEMBL540048 | N-(4-{[3-(1-Imino-ethyl)-benzylamin...)Show SMILES CC(N)=Nc1ccc(CNCc2cccc(c2)C(C)=N)cc1 |w:3.3| Show InChI InChI=1S/C18H22N4/c1-13(19)17-5-3-4-16(10-17)12-21-11-15-6-8-18(9-7-15)22-14(2)20/h3-10,19,21H,11-12H2,1-2H3,(H2,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002865
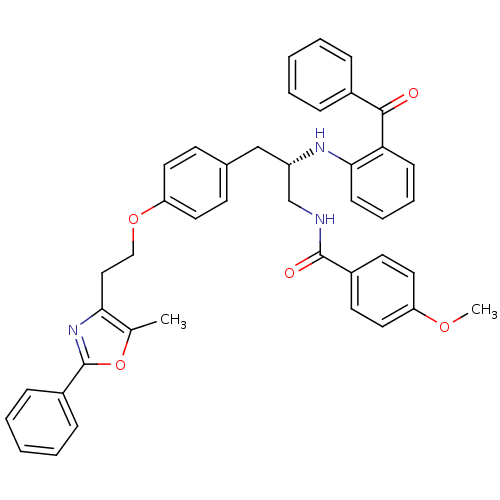
(CHEMBL230730)Show SMILES COc1ccc(cc1)C(=O)NC[C@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C42H39N3O5/c1-29-38(45-42(50-29)33-13-7-4-8-14-33)25-26-49-36-21-17-30(18-22-36)27-34(28-43-41(47)32-19-23-35(48-2)24-20-32)44-39-16-10-9-15-37(39)40(46)31-11-5-3-6-12-31/h3-24,34,44H,25-28H2,1-2H3,(H,43,47)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002864
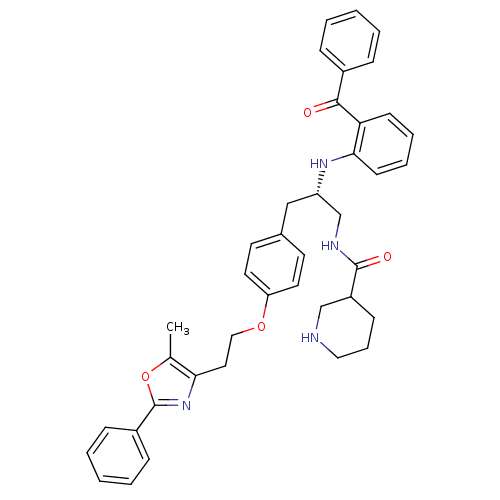
(CHEMBL398183)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)C2CCCNC2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C40H42N4O4/c1-28-36(44-40(48-28)31-13-6-3-7-14-31)22-24-47-34-20-18-29(19-21-34)25-33(27-42-39(46)32-15-10-23-41-26-32)43-37-17-9-8-16-35(37)38(45)30-11-4-2-5-12-30/h2-9,11-14,16-21,32-33,41,43H,10,15,22-27H2,1H3,(H,42,46)/t32?,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50423009
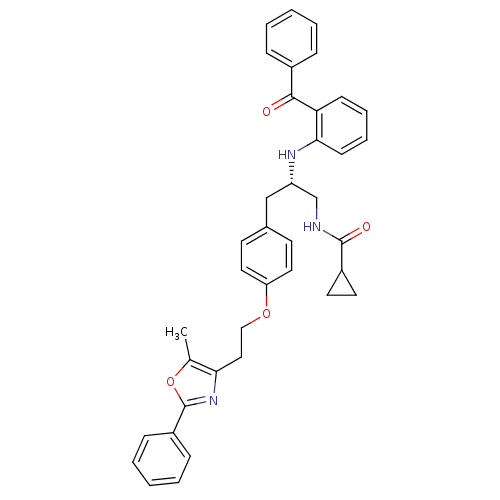
(CHEMBL230731)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)C2CC2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C38H37N3O4/c1-26-34(41-38(45-26)30-12-6-3-7-13-30)22-23-44-32-20-16-27(17-21-32)24-31(25-39-37(43)29-18-19-29)40-35-15-9-8-14-33(35)36(42)28-10-4-2-5-11-28/h2-17,20-21,29,31,40H,18-19,22-25H2,1H3,(H,39,43)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065848
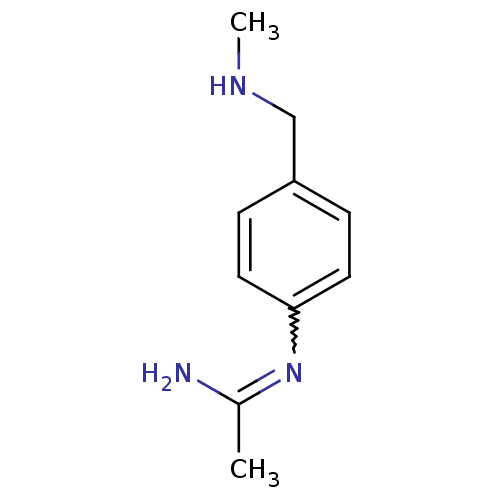
(CHEMBL539793 | N-(4-Methylaminomethyl-phenyl)-acet...)Show InChI InChI=1S/C10H15N3/c1-8(11)13-10-5-3-9(4-6-10)7-12-2/h3-6,12H,7H2,1-2H3,(H2,11,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002863
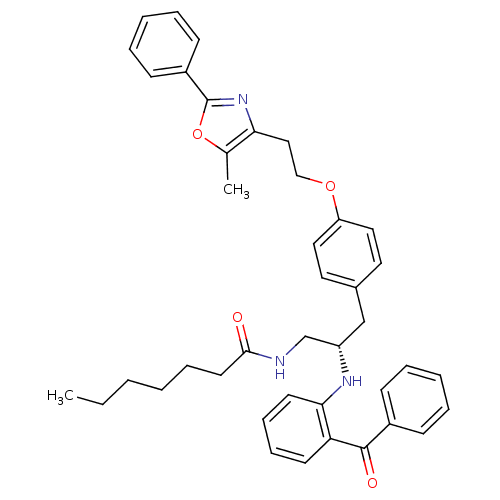
(CHEMBL230732)Show SMILES CCCCCCC(=O)NC[C@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C41H45N3O4/c1-3-4-5-12-21-39(45)42-29-34(43-38-20-14-13-19-36(38)40(46)32-15-8-6-9-16-32)28-31-22-24-35(25-23-31)47-27-26-37-30(2)48-41(44-37)33-17-10-7-11-18-33/h6-11,13-20,22-25,34,43H,3-5,12,21,26-29H2,1-2H3,(H,42,45)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50423013
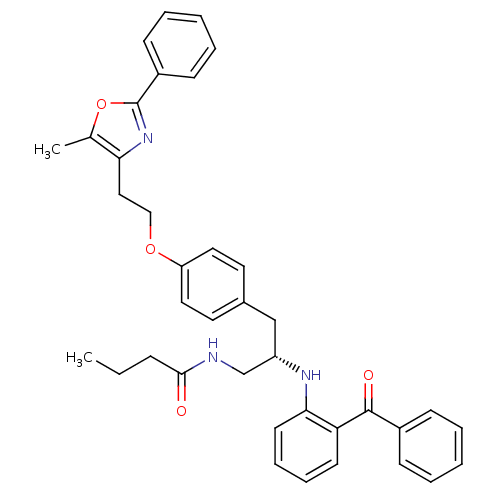
(CHEMBL395527)Show SMILES CCCC(=O)NC[C@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C38H39N3O4/c1-3-12-36(42)39-26-31(40-35-18-11-10-17-33(35)37(43)29-13-6-4-7-14-29)25-28-19-21-32(22-20-28)44-24-23-34-27(2)45-38(41-34)30-15-8-5-9-16-30/h4-11,13-22,31,40H,3,12,23-26H2,1-2H3,(H,39,42)/t31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50423011
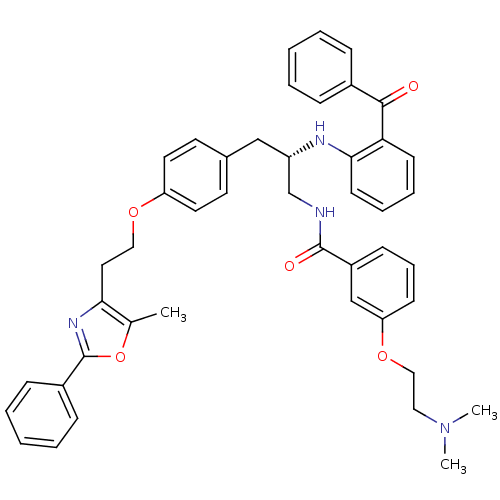
(CHEMBL267996)Show SMILES CN(C)CCOc1cccc(c1)C(=O)NC[C@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1 Show InChI InChI=1S/C45H46N4O5/c1-32-41(48-45(54-32)35-15-8-5-9-16-35)25-27-52-38-23-21-33(22-24-38)29-37(31-46-44(51)36-17-12-18-39(30-36)53-28-26-49(2)3)47-42-20-11-10-19-40(42)43(50)34-13-6-4-7-14-34/h4-24,30,37,47H,25-29,31H2,1-3H3,(H,46,51)/t37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002862
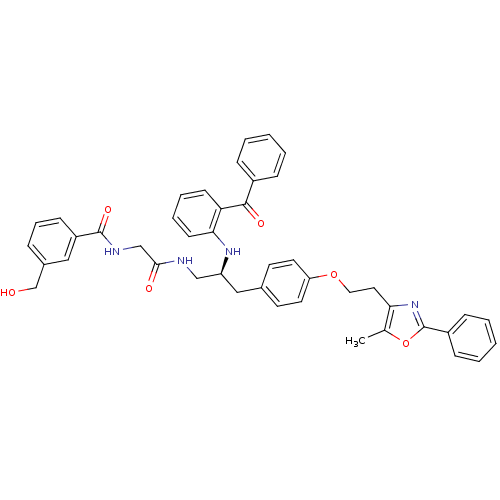
(CHEMBL396220)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)CNC(=O)c2cccc(CO)c2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C44H42N4O6/c1-30-39(48-44(54-30)34-14-6-3-7-15-34)23-24-53-37-21-19-31(20-22-37)26-36(27-45-41(50)28-46-43(52)35-16-10-11-32(25-35)29-49)47-40-18-9-8-17-38(40)42(51)33-12-4-2-5-13-33/h2-22,25,36,47,49H,23-24,26-29H2,1H3,(H,45,50)(H,46,52)/t36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002860
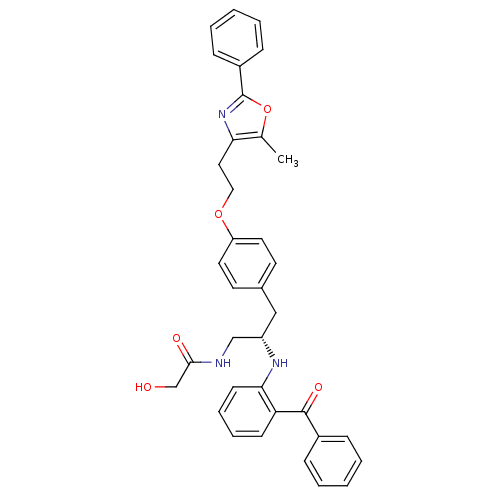
(CHEMBL231656)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)CO)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C36H35N3O5/c1-25-32(39-36(44-25)28-12-6-3-7-13-28)20-21-43-30-18-16-26(17-19-30)22-29(23-37-34(41)24-40)38-33-15-9-8-14-31(33)35(42)27-10-4-2-5-11-27/h2-19,29,38,40H,20-24H2,1H3,(H,37,41)/t29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002868

(CHEMBL230523)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)c2ccccc2C(O)=O)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C42H37N3O6/c1-28-37(45-41(51-28)31-14-6-3-7-15-31)24-25-50-33-22-20-29(21-23-33)26-32(27-43-40(47)34-16-8-9-17-35(34)42(48)49)44-38-19-11-10-18-36(38)39(46)30-12-4-2-5-13-30/h2-23,32,44H,24-27H2,1H3,(H,43,47)(H,48,49)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058459
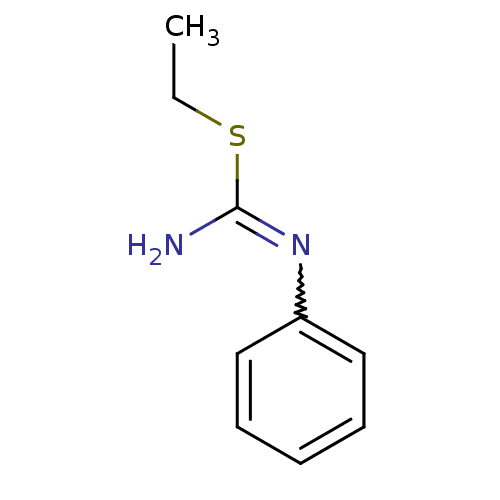
(2-Ethyl-1-phenyl-isothiourea; hydriodide | CHEMBL4...)Show InChI InChI=1S/C9H12N2S/c1-2-12-9(10)11-8-6-4-3-5-7-8/h3-7H,2H2,1H3,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065843

(CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...)Show InChI InChI=1S/C12H13N3S/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50423010
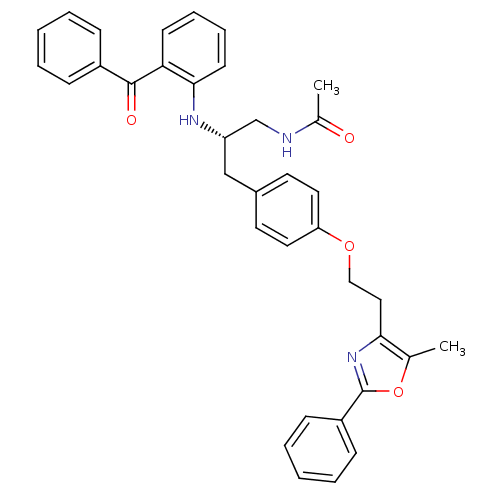
(CHEMBL230259)Show SMILES CC(=O)NC[C@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1 |r| Show InChI InChI=1S/C36H35N3O4/c1-25-33(39-36(43-25)29-13-7-4-8-14-29)21-22-42-31-19-17-27(18-20-31)23-30(24-37-26(2)40)38-34-16-10-9-15-32(34)35(41)28-11-5-3-6-12-28/h3-20,30,38H,21-24H2,1-2H3,(H,37,40)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065805
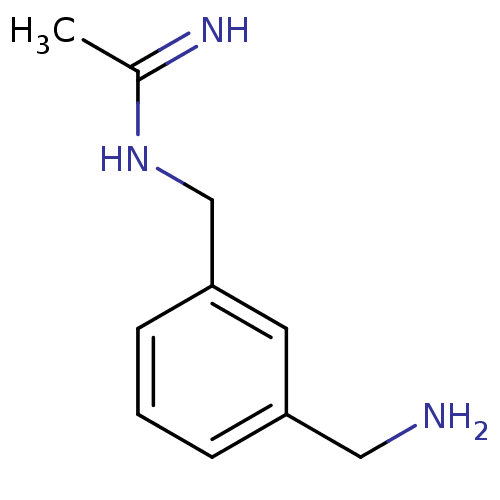
(CHEMBL544788 | N-(3-Aminomethyl-benzyl)-acetamidin...)Show InChI InChI=1S/C10H15N3/c1-8(12)13-7-10-4-2-3-9(5-10)6-11/h2-5H,6-7,11H2,1H3,(H2,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058469
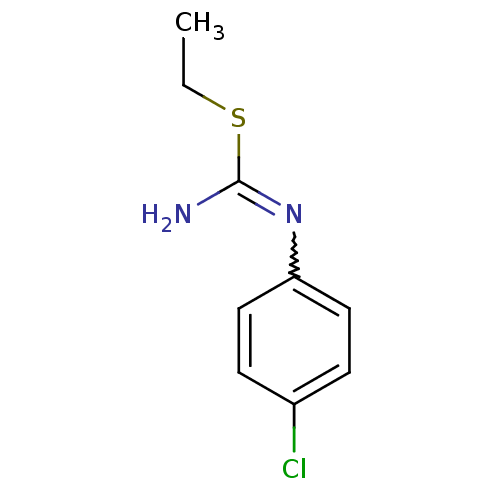
(1-(4-Chloro-phenyl)-2-ethyl-isothiourea; hydrochlo...)Show InChI InChI=1S/C9H11ClN2S/c1-2-13-9(11)12-8-5-3-7(10)4-6-8/h3-6H,2H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002858

(CHEMBL230524)Show SMILES CC(CC(C)C(=O)NC[C@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C41H43N3O6/c1-27(24-28(2)41(47)48)39(46)42-26-33(43-37-17-11-10-16-35(37)38(45)31-12-6-4-7-13-31)25-30-18-20-34(21-19-30)49-23-22-36-29(3)50-40(44-36)32-14-8-5-9-15-32/h4-21,27-28,33,43H,22-26H2,1-3H3,(H,42,46)(H,47,48)/t27?,28?,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002859
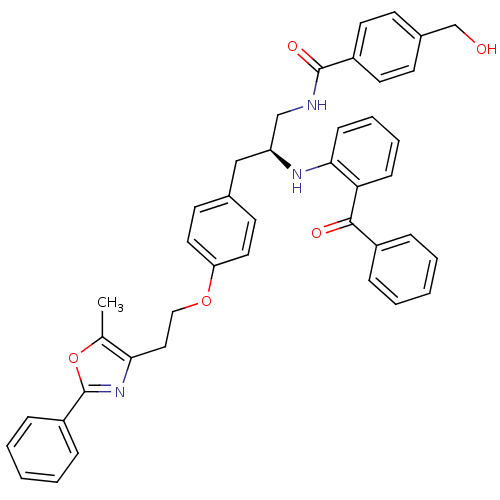
(CHEMBL396480)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)c2ccc(CO)cc2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C42H39N3O5/c1-29-38(45-42(50-29)34-12-6-3-7-13-34)24-25-49-36-22-18-30(19-23-36)26-35(27-43-41(48)33-20-16-31(28-46)17-21-33)44-39-15-9-8-14-37(39)40(47)32-10-4-2-5-11-32/h2-23,35,44,46H,24-28H2,1H3,(H,43,48)/t35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50065807

(CHEMBL552825 | N-(3-Aminomethyl-phenyl)-furan-2-ca...)Show InChI InChI=1S/C12H13N3O/c13-8-9-3-1-4-10(7-9)15-12(14)11-5-2-6-16-11/h1-7H,8,13H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human inducible nitric oxide synthase (iNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002866
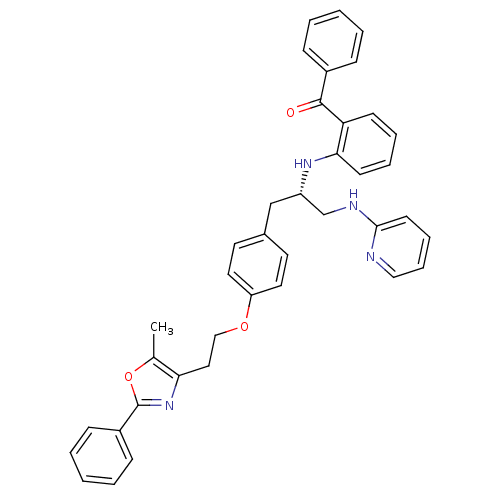
(CHEMBL266741)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNc2ccccn2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C39H36N4O3/c1-28-35(43-39(46-28)31-14-6-3-7-15-31)23-25-45-33-21-19-29(20-22-33)26-32(27-41-37-18-10-11-24-40-37)42-36-17-9-8-16-34(36)38(44)30-12-4-2-5-13-30/h2-22,24,32,42H,23,25-27H2,1H3,(H,40,41)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065818
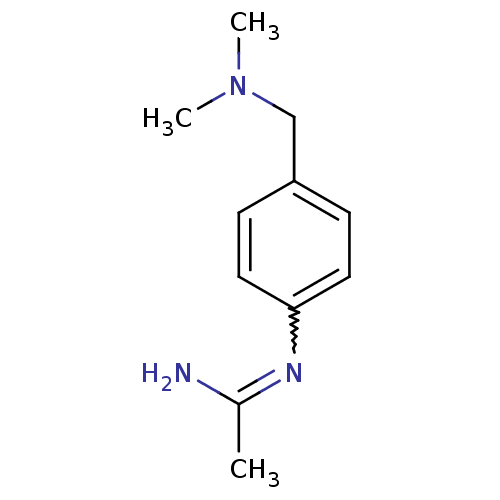
(CHEMBL554201 | N-(4-Dimethylaminomethyl-phenyl)-ac...)Show InChI InChI=1S/C11H17N3/c1-9(12)13-11-6-4-10(5-7-11)8-14(2)3/h4-7H,8H2,1-3H3,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058441
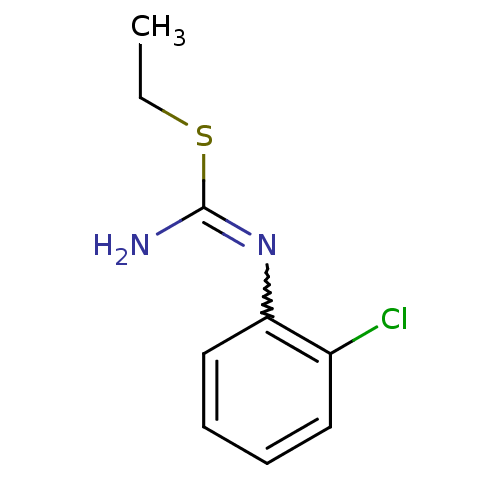
(1-(2-Chloro-phenyl)-2-ethyl-isothiourea; hydrochlo...)Show InChI InChI=1S/C9H11ClN2S/c1-2-13-9(11)12-8-6-4-3-5-7(8)10/h3-6H,2H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058448
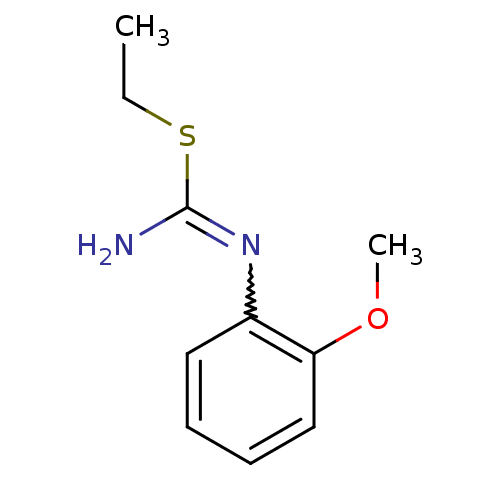
(2-Ethyl-1-(2-methoxy-phenyl)-isothiourea; hydrochl...)Show InChI InChI=1S/C10H14N2OS/c1-3-14-10(11)12-8-6-4-5-7-9(8)13-2/h4-7H,3H2,1-2H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002867
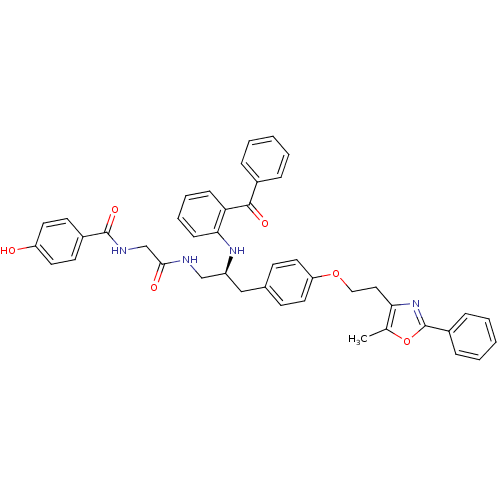
(CHEMBL396481)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)CNC(=O)c2ccc(O)cc2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C43H40N4O6/c1-29-38(47-43(53-29)33-12-6-3-7-13-33)24-25-52-36-22-16-30(17-23-36)26-34(27-44-40(49)28-45-42(51)32-18-20-35(48)21-19-32)46-39-15-9-8-14-37(39)41(50)31-10-4-2-5-11-31/h2-23,34,46,48H,24-28H2,1H3,(H,44,49)(H,45,51)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058443
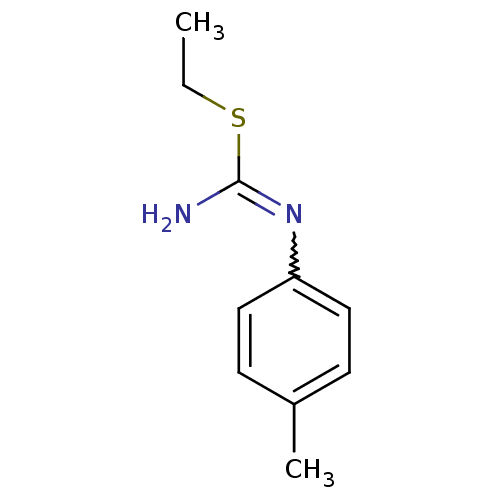
(2-Ethyl-1-p-tolyl-isothiourea; hydrochloride | CHE...)Show InChI InChI=1S/C10H14N2S/c1-3-13-10(11)12-9-6-4-8(2)5-7-9/h4-7H,3H2,1-2H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065817
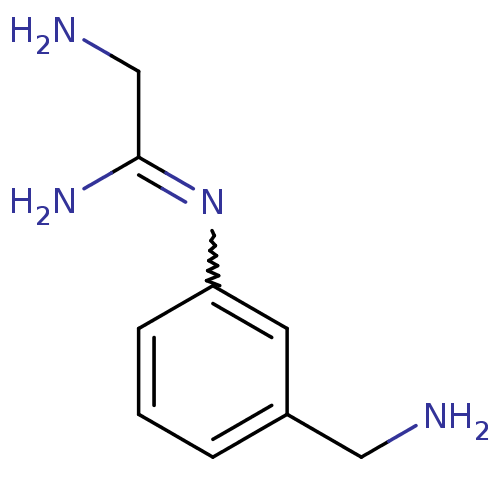
(2-Amino-N-(3-aminomethyl-phenyl)-acetamidine | CHE...)Show InChI InChI=1S/C9H14N4/c10-5-7-2-1-3-8(4-7)13-9(12)6-11/h1-4H,5-6,10-11H2,(H2,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058445
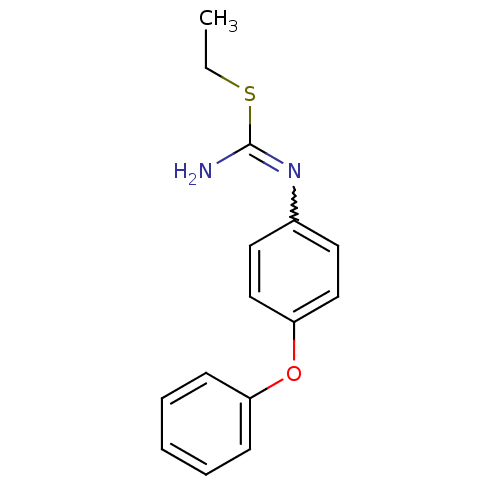
(2-Ethyl-1-(4-phenoxy-phenyl)-isothiourea; hydriodi...)Show InChI InChI=1S/C15H16N2OS/c1-2-19-15(16)17-12-8-10-14(11-9-12)18-13-6-4-3-5-7-13/h3-11H,2H2,1H3,(H2,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50423012
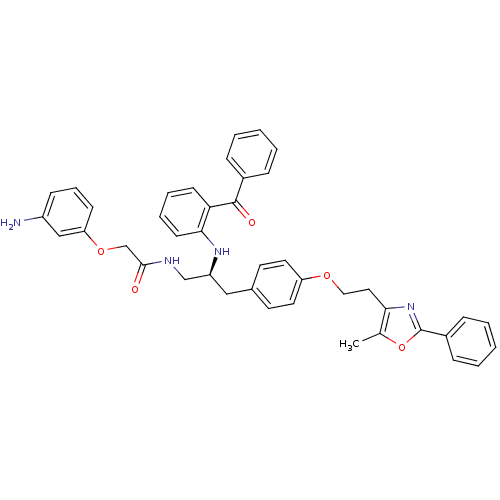
(CHEMBL266742)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)COc2cccc(N)c2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C42H40N4O5/c1-29-38(46-42(51-29)32-13-6-3-7-14-32)23-24-49-35-21-19-30(20-22-35)25-34(27-44-40(47)28-50-36-16-10-15-33(43)26-36)45-39-18-9-8-17-37(39)41(48)31-11-4-2-5-12-31/h2-22,26,34,45H,23-25,27-28,43H2,1H3,(H,44,47)/t34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002869
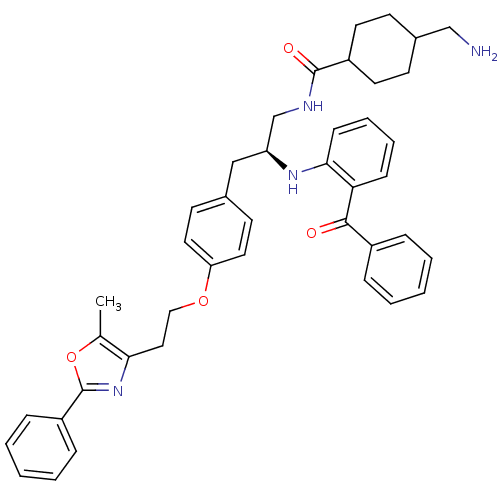
(CHEMBL230522)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)C2CCC(CN)CC2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 |wU:14.28,(-1.42,-12.31,;-2.65,-13.24,;-4.13,-12.79,;-5.02,-14.06,;-4.08,-15.28,;-2.62,-14.78,;-1.29,-15.55,;.04,-14.78,;1.37,-15.55,;2.71,-14.78,;4.04,-15.56,;5.37,-14.79,;5.38,-13.25,;6.71,-12.48,;8.04,-13.25,;9.38,-12.48,;10.71,-13.25,;12.05,-12.48,;12.05,-10.94,;13.38,-13.25,;13.37,-14.78,;14.7,-15.56,;16.04,-14.79,;17.37,-15.57,;17.36,-17.11,;16.05,-13.25,;14.71,-12.48,;8.05,-14.79,;9.38,-15.56,;10.71,-14.78,;12.04,-15.55,;12.04,-17.1,;10.71,-17.87,;9.38,-17.1,;8.05,-17.87,;6.71,-17.1,;8.04,-19.41,;6.71,-20.17,;6.7,-21.71,;8.04,-22.48,;9.38,-21.7,;9.37,-20.17,;4.04,-12.47,;2.71,-13.25,;-6.56,-14.08,;-7.34,-12.77,;-8.88,-12.79,;-9.64,-14.13,;-8.85,-15.46,;-7.3,-15.43,)| Show InChI InChI=1S/C42H46N4O4/c1-29-38(46-42(50-29)34-12-6-3-7-13-34)24-25-49-36-22-18-30(19-23-36)26-35(28-44-41(48)33-20-16-31(27-43)17-21-33)45-39-15-9-8-14-37(39)40(47)32-10-4-2-5-11-32/h2-15,18-19,22-23,31,33,35,45H,16-17,20-21,24-28,43H2,1H3,(H,44,48)/t31?,33?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058468
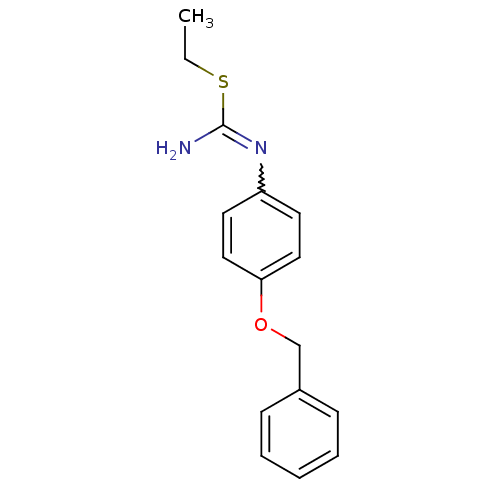
(1-(4-Benzyloxy-phenyl)-2-ethyl-isothiourea; hydrio...)Show InChI InChI=1S/C16H18N2OS/c1-2-20-16(17)18-14-8-10-15(11-9-14)19-12-13-6-4-3-5-7-13/h3-11H,2,12H2,1H3,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50072297
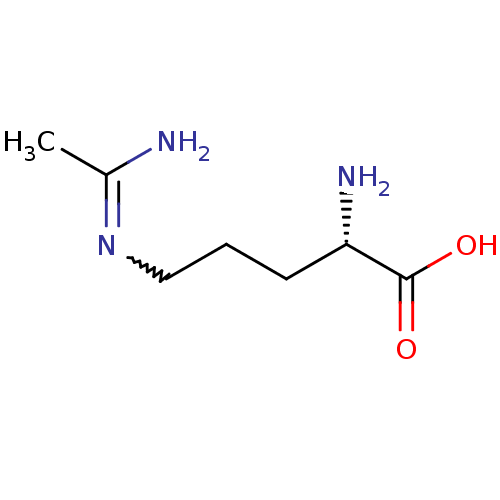
((S)-5-Acetimidoylamino-2-amino-pent | (S)-5-Acetim...)Show InChI InChI=1S/C7H15N3O2/c1-5(8)10-4-2-3-6(9)7(11)12/h6H,2-4,9H2,1H3,(H2,8,10)(H,11,12)/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant for the inhibition of human Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 7: 1763-1768 (1997)
Article DOI: 10.1016/S0960-894X(97)00309-0
BindingDB Entry DOI: 10.7270/Q2TQ61J5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50289680
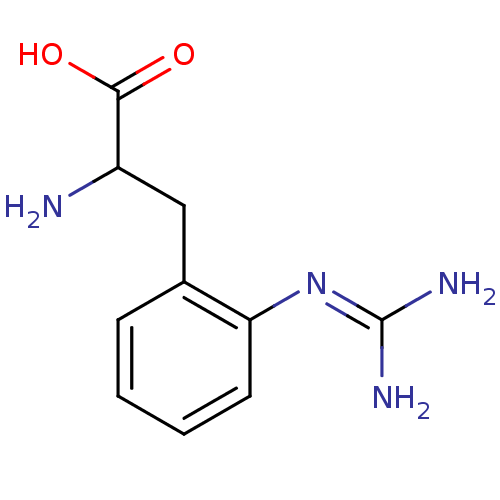
(2-Amino-3-(2-guanidino-phenyl)-propionic acid | CH...)Show SMILES [#7]-[#6](-[#6]-c1ccccc1\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C10H14N4O2/c11-7(9(15)16)5-6-3-1-2-4-8(6)14-10(12)13/h1-4,7H,5,11H2,(H,15,16)(H4,12,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant for the inhibition of human Endothelial nitric oxide synthase |
Bioorg Med Chem Lett 7: 1763-1768 (1997)
Article DOI: 10.1016/S0960-894X(97)00309-0
BindingDB Entry DOI: 10.7270/Q2TQ61J5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058440
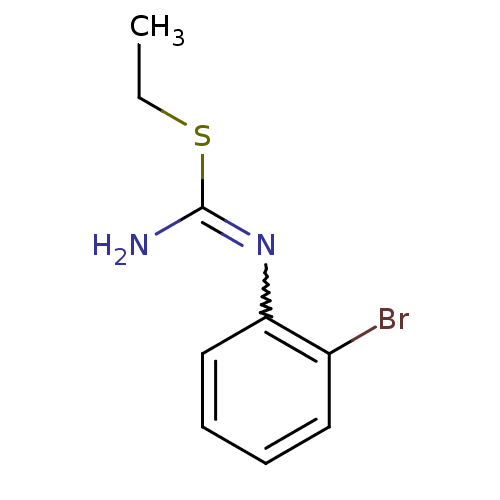
(1-(2-Bromo-phenyl)-2-ethyl-isothiourea; hydriodide...)Show InChI InChI=1S/C9H11BrN2S/c1-2-13-9(11)12-8-6-4-3-5-7(8)10/h3-6H,2H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50423008
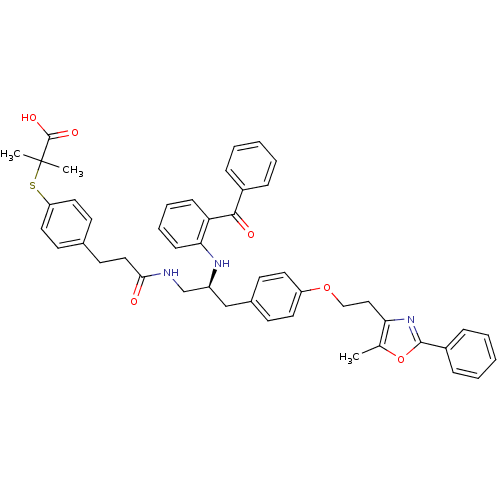
(CHEMBL230804)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)CCc2ccc(SC(C)(C)C(O)=O)cc2)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C47H47N3O6S/c1-32-41(50-45(56-32)36-14-8-5-9-15-36)28-29-55-38-23-18-34(19-24-38)30-37(49-42-17-11-10-16-40(42)44(52)35-12-6-4-7-13-35)31-48-43(51)27-22-33-20-25-39(26-21-33)57-47(2,3)46(53)54/h4-21,23-26,37,49H,22,27-31H2,1-3H3,(H,48,51)(H,53,54)/t37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50002870
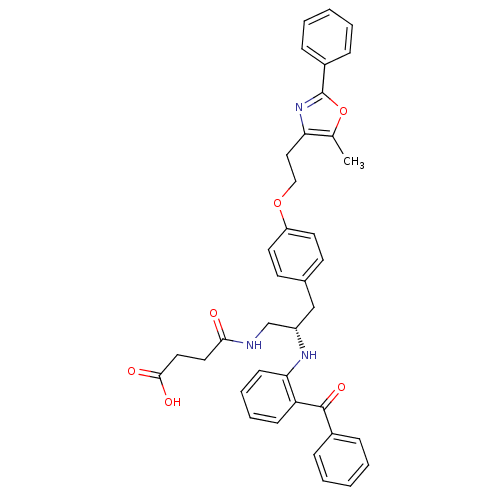
(CHEMBL266985)Show SMILES Cc1oc(nc1CCOc1ccc(C[C@@H](CNC(=O)CCC(O)=O)Nc2ccccc2C(=O)c2ccccc2)cc1)-c1ccccc1 Show InChI InChI=1S/C38H37N3O6/c1-26-33(41-38(47-26)29-12-6-3-7-13-29)22-23-46-31-18-16-27(17-19-31)24-30(25-39-35(42)20-21-36(43)44)40-34-15-9-8-14-32(34)37(45)28-10-4-2-5-11-28/h2-19,30,40H,20-25H2,1H3,(H,39,42)(H,43,44)/t30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 269 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]BRL49653 from PPARgamma by scintillation proximity assay |
Bioorg Med Chem Lett 17: 3916-20 (2007)
Checked by Author
Article DOI: 10.1016/j.bmcl.2007.04.111
BindingDB Entry DOI: 10.7270/Q2R49S33 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50289681
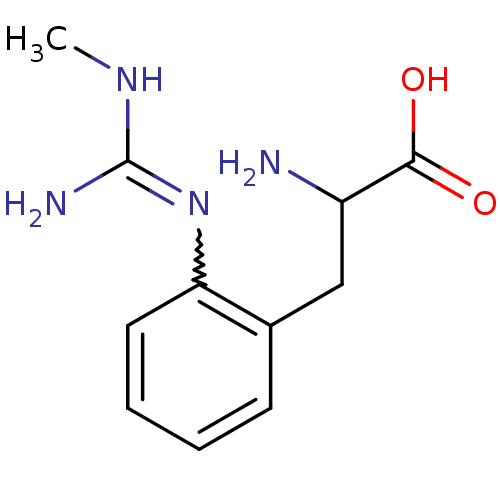
(2-Amino-3-[2-(N'-methyl-guanidino)-phenyl]-propion...)Show InChI InChI=1S/C11H16N4O2/c1-14-11(13)15-9-5-3-2-4-7(9)6-8(12)10(16)17/h2-5,8H,6,12H2,1H3,(H,16,17)(H3,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant for the inhibition of human Endothelial nitric oxide synthase |
Bioorg Med Chem Lett 7: 1763-1768 (1997)
Article DOI: 10.1016/S0960-894X(97)00309-0
BindingDB Entry DOI: 10.7270/Q2TQ61J5 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50058447
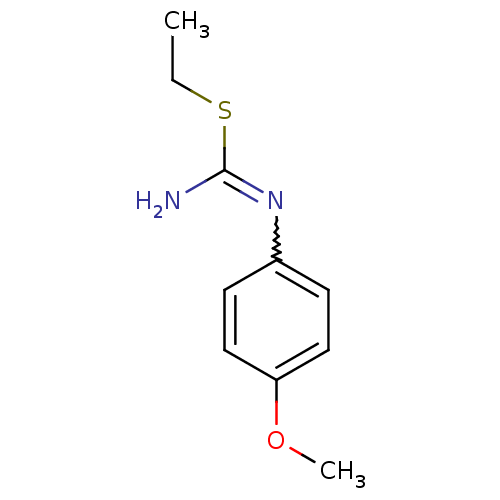
(2-Ethyl-1-(4-methoxy-phenyl)-isothiourea; hydriodi...)Show InChI InChI=1S/C10H14N2OS/c1-3-14-10(11)12-8-4-6-9(13-2)7-5-8/h4-7H,3H2,1-2H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase in brain (nNOS). |
J Med Chem 40: 1901-5 (1997)
Article DOI: 10.1021/jm960785c
BindingDB Entry DOI: 10.7270/Q2SJ1M94 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50065847
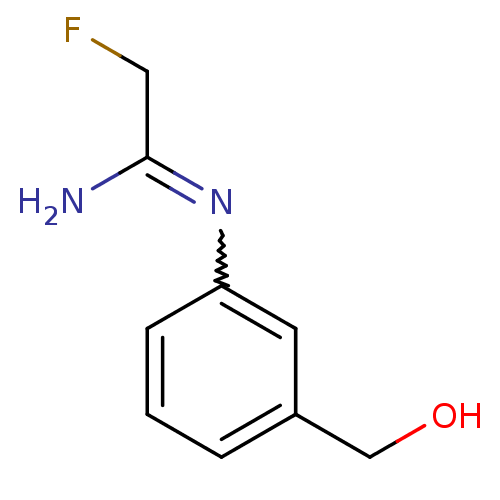
(2-Fluoro-N-(3-hydroxymethyl-phenyl)-acetamidine | ...)Show InChI InChI=1S/C9H11FN2O/c10-5-9(11)12-8-3-1-2-7(4-8)6-13/h1-4,13H,5-6H2,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme. |
J Med Chem 41: 2858-71 (1998)
Article DOI: 10.1021/jm980072p
BindingDB Entry DOI: 10.7270/Q2862H4X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data