

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (GUINEA PIG) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 19.5+/-12.0 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50366924 (CHEMBL609539) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 117+/-46 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452006 (CHEMBL610371) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 158+/-21 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451992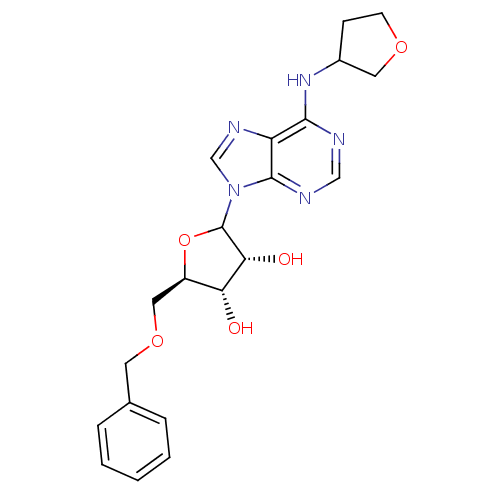 (CHEMBL609537) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451996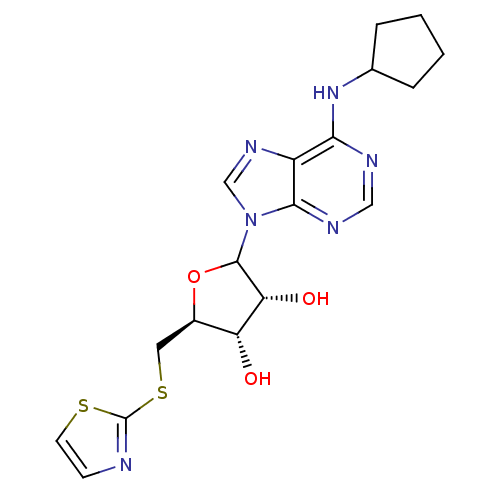 (CHEMBL608020) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 166+/-75 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451986 (CHEMBL608343) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451998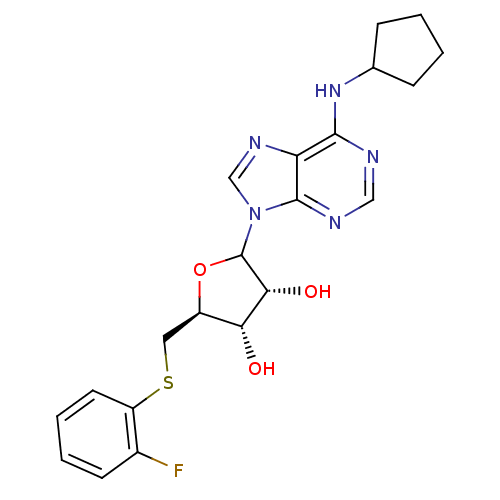 (CHEMBL611856) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 287+/-125 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451989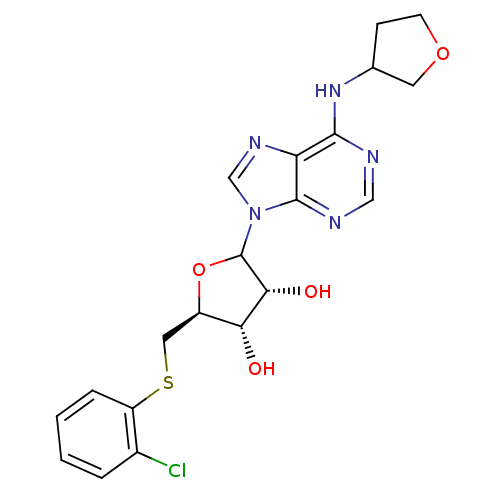 (CHEMBL608642) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 338 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 338+/-98 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451983 (CHEMBL610953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 343 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 343+/-137 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452003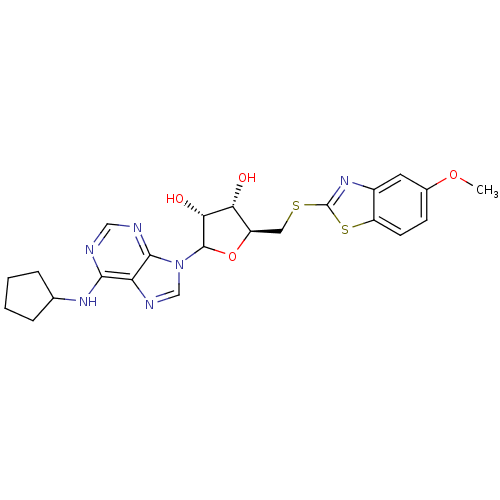 (CHEMBL609532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 354+/-113 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451994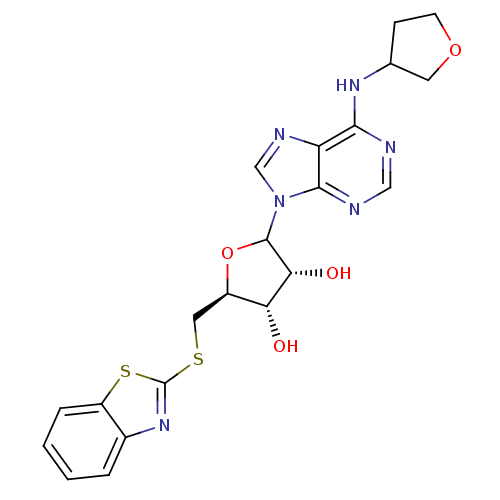 (CHEMBL608940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 400+/-38 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452005 (CHEMBL612173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 473 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 473+/-329 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451982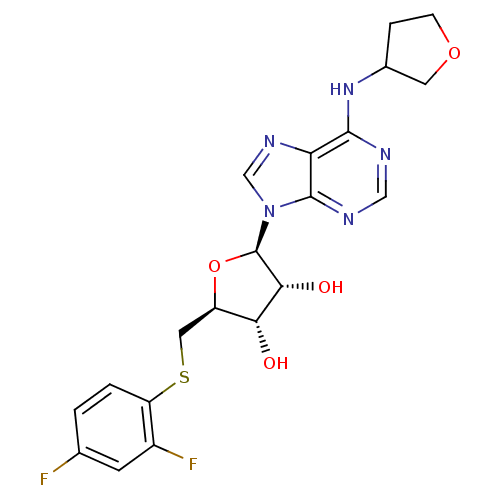 (CHEMBL2092779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Agonistic activity against adenosine A1 receptor in guinea pig isolated hearts | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451985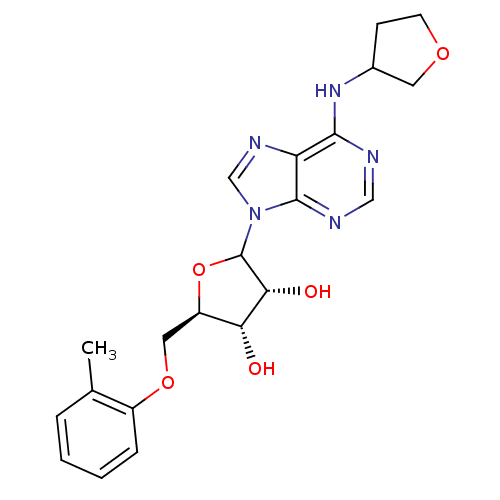 (CHEMBL612179) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 503 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 503+/-262 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50267574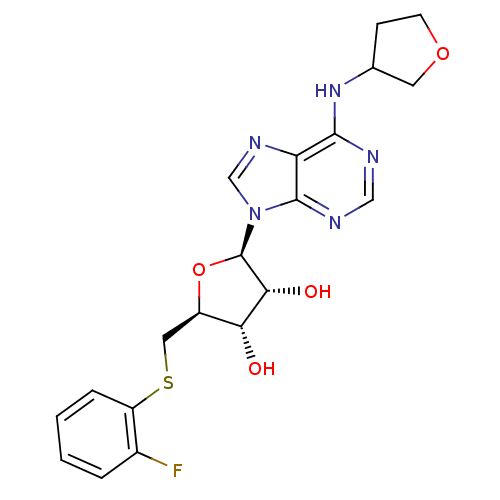 (CHEMBL489638 | N6-Tetrahydrofuranyl-9H-[5-deoxy-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 506 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 506+/-111 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50138530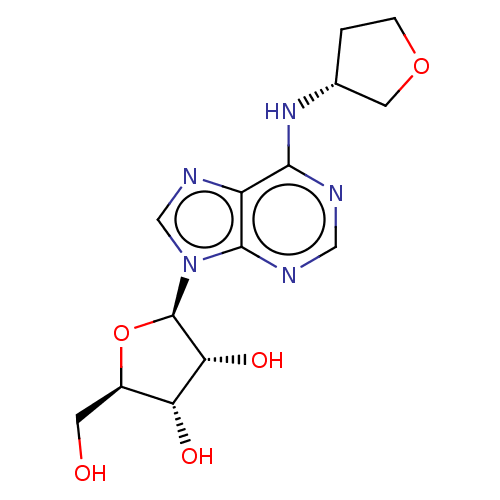 ((2R,3R,5R)-2-Hydroxymethyl-5-{6-[(R)-(tetrahydro-f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Agonistic activity against adenosine A1 receptor in guinea pig isolated hearts | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451981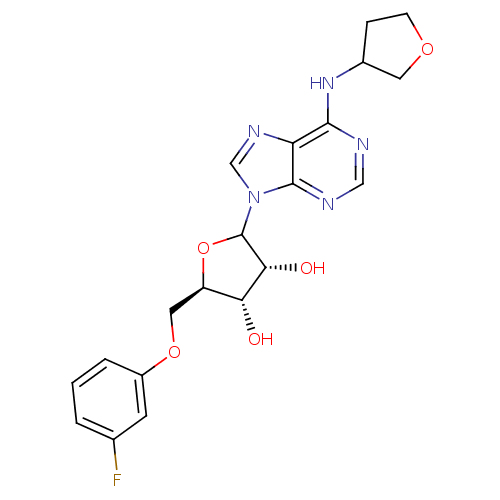 (CHEMBL608611) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 604 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 604+/-241 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451990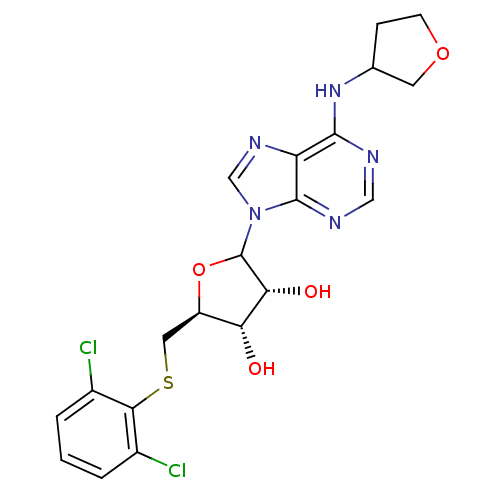 (CHEMBL609538) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 614+/-78 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452001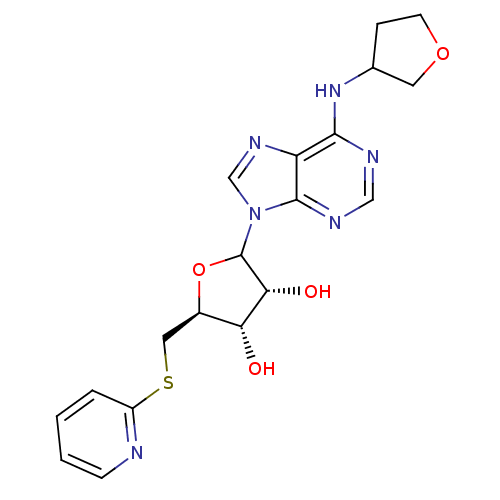 (CHEMBL608941) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 894 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 894+/-659 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451999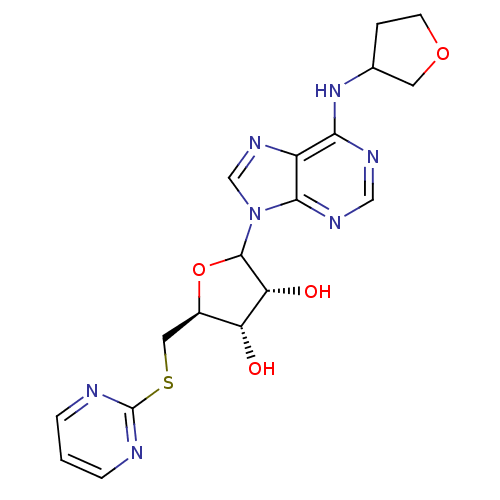 (CHEMBL608018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 913 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 913+/-517 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452002 (CHEMBL609235) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 1143+/-426 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452004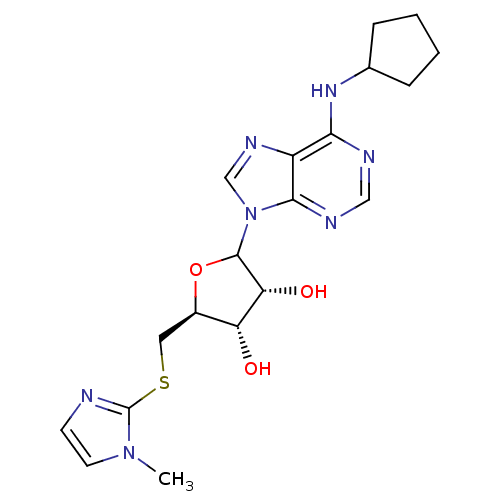 (CHEMBL609233) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451991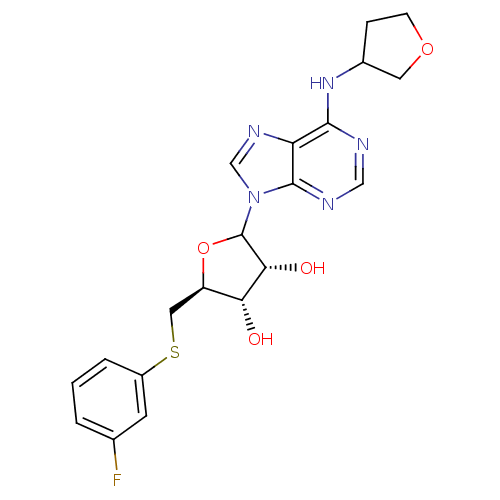 (CHEMBL608938) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 1571+/-245 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451984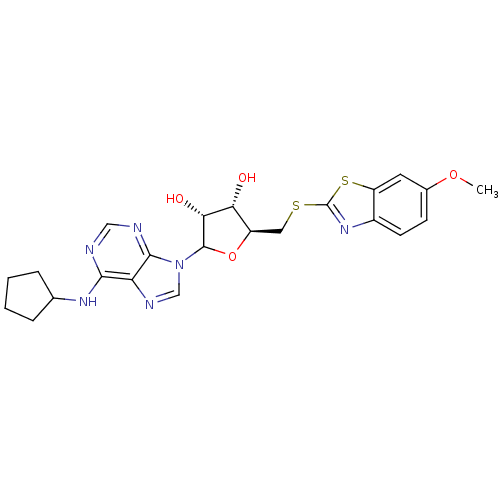 (CHEMBL609820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 1666+/-556 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451987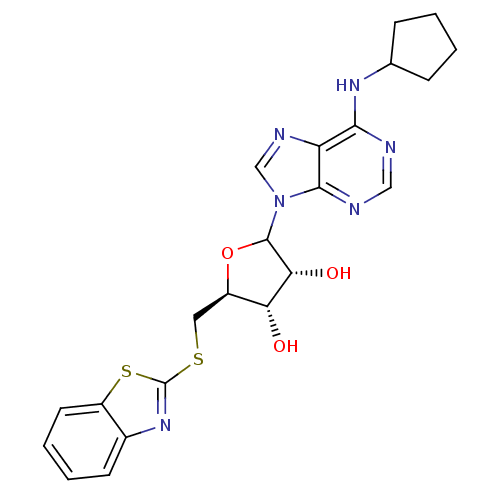 (CHEMBL607733) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 1773+/-209 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451980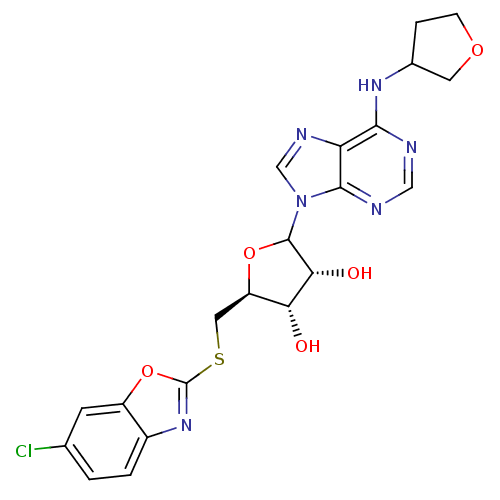 (CHEMBL609817) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 1945+/-1316 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50138533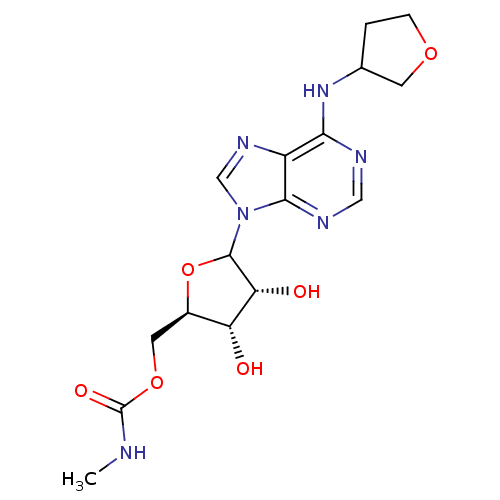 (CHEMBL150950 | Methyl-carbamic acid (2R,3R,5R)-3,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Agonistic activity against adenosine A1 receptor in guinea pig isolated hearts | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451997 (CHEMBL608340) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]-CPX in guinea pig DDT membrane; 4191+/-922 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451993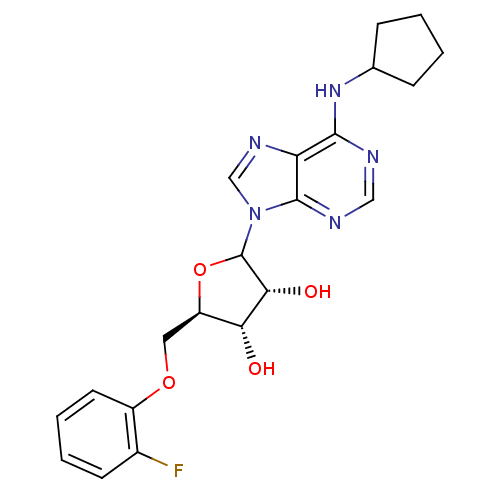 (CHEMBL609536) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451988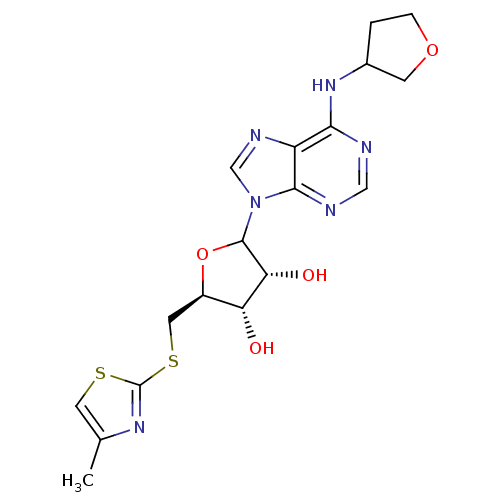 (CHEMBL607734) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451982 (CHEMBL2092779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 5497+/-180 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451995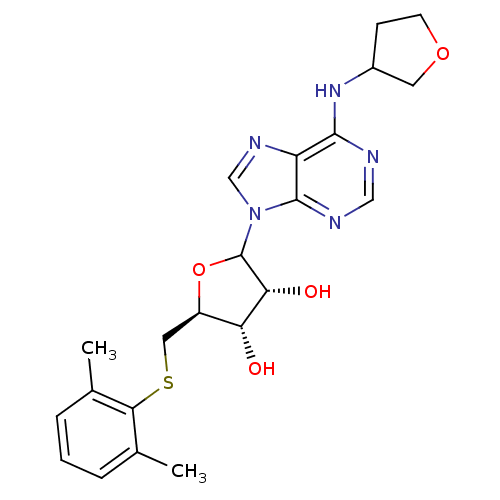 (CHEMBL608341) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 5855+/-1208 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50452000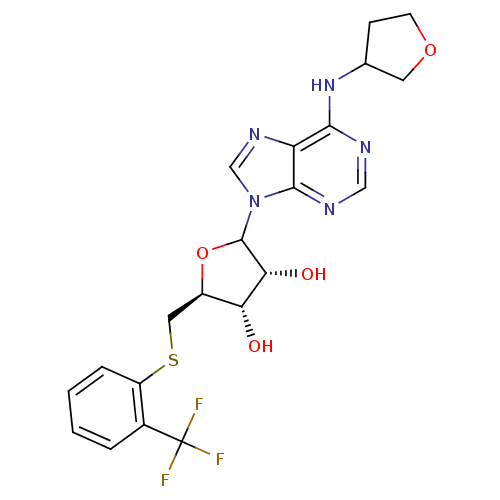 (CHEMBL609793) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 6250+/-1767 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50451979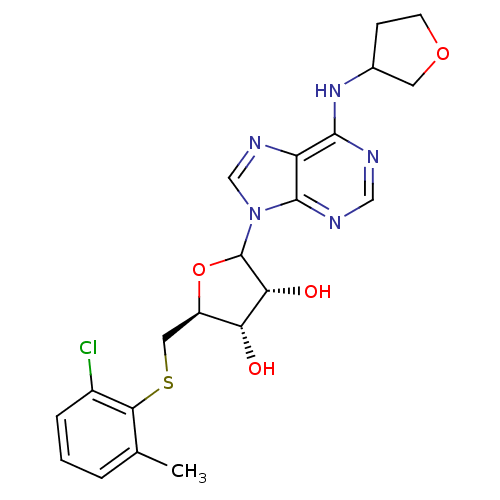 (CHEMBL610411) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor using [3H]CPX in guinea pig DDT membrane; 7292+/-3241 | Bioorg Med Chem Lett 14: 3793-7 (2004) Article DOI: 10.1016/j.bmcl.2004.04.096 BindingDB Entry DOI: 10.7270/Q20Z73TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277781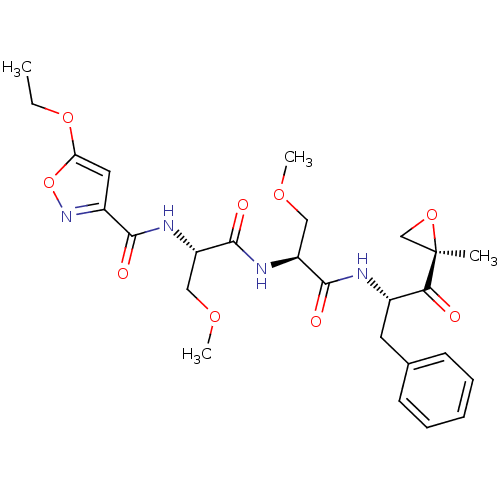 ((2S)-2-[(2S)-2-[(5-ethoxy-1,2-oxazol-3-yl)formamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277889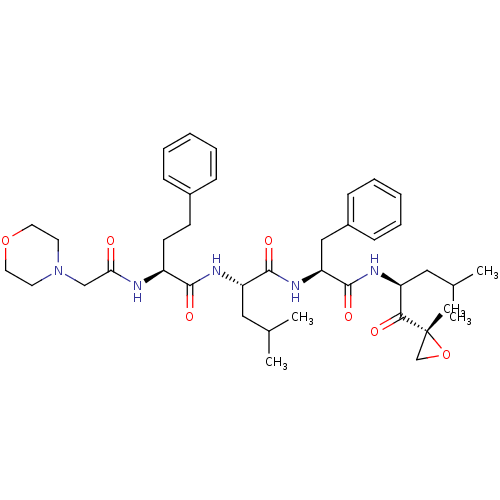 (CARFILZOMIB | CHEMBL451887) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277779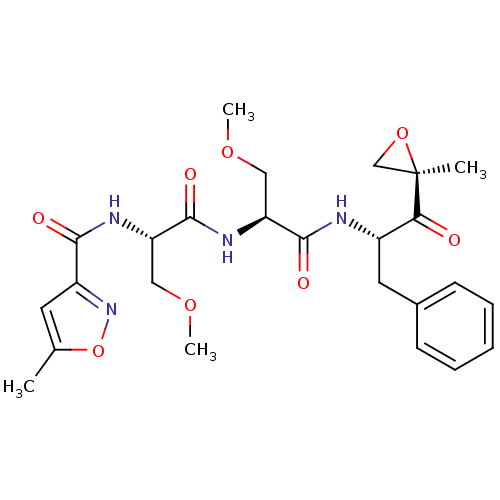 (CHEMBL484003 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277815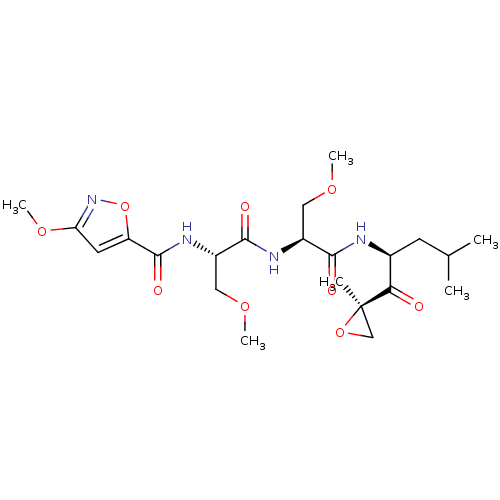 (3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277816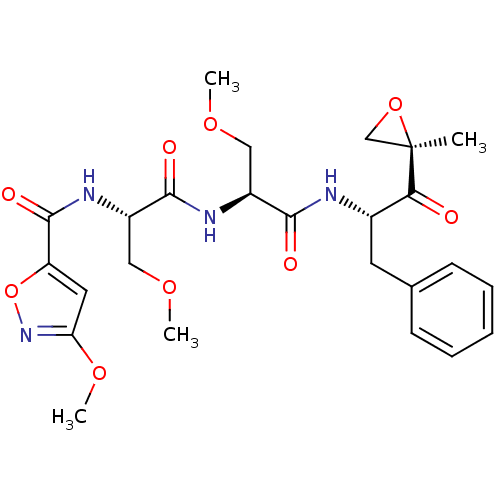 (3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277780 (5-ethoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138607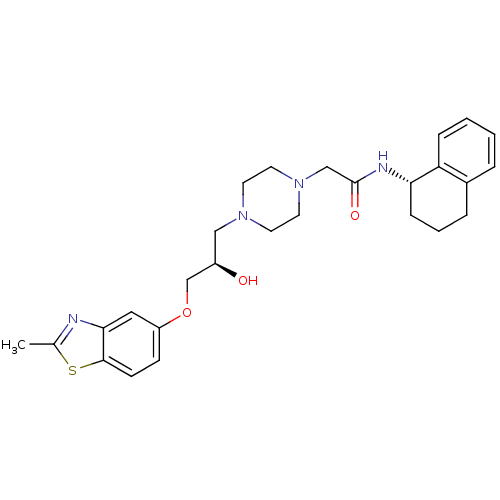 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277818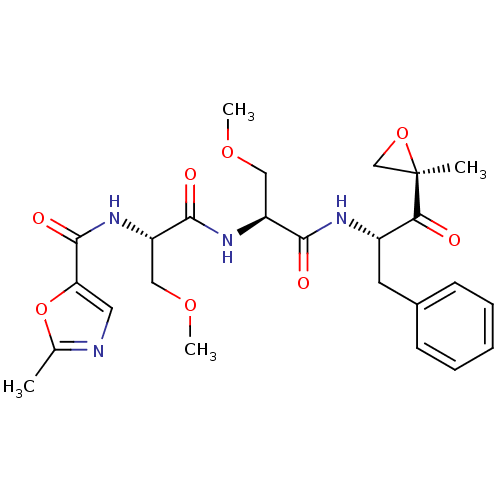 (2-Me-5-thiazole-Ser(OMe)-Ser(OMe)-Phe-ketoepoxide ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-8 (Homo sapiens (Human)) | BDBM50277778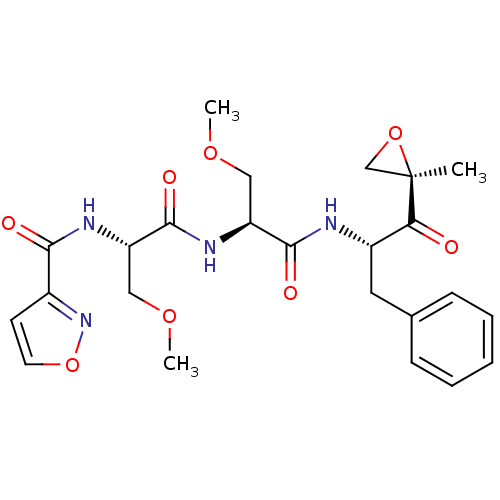 (CHEMBL484002 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc. Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA | J Med Chem 52: 3028-38 (2009) Article DOI: 10.1021/jm801329v BindingDB Entry DOI: 10.7270/Q2ZW1KS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138628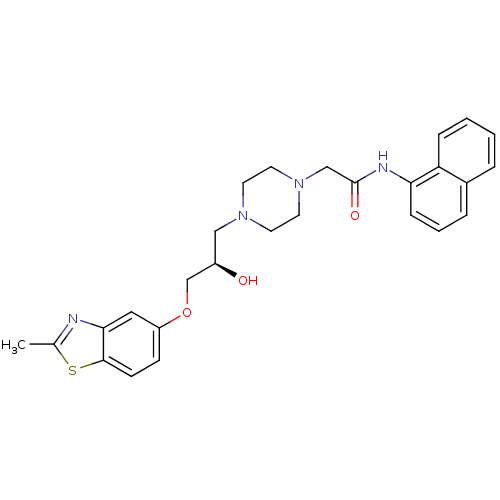 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50156430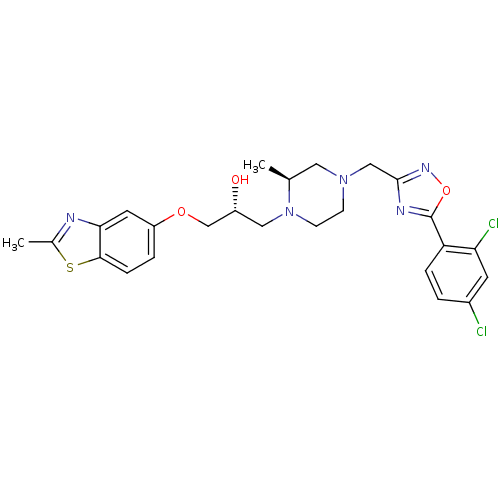 ((R)-1-{(S)-4-[5-(2,4-Dichloro-phenyl)-[1,2,4]oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria | Bioorg Med Chem Lett 14: 6017-21 (2004) Article DOI: 10.1016/j.bmcl.2004.09.077 BindingDB Entry DOI: 10.7270/Q2ZS2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50156430 ((R)-1-{(S)-4-[5-(2,4-Dichloro-phenyl)-[1,2,4]oxadi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria | Bioorg Med Chem Lett 14: 6017-21 (2004) Article DOI: 10.1016/j.bmcl.2004.09.077 BindingDB Entry DOI: 10.7270/Q2ZS2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50156421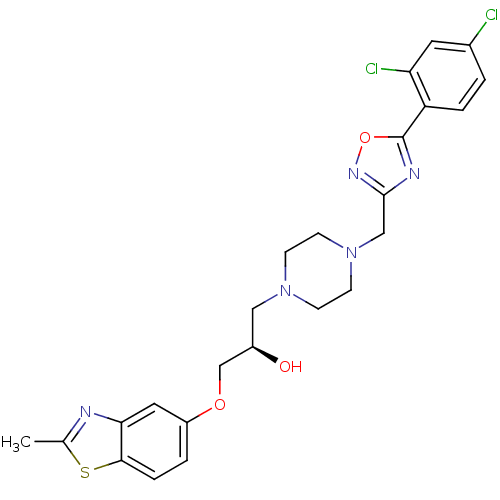 ((R)-1-{4-[5-(2,4-Dichloro-phenyl)-[1,2,4]oxadiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of palmitoyl-CoA oxidation in rat heart mitochondria | Bioorg Med Chem Lett 14: 6017-21 (2004) Article DOI: 10.1016/j.bmcl.2004.09.077 BindingDB Entry DOI: 10.7270/Q2ZS2W0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138619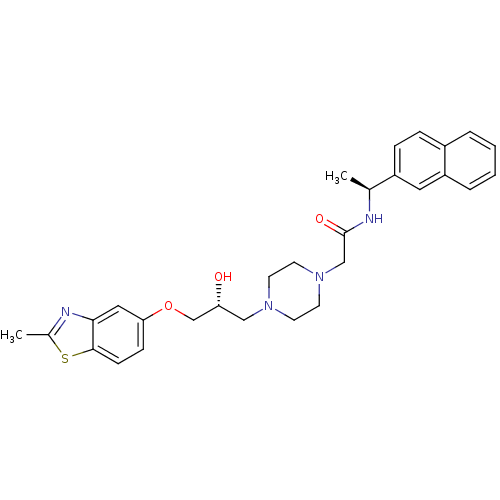 (2-{4-[(R)-2-Hydroxy-3-(2-methyl-benzothiazol-5-ylo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal acyl-coenzyme A oxidase 1 (Rattus norvegicus) | BDBM50138602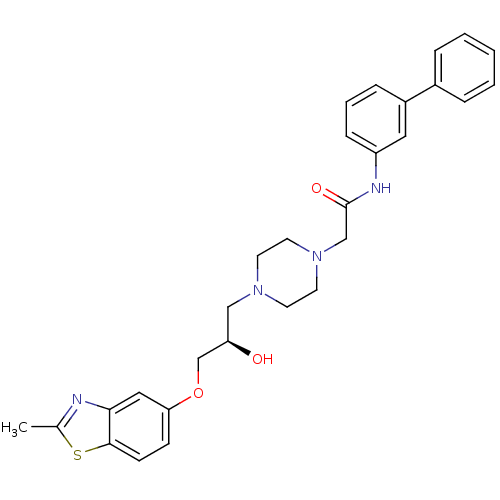 (CHEMBL152968 | N-Biphenyl-3-yl-2-{4-[(R)-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibitory activity against Palmitoyl-CoA oxidase to inhibit rat heart mitochondrial Palmitoyl-CoA oxidation. | Bioorg Med Chem Lett 14: 549-52 (2003) BindingDB Entry DOI: 10.7270/Q2GH9HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 128 total ) | Next | Last >> |