

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775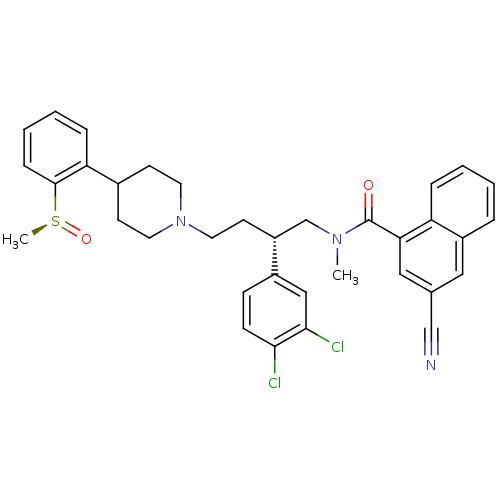 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50105595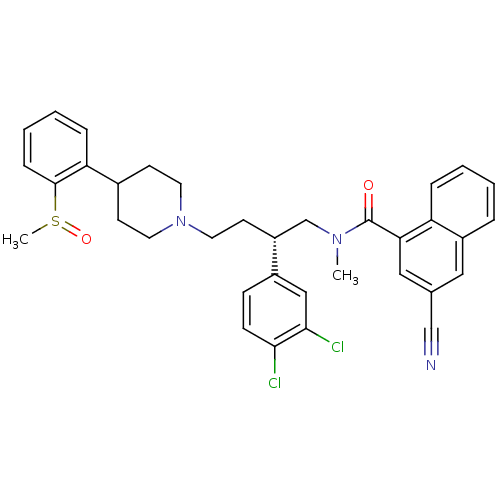 ((R,S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 307-15 (2001) BindingDB Entry DOI: 10.7270/Q2H130JX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118098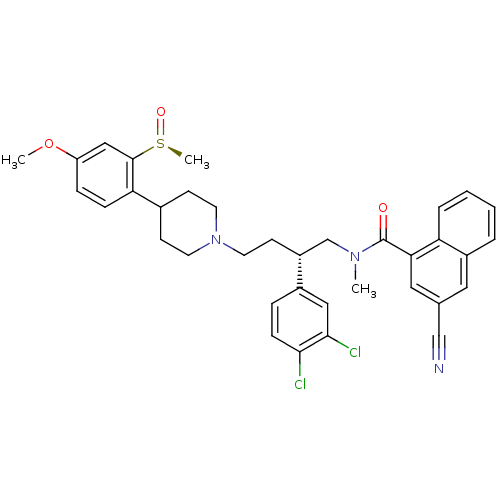 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118100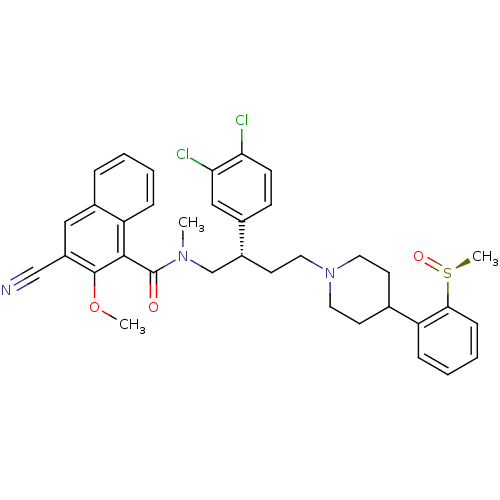 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118099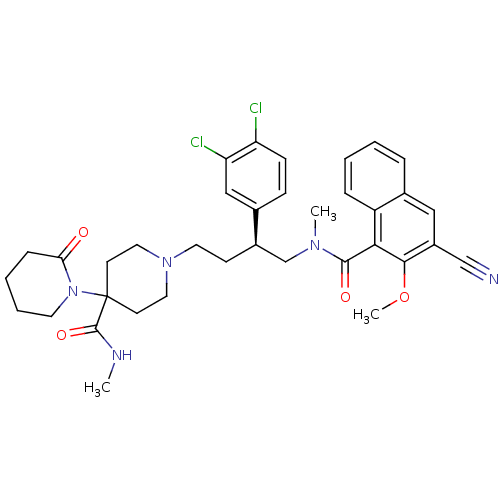 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50175494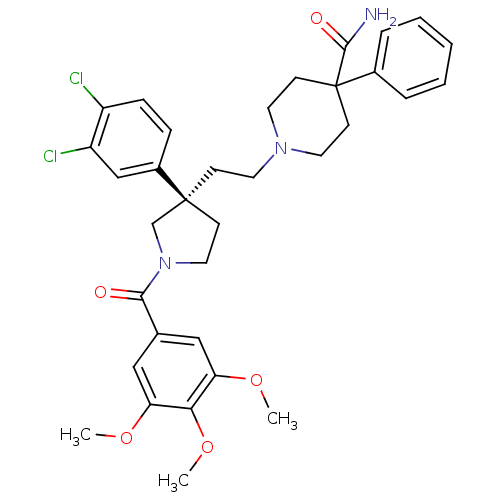 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50040237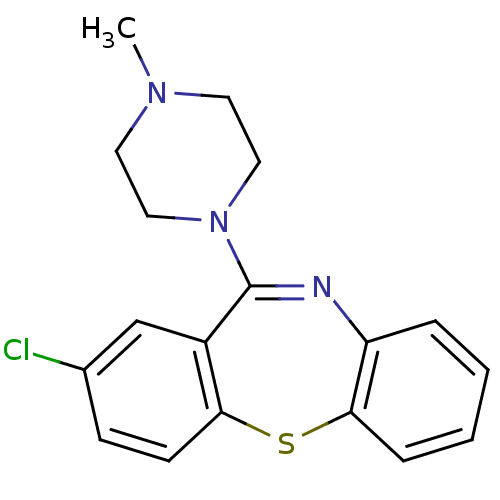 (2-Chloro-11-(4-methyl-piperazin-1-yl)-dibenzo[b,f]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 2 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50105595 ((R,S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 307-15 (2001) BindingDB Entry DOI: 10.7270/Q2H130JX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141314 (US8653257, 2-Chloro-11-piperazin-1-yl-dibenzo[b,f]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50403654 (CHEMBL2115073) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50409575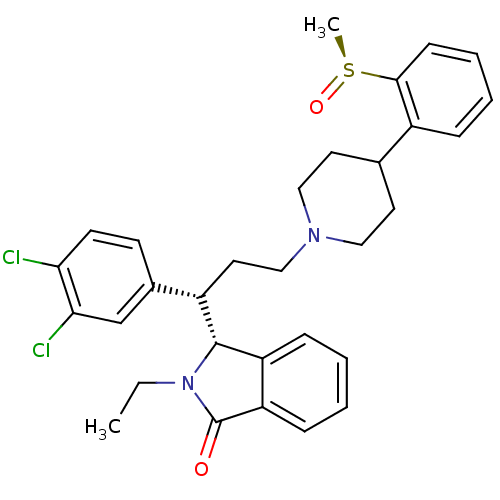 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 2 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141315 (US8653257, 2-Fluoro-11-(4-methyl-piperazin-1-yl)-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071118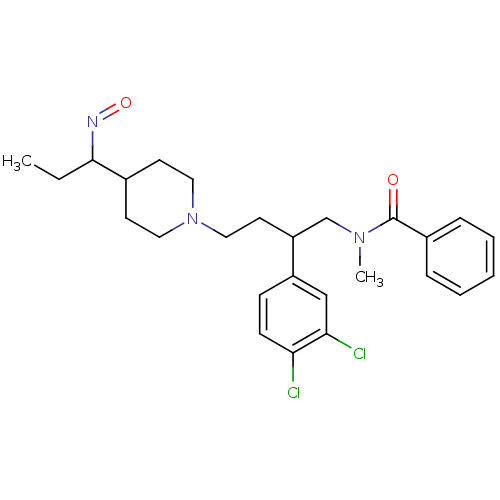 (CHEMBL2111522 | CHEMBL443264 | N-[2-(3,4-Dichloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071112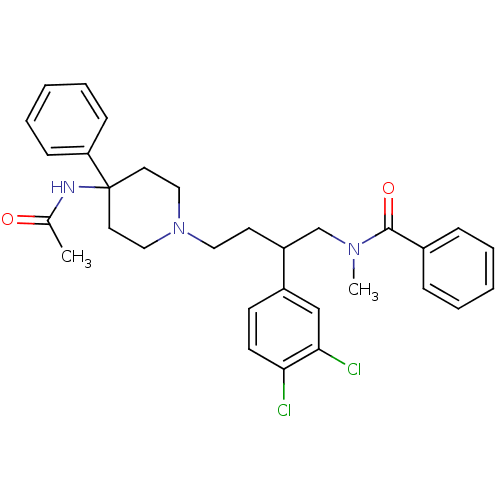 (CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071112 (CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50216091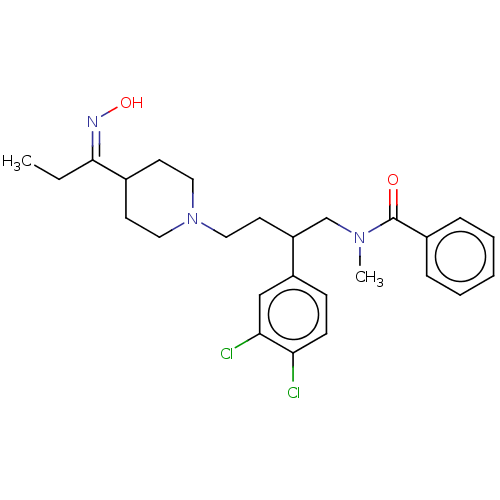 (CHEMBL2111522) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071118 (CHEMBL2111522 | CHEMBL443264 | N-[2-(3,4-Dichloro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071125 (CHEMBL56481 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(1-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071120 (CHEMBL56788 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(1-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071119 (CHEMBL56143 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(1-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071115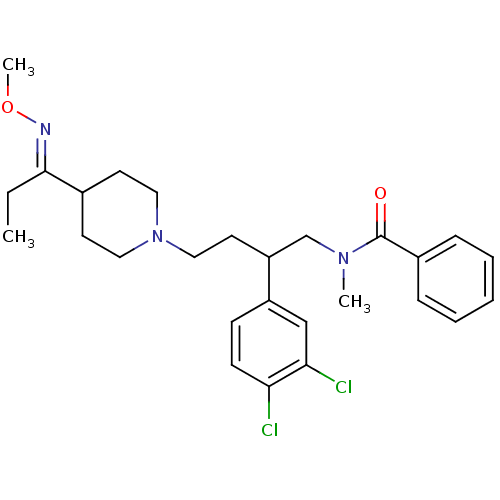 (CHEMBL58638 | N-[2-(3,4-Dichloro-phenyl)-4-(4-{1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071121 (CHEMBL301437 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071122 (CHEMBL417940 | N-[2-(3,4-Dichloro-phenyl)-4-(4-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118099 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071126 (CHEMBL59542 | N-[2-(3,4-Dichloro-phenyl)-4-(4-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141312 (US8653257, 2-Fluoro-11-piperazin-1-yl-dibenzo[b,f]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071124 (CHEMBL56739 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(1-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071123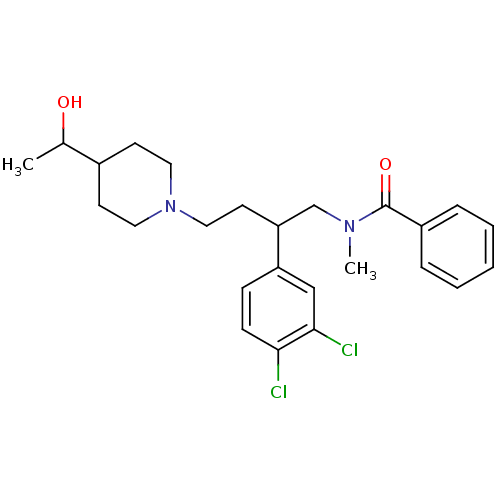 (CHEMBL56274 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(1-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118099 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM141313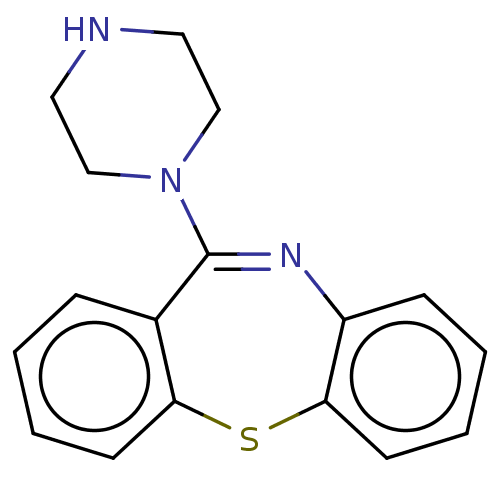 (US8653257, 11-Piperazin-1-yl-dibenzo[b,f][1,4]thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca AB US Patent | Assay Description The ability of test compounds to displace 3H-raclopride at the D2s receptor can be determined on membranes from D2s-transfected CHO cells (Bmax 13 pm... | US Patent US8653257 (2014) BindingDB Entry DOI: 10.7270/Q2DR2T69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071113 (Acetic acid 2-{1-[4-(benzoyl-methyl-amino)-3-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071117 (CHEMBL292415 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071116 (CHEMBL58331 | N-[4-[4-(2-Acetylamino-ethyl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118100 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 3 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071114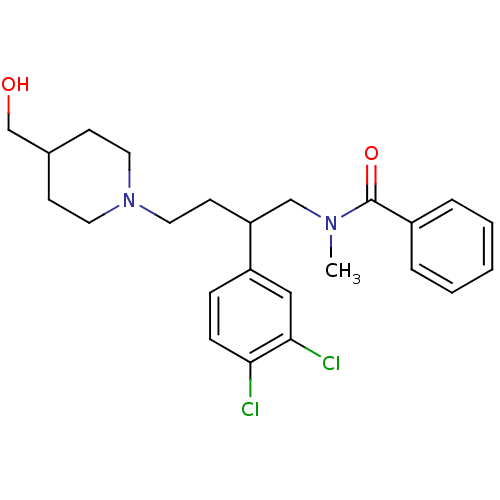 (CHEMBL57224 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 142 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]- NKA from human NK-2 receptor expressed in MEL cells | Bioorg Med Chem Lett 8: 1935-40 (1999) BindingDB Entry DOI: 10.7270/Q2J38RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 3 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118100 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | CHEMBL5275914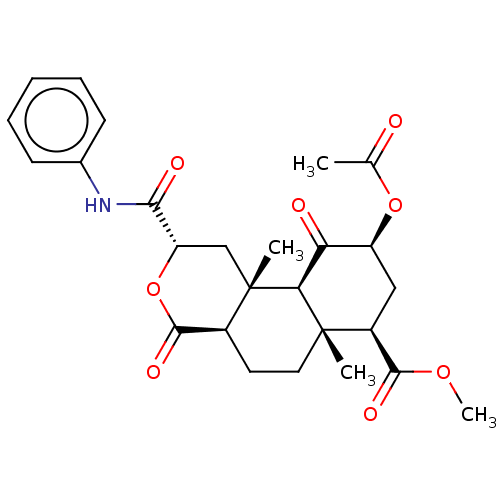 | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50409575 (CHEMBL339767 | ZD-7944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to human Tachykinin receptor 1 expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50409575 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM21130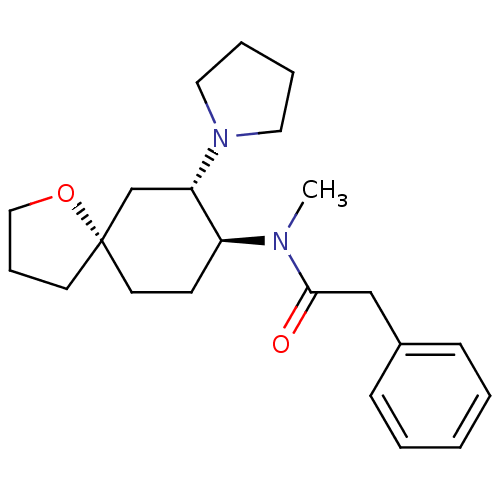 (N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by HTRF assay | Bioorg Med Chem Lett 28: 2770-2772 (2018) Article DOI: 10.1016/j.bmcl.2018.01.055 BindingDB Entry DOI: 10.7270/Q2V40XV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50462205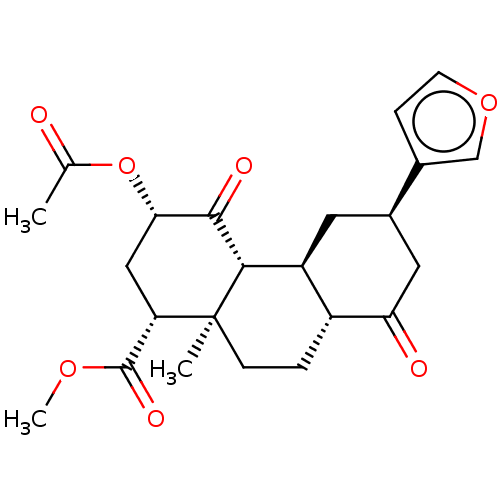 (CHEMBL4250476) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by HTRF assay | Bioorg Med Chem Lett 28: 2770-2772 (2018) Article DOI: 10.1016/j.bmcl.2018.01.055 BindingDB Entry DOI: 10.7270/Q2V40XV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |