 Found 275 hits with Last Name = 'shepard' and Initial = 'rm'
Found 275 hits with Last Name = 'shepard' and Initial = 'rm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352059
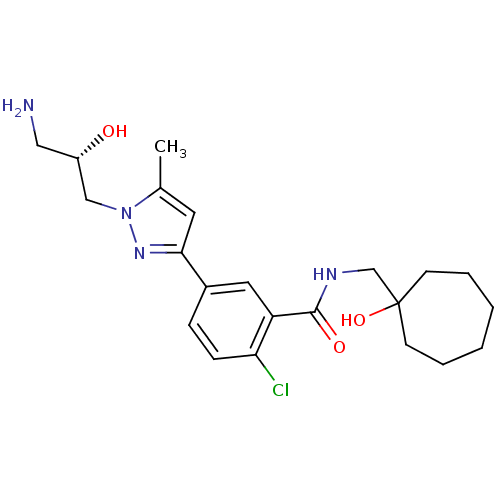
(CHEMBL1824026)Show SMILES Cc1cc(nn1C[C@@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144428

(CHEMBL66159 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NN Show InChI InChI=1S/C26H32FN5O3/c1-26(2,27)13-12-18(24(34)32-28)15-23(33)21(14-17-8-4-3-5-9-17)31-25(35)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,33H,12-15,28H2,1-2H3,(H,31,35)(H,32,34)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352039

(CHEMBL1823817)Show SMILES COC[C@H](O)Cn1c(=O)cnn(-c2ccc(Cl)c(c2)C(=O)NCC2(O)CCCCCC2)c1=O |r| Show InChI InChI=1S/C22H29ClN4O6/c1-33-13-16(28)12-26-19(29)11-25-27(21(26)31)15-6-7-18(23)17(10-15)20(30)24-14-22(32)8-4-2-3-5-9-22/h6-7,10-11,16,28,32H,2-5,8-9,12-14H2,1H3,(H,24,30)/t16-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352059

(CHEMBL1824026)Show SMILES Cc1cc(nn1C[C@@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144400

(CHEMBL304358 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H32N4O3/c1-17(2)12-13-19(25(27)32)15-24(31)22(14-18-8-4-3-5-9-18)30-26(33)23-16-28-20-10-6-7-11-21(20)29-23/h3-11,16-17,19,22,24,31H,12-15H2,1-2H3,(H2,27,32)(H,30,33)/t19-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352060

(CHEMBL1824027)Show SMILES Cc1cc(nn1C[C@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352058
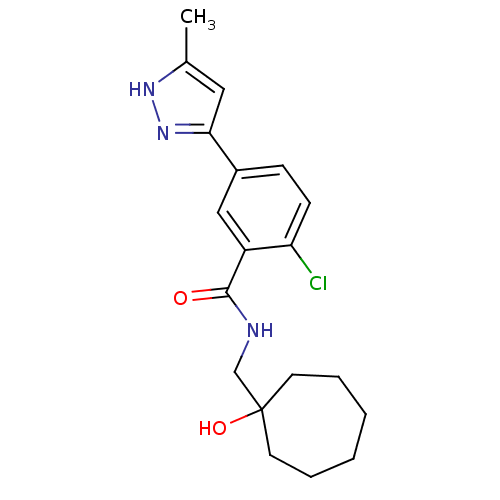
(CHEMBL560241)Show SMILES Cc1cc(n[nH]1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H24ClN3O2/c1-13-10-17(23-22-13)14-6-7-16(20)15(11-14)18(24)21-12-19(25)8-4-2-3-5-9-19/h6-7,10-11,25H,2-5,8-9,12H2,1H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352059

(CHEMBL1824026)Show SMILES Cc1cc(nn1C[C@@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL-1beta release |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144420
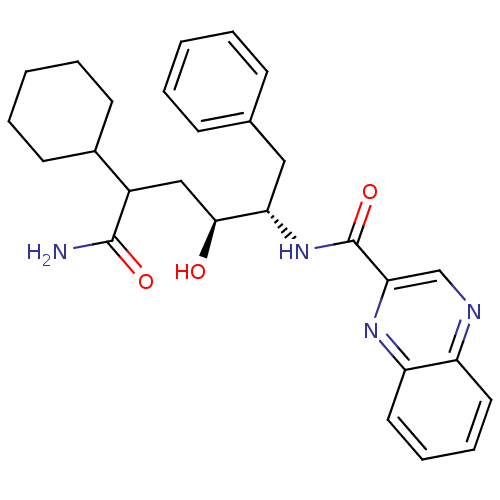
(CHEMBL68145 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCCCC1 Show InChI InChI=1S/C27H32N4O3/c28-26(33)20(19-11-5-2-6-12-19)16-25(32)23(15-18-9-3-1-4-10-18)31-27(34)24-17-29-21-13-7-8-14-22(21)30-24/h1,3-4,7-10,13-14,17,19-20,23,25,32H,2,5-6,11-12,15-16H2,(H2,28,33)(H,31,34)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352052

(CHEMBL1824020)Show SMILES Cc1nc(cs1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H23ClN2O2S/c1-13-22-17(11-25-13)14-6-7-16(20)15(10-14)18(23)21-12-19(24)8-4-2-3-5-9-19/h6-7,10-11,24H,2-5,8-9,12H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144431

(CHEMBL303673 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES ONC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O4/c28-27(29)12-10-18(11-13-27)19(25(35)33-37)15-24(34)22(14-17-6-2-1-3-7-17)32-26(36)23-16-30-20-8-4-5-9-21(20)31-23/h1-9,16,18-19,22,24,34,37H,10-15H2,(H,32,36)(H,33,35)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352060

(CHEMBL1824027)Show SMILES Cc1cc(nn1C[C@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352058

(CHEMBL560241)Show SMILES Cc1cc(n[nH]1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H24ClN3O2/c1-13-10-17(23-22-13)14-6-7-16(20)15(11-14)18(24)21-12-19(25)8-4-2-3-5-9-19/h6-7,10-11,25H,2-5,8-9,12H2,1H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144428

(CHEMBL66159 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NN Show InChI InChI=1S/C26H32FN5O3/c1-26(2,27)13-12-18(24(34)32-28)15-23(33)21(14-17-8-4-3-5-9-17)31-25(35)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,33H,12-15,28H2,1-2H3,(H,31,35)(H,32,34)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209006
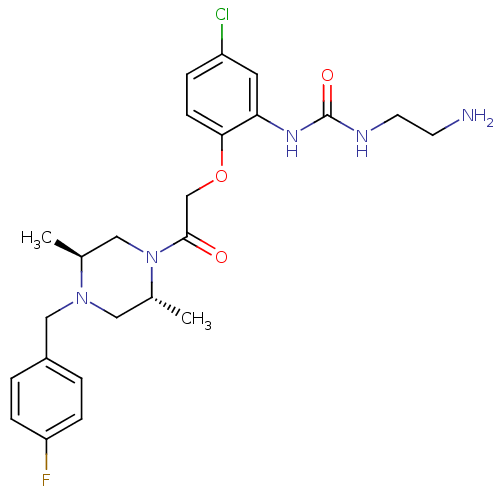
(1-(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpi...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)COc1ccc(Cl)cc1NC(=O)NCCN Show InChI InChI=1S/C24H31ClFN5O3/c1-16-13-31(17(2)12-30(16)14-18-3-6-20(26)7-4-18)23(32)15-34-22-8-5-19(25)11-21(22)29-24(33)28-10-9-27/h3-8,11,16-17H,9-10,12-15,27H2,1-2H3,(H2,28,29,33)/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144431

(CHEMBL303673 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES ONC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O4/c28-27(29)12-10-18(11-13-27)19(25(35)33-37)15-24(34)22(14-17-6-2-1-3-7-17)32-26(36)23-16-30-20-8-4-5-9-21(20)31-23/h1-9,16,18-19,22,24,34,37H,10-15H2,(H,32,36)(H,33,35)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144420

(CHEMBL68145 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCCCC1 Show InChI InChI=1S/C27H32N4O3/c28-26(33)20(19-11-5-2-6-12-19)16-25(32)23(15-18-9-3-1-4-10-18)31-27(34)24-17-29-21-13-7-8-14-22(21)30-24/h1,3-4,7-10,13-14,17,19-20,23,25,32H,2,5-6,11-12,15-16H2,(H2,28,33)(H,31,34)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144417
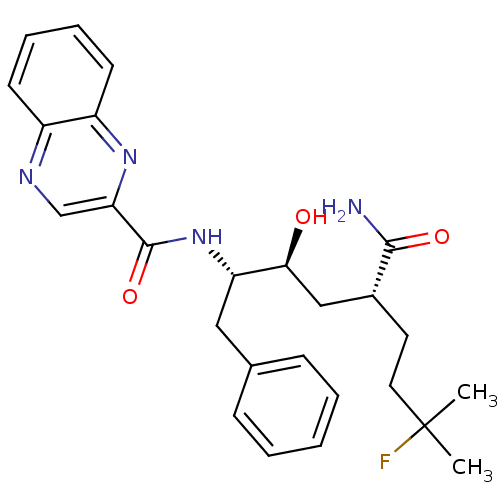
(CHEMBL68366 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O3/c1-26(2,27)13-12-18(24(28)33)15-23(32)21(14-17-8-4-3-5-9-17)31-25(34)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,32H,12-15H2,1-2H3,(H2,28,33)(H,31,34)/t18-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352060

(CHEMBL1824027)Show SMILES Cc1cc(nn1C[C@H](O)CN)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 |r| Show InChI InChI=1S/C22H31ClN4O3/c1-15-10-20(26-27(15)13-17(28)12-24)16-6-7-19(23)18(11-16)21(29)25-14-22(30)8-4-2-3-5-9-22/h6-7,10-11,17,28,30H,2-5,8-9,12-14,24H2,1H3,(H,25,29)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL-1beta release |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352055

(CHEMBL549618)Show InChI InChI=1S/C18H22ClN3O2/c19-15-6-5-13(16-7-10-21-22-16)11-14(15)17(23)20-12-18(24)8-3-1-2-4-9-18/h5-7,10-11,24H,1-4,8-9,12H2,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144417

(CHEMBL68366 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CC(C)(F)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O3/c1-26(2,27)13-12-18(24(28)33)15-23(32)21(14-17-8-4-3-5-9-17)31-25(34)22-16-29-19-10-6-7-11-20(19)30-22/h3-11,16,18,21,23,32H,12-15H2,1-2H3,(H2,28,33)(H,31,34)/t18-,21+,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209001
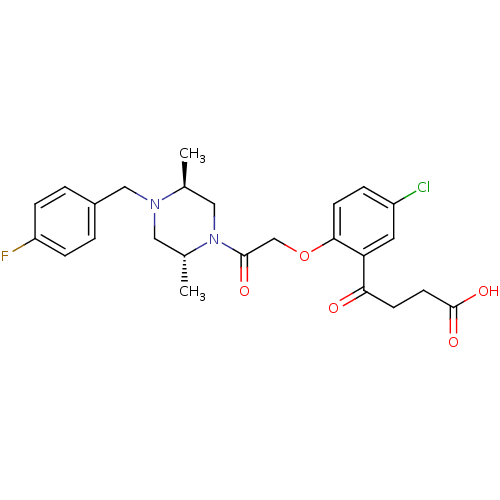
(4-(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpi...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)COc1ccc(Cl)cc1C(=O)CCC(O)=O Show InChI InChI=1S/C25H28ClFN2O5/c1-16-13-29(17(2)12-28(16)14-18-3-6-20(27)7-4-18)24(31)15-34-23-9-5-19(26)11-21(23)22(30)8-10-25(32)33/h3-7,9,11,16-17H,8,10,12-15H2,1-2H3,(H,32,33)/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144421

(CHEMBL302533 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O3/c28-27(29)12-10-18(11-13-27)19(25(30)35)15-24(34)22(14-17-6-2-1-3-7-17)33-26(36)23-16-31-20-8-4-5-9-21(20)32-23/h1-9,16,18-19,22,24,34H,10-15H2,(H2,30,35)(H,33,36)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209003

((2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpipe...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)COc1ccc(Cl)cc1CS(O)(=O)=O Show InChI InChI=1S/C22H26ClFN2O5S/c1-15-11-26(16(2)10-25(15)12-17-3-6-20(24)7-4-17)22(27)13-31-21-8-5-19(23)9-18(21)14-32(28,29)30/h3-9,15-16H,10-14H2,1-2H3,(H,28,29,30)/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144419

(CHEMBL305423 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NO Show InChI InChI=1S/C26H31FN4O4/c1-26(2,27)13-12-18(24(33)31-35)15-23(32)21(14-17-8-4-3-5-9-17)30-25(34)22-16-28-19-10-6-7-11-20(19)29-22/h3-11,16,18,21,23,32,35H,12-15H2,1-2H3,(H,30,34)(H,31,33)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144400

(CHEMBL304358 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)CC[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H32N4O3/c1-17(2)12-13-19(25(27)32)15-24(31)22(14-18-8-4-3-5-9-18)30-26(33)23-16-28-20-10-6-7-11-21(20)29-23/h3-11,16-17,19,22,24,31H,12-15H2,1-2H3,(H2,27,32)(H,30,33)/t19-,22+,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352061

(CHEMBL1824022)Show SMILES COc1ccc(nn1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C20H24ClN3O3/c1-27-18-9-8-17(23-24-18)14-6-7-16(21)15(12-14)19(25)22-13-20(26)10-4-2-3-5-11-20/h6-9,12,26H,2-5,10-11,13H2,1H3,(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209000

(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpiper...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)COc1ccc(Cl)cc1C(=O)NCCN Show InChI InChI=1S/C24H30ClFN4O3/c1-16-13-30(17(2)12-29(16)14-18-3-6-20(26)7-4-18)23(31)15-33-22-8-5-19(25)11-21(22)24(32)28-10-9-27/h3-8,11,16-17H,9-10,12-15,27H2,1-2H3,(H,28,32)/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144419

(CHEMBL305423 | Quinoxaline-2-carboxylic acid ((1S,...)Show SMILES CC(C)(F)CCC(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C(=O)NO Show InChI InChI=1S/C26H31FN4O4/c1-26(2,27)13-12-18(24(33)31-35)15-23(32)21(14-17-8-4-3-5-9-17)30-25(34)22-16-28-19-10-6-7-11-20(19)29-22/h3-11,16,18,21,23,32,35H,12-15H2,1-2H3,(H,30,34)(H,31,33)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50318005
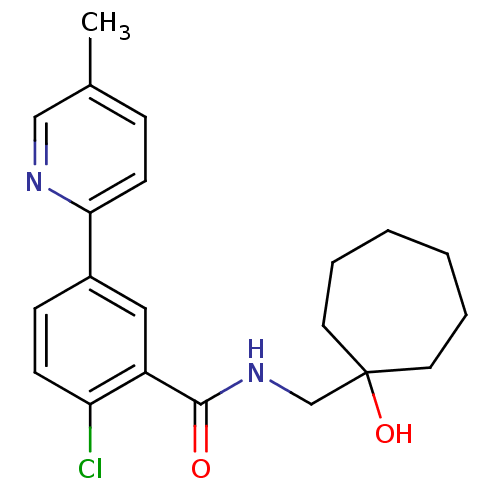
(2-chloro-N-((1-hydroxycycloheptyl)methyl)-5-(5-met...)Show SMILES Cc1ccc(nc1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C21H25ClN2O2/c1-15-6-9-19(23-13-15)16-7-8-18(22)17(12-16)20(25)24-14-21(26)10-4-2-3-5-11-21/h6-9,12-13,26H,2-5,10-11,14H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208999
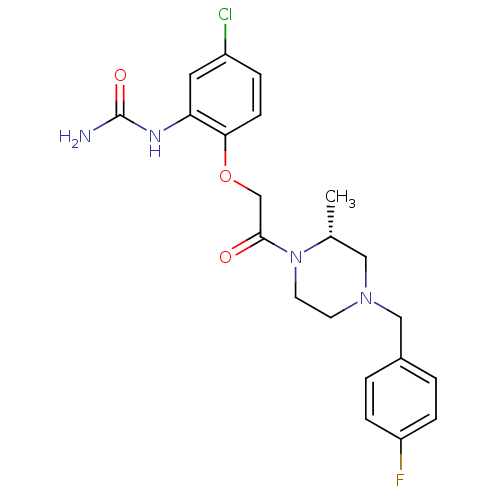
((R)-1-(2-(2-(4-(4-fluorobenzyl)-2-methylpiperazin-...)Show SMILES C[C@@H]1CN(Cc2ccc(F)cc2)CCN1C(=O)COc1ccc(Cl)cc1NC(N)=O |r| Show InChI InChI=1S/C21H24ClFN4O3/c1-14-11-26(12-15-2-5-17(23)6-3-15)8-9-27(14)20(28)13-30-19-7-4-16(22)10-18(19)25-21(24)29/h2-7,10,14H,8-9,11-13H2,1H3,(H3,24,25,29)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209004
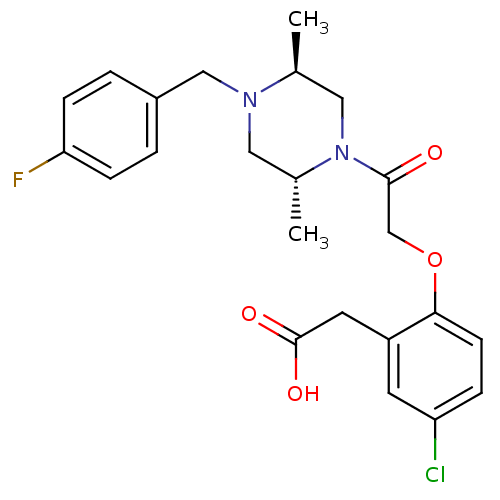
(2-(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpi...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)COc1ccc(Cl)cc1CC(O)=O Show InChI InChI=1S/C23H26ClFN2O4/c1-15-12-27(16(2)11-26(15)13-17-3-6-20(25)7-4-17)22(28)14-31-21-8-5-19(24)9-18(21)10-23(29)30/h3-9,15-16H,10-14H2,1-2H3,(H,29,30)/t15-,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50209002
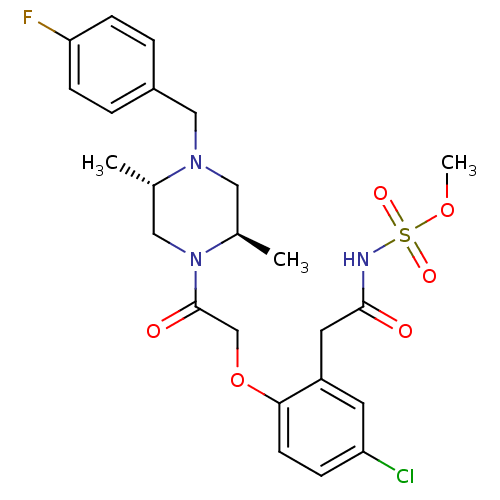
(CHEMBL391239 | methyl 2-(2-(2-((2R,5S)-4-(4-fluoro...)Show SMILES COS(=O)(=O)NC(=O)Cc1cc(Cl)ccc1OCC(=O)N1C[C@H](C)N(Cc2ccc(F)cc2)C[C@H]1C Show InChI InChI=1S/C24H29ClFN3O6S/c1-16-13-29(17(2)12-28(16)14-18-4-7-21(26)8-5-18)24(31)15-35-22-9-6-20(25)10-19(22)11-23(30)27-36(32,33)34-3/h4-10,16-17H,11-15H2,1-3H3,(H,27,30)/t16-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144422

(CHEMBL308473 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES CC(C)(O)CCC(C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144425
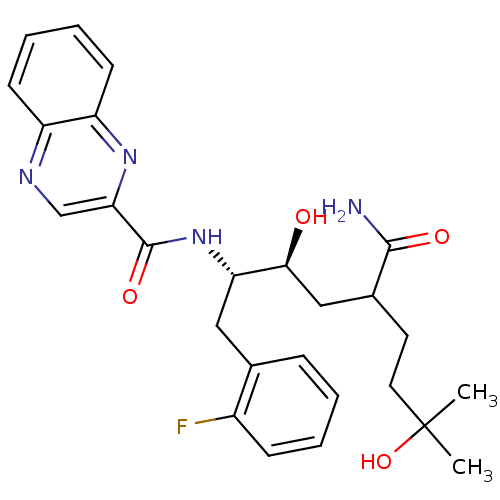
(CHEMBL304053 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES CC(C)(O)CCC(C[C@H](O)[C@H](Cc1ccccc1F)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)12-11-17(24(28)33)14-23(32)21(13-16-7-3-4-8-18(16)27)31-25(34)22-15-29-19-9-5-6-10-20(19)30-22/h3-10,15,17,21,23,32,35H,11-14H2,1-2H3,(H2,28,33)(H,31,34)/t17?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50208998

(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpiper...)Show SMILES C[C@H]1CN([C@H](C)CN1Cc1ccc(F)cc1)C(=O)COc1ccc(Cl)cc1C(O)=O Show InChI InChI=1S/C22H24ClFN2O4/c1-14-11-26(15(2)10-25(14)12-16-3-6-18(24)7-4-16)21(27)13-30-20-8-5-17(23)9-19(20)22(28)29/h3-9,14-15H,10-13H2,1-2H3,(H,28,29)/t14-,15+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding Affinity at CCL3 |
Bioorg Med Chem Lett 17: 3109-12 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.037
BindingDB Entry DOI: 10.7270/Q2CF9PSH |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144422

(CHEMBL308473 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES CC(C)(O)CCC(C[C@H](O)[C@H](Cc1cccc(F)c1)NC(=O)c1cnc2ccccc2n1)C(N)=O Show InChI InChI=1S/C26H31FN4O4/c1-26(2,35)11-10-17(24(28)33)14-23(32)21(13-16-6-5-7-18(27)12-16)31-25(34)22-15-29-19-8-3-4-9-20(19)30-22/h3-9,12,15,17,21,23,32,35H,10-11,13-14H2,1-2H3,(H2,28,33)(H,31,34)/t17?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144423

(CHEMBL302320 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1(O)CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O4/c28-27(29)12-10-26(37,11-13-27)18(24(30)35)15-23(34)21(14-17-6-2-1-3-7-17)33-25(36)22-16-31-19-8-4-5-9-20(19)32-22/h1-9,16,18,21,23,34,37H,10-15H2,(H2,30,35)(H,33,36)/t18?,21-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144416
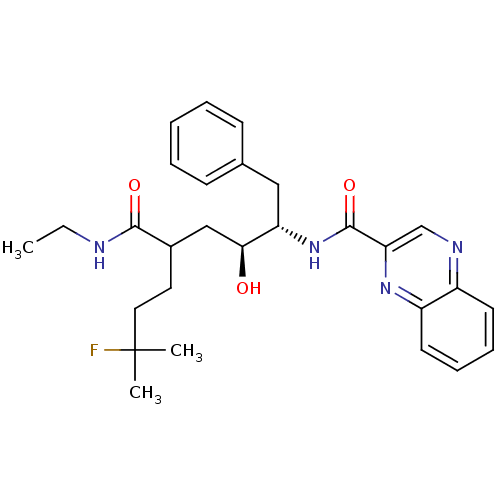
(CHEMBL69123 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CCNC(=O)C(CCC(C)(C)F)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C28H35FN4O3/c1-4-30-26(35)20(14-15-28(2,3)29)17-25(34)23(16-19-10-6-5-7-11-19)33-27(36)24-18-31-21-12-8-9-13-22(21)32-24/h5-13,18,20,23,25,34H,4,14-17H2,1-3H3,(H,30,35)(H,33,36)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 binding to C-C chemokine receptor type 1 |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50318005

(2-chloro-N-((1-hydroxycycloheptyl)methyl)-5-(5-met...)Show SMILES Cc1ccc(nc1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C21H25ClN2O2/c1-15-6-9-19(23-13-15)16-7-8-18(22)17(12-16)20(25)24-14-21(26)10-4-2-3-5-11-21/h6-9,12-13,26H,2-5,10-11,14H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352040

(CHEMBL1823818)Show SMILES Cc1ccc(nc1)-c1ccc(Cl)c(c1)C(=O)NCCc1ccccc1Cl Show InChI InChI=1S/C21H18Cl2N2O/c1-14-6-9-20(25-13-14)16-7-8-19(23)17(12-16)21(26)24-11-10-15-4-2-3-5-18(15)22/h2-9,12-13H,10-11H2,1H3,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144424
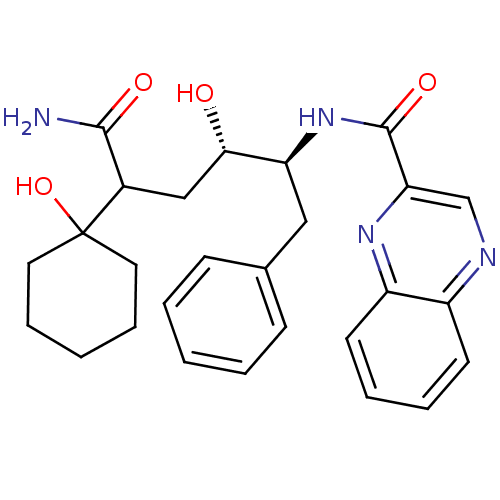
(CHEMBL306457 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1(O)CCCCC1 Show InChI InChI=1S/C27H32N4O4/c28-25(33)19(27(35)13-7-2-8-14-27)16-24(32)22(15-18-9-3-1-4-10-18)31-26(34)23-17-29-20-11-5-6-12-21(20)30-23/h1,3-6,9-12,17,19,22,24,32,35H,2,7-8,13-16H2,(H2,28,33)(H,31,34)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352058

(CHEMBL560241)Show SMILES Cc1cc(n[nH]1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H24ClN3O2/c1-13-10-17(23-22-13)14-6-7-16(20)15(11-14)18(24)21-12-19(25)8-4-2-3-5-9-19/h6-7,10-11,25H,2-5,8-9,12H2,1H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-stimulated human whole blood assessed as inhibition of ATP-induced IL-1beta release |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144416

(CHEMBL69123 | Quinoxaline-2-carboxylic acid ((1S,2...)Show SMILES CCNC(=O)C(CCC(C)(C)F)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1 Show InChI InChI=1S/C28H35FN4O3/c1-4-30-26(35)20(14-15-28(2,3)29)17-25(34)23(16-19-10-6-5-7-11-19)33-27(36)24-18-31-21-12-8-9-13-22(21)32-24/h5-13,18,20,23,25,34H,4,14-17H2,1-3H3,(H,30,35)(H,33,36)/t20?,23-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352054

(CHEMBL1824023)Show SMILES OC1(CNC(=O)c2cc(ccc2Cl)-c2ccc(=O)[nH]n2)CCCCCC1 Show InChI InChI=1S/C19H22ClN3O3/c20-15-6-5-13(16-7-8-17(24)23-22-16)11-14(15)18(25)21-12-19(26)9-3-1-2-4-10-19/h5-8,11,26H,1-4,9-10,12H2,(H,21,25)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in HEK293 cells assessed as inhibition of ATP-induced YO-PRO-1 uptake |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352055

(CHEMBL549618)Show InChI InChI=1S/C18H22ClN3O2/c19-15-6-5-13(16-7-10-21-22-16)11-14(15)17(23)20-12-18(24)8-3-1-2-4-9-18/h5-7,10-11,24H,1-4,8-9,12H2,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50144421

(CHEMBL302533 | Quinoxaline-2-carboxylic acid [(1S,...)Show SMILES NC(=O)C(C[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cnc2ccccc2n1)C1CCC(F)(F)CC1 Show InChI InChI=1S/C27H30F2N4O3/c28-27(29)12-10-18(11-13-27)19(25(30)35)15-24(34)22(14-17-6-2-1-3-7-17)33-26(36)23-16-31-20-8-4-5-9-21(20)32-23/h1-9,16,18-19,22,24,34H,10-15H2,(H2,30,35)(H,33,36)/t19?,22-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CCL3 induced chemotaxis in human T lymphocytes |
Bioorg Med Chem Lett 14: 2175-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.022
BindingDB Entry DOI: 10.7270/Q24B30SR |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352056
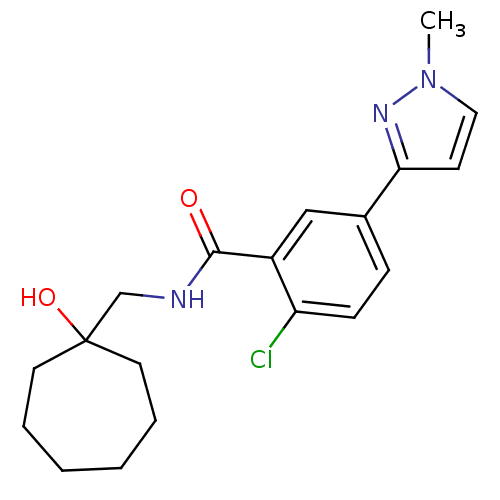
(CHEMBL1824024)Show SMILES Cn1ccc(n1)-c1ccc(Cl)c(c1)C(=O)NCC1(O)CCCCCC1 Show InChI InChI=1S/C19H24ClN3O2/c1-23-11-8-17(22-23)14-6-7-16(20)15(12-14)18(24)21-13-19(25)9-4-2-3-5-10-19/h6-8,11-12,25H,2-5,9-10,13H2,1H3,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352053
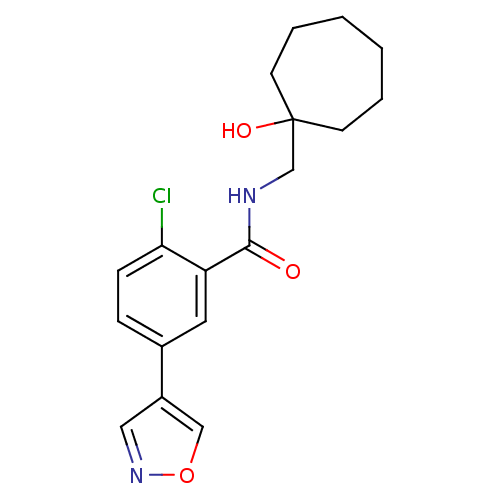
(CHEMBL1824021)Show InChI InChI=1S/C18H21ClN2O3/c19-16-6-5-13(14-10-21-24-11-14)9-15(16)17(22)20-12-18(23)7-3-1-2-4-8-18/h5-6,9-11,23H,1-4,7-8,12H2,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50352048

(CHEMBL1824016)Show SMILES Cc1ccc(nn1)-c1ccc(Cl)c(c1)C(=O)NCCc1ccccc1Cl Show InChI InChI=1S/C20H17Cl2N3O/c1-13-6-9-19(25-24-13)15-7-8-18(22)16(12-15)20(26)23-11-10-14-4-2-3-5-17(14)21/h2-9,12H,10-11H2,1H3,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7R expressed in LPS-activated human monocytes assessed as inhibition of ATP-induced IL-1beta release in presence of low ser... |
Bioorg Med Chem Lett 21: 5475-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.117
BindingDB Entry DOI: 10.7270/Q2PC32RF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data