

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101827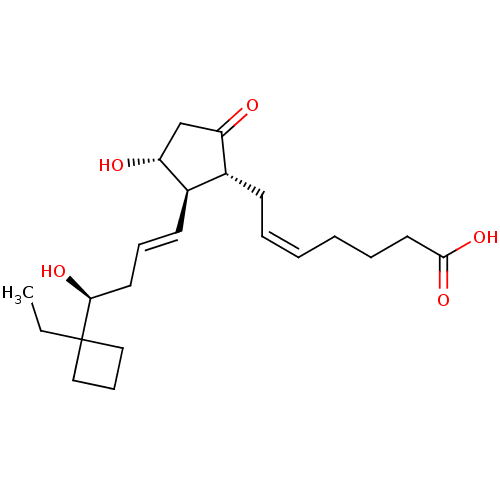 ((Z)-7-{(1R,2R,3R)-2-[(E)-(S)-4-(1-Ethyl-cyclobutyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101832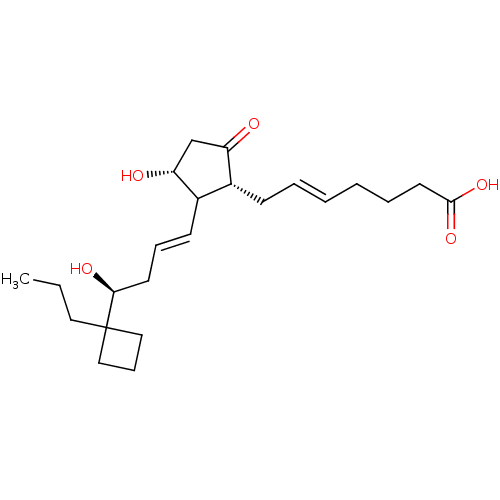 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101833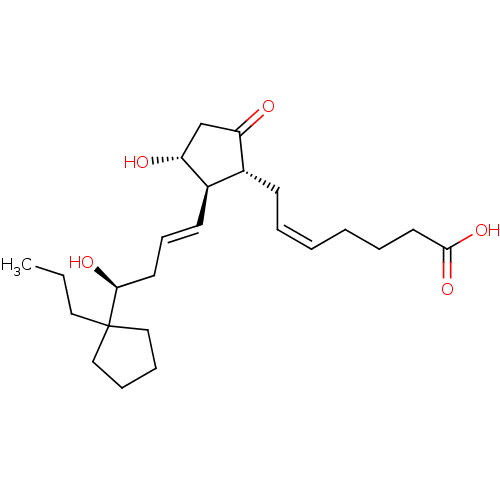 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101823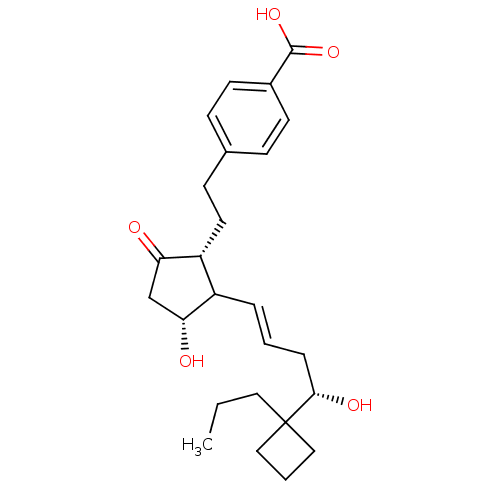 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101823 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101826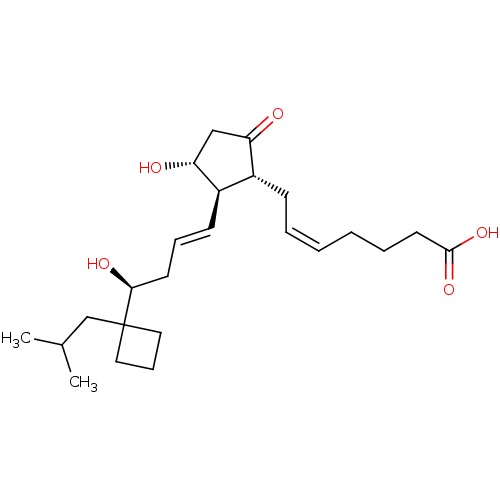 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235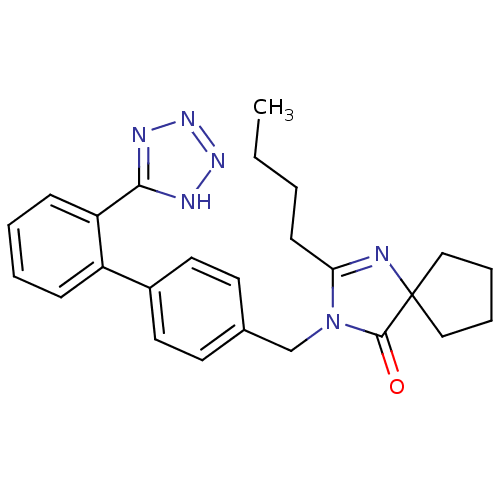 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105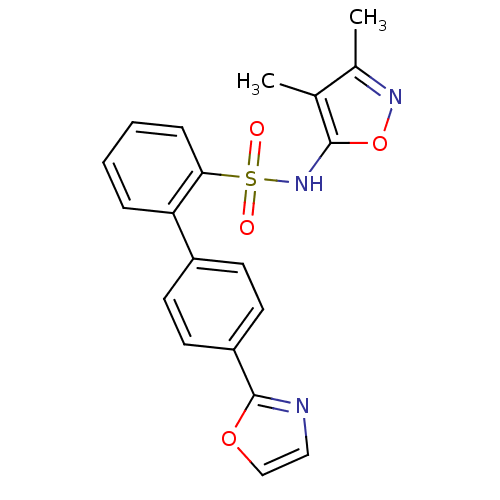 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101832 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417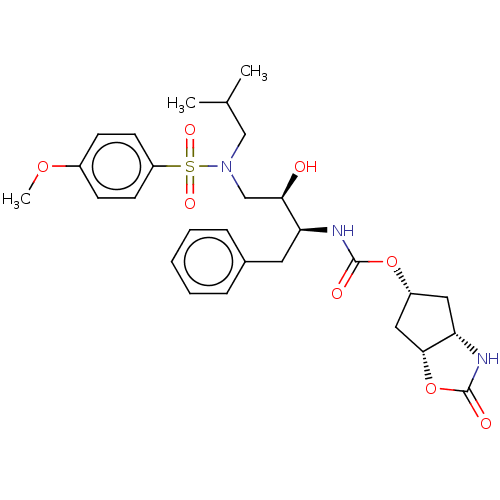 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498523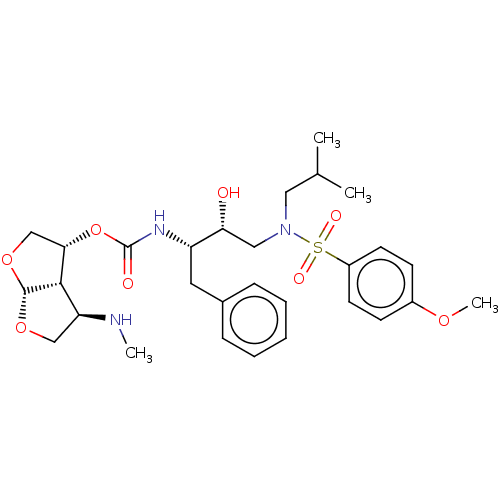 (CHEMBL3605643) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719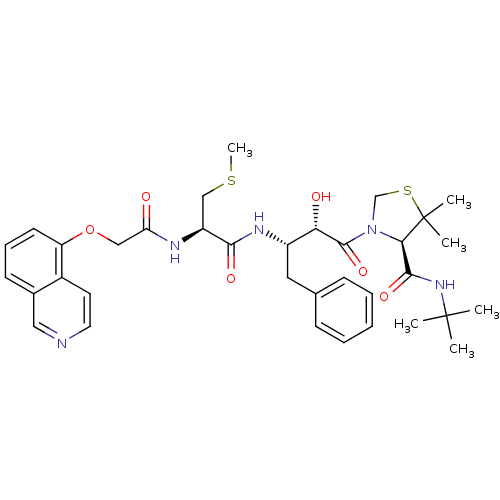 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00230 | -69.1 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute | Assay Description Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... | Antimicrob Agents Chemother 37: 810-7 (1993) Article DOI: 10.1128/aac.37.4.810 BindingDB Entry DOI: 10.7270/Q2KH0KHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498524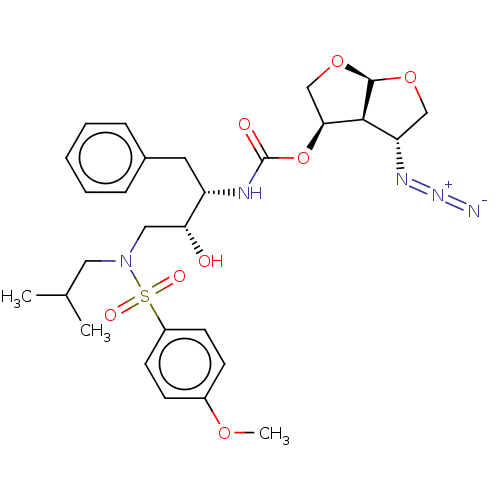 (CHEMBL3605638) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033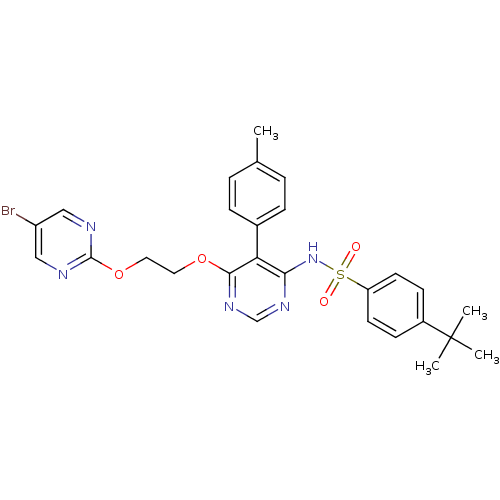 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105033 (CHEMBL112531 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579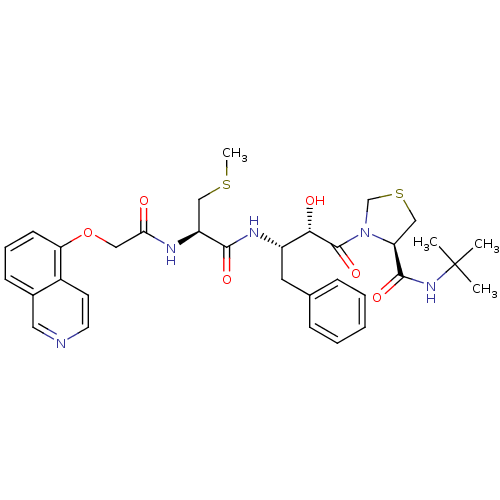 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00550 | -66.9 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute | Assay Description Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... | Antimicrob Agents Chemother 37: 810-7 (1993) Article DOI: 10.1128/aac.37.4.810 BindingDB Entry DOI: 10.7270/Q2KH0KHT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498534 (CHEMBL3605635) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM793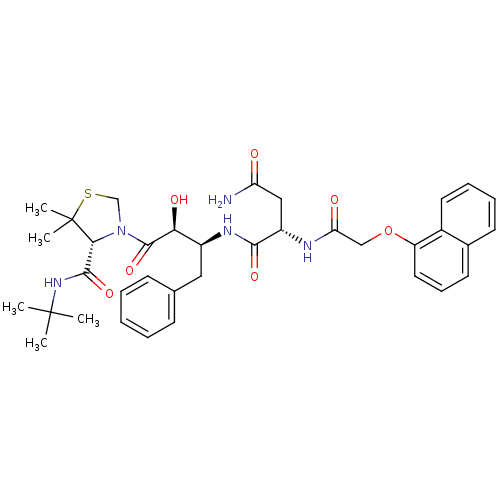 ((2S)-N-[(2S,3S)-4-[(4R)-4-(tert-butylcarbamoyl)-5,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00680 | -66.3 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute | Assay Description Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... | Antimicrob Agents Chemother 37: 810-7 (1993) Article DOI: 10.1128/aac.37.4.810 BindingDB Entry DOI: 10.7270/Q2KH0KHT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498528 (CHEMBL3605644) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50366138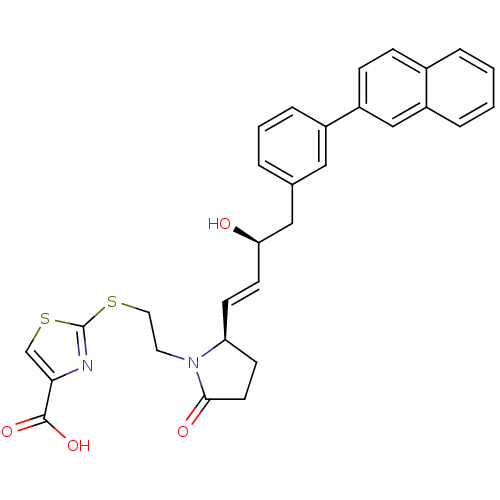 (CHEMBL1957437) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-PGE2 from mouse EP4 receptor expressed in CHO cells after 60 mins by scintillation counting | Bioorg Med Chem 20: 2235-51 (2012) Article DOI: 10.1016/j.bmc.2012.02.018 BindingDB Entry DOI: 10.7270/Q2542P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498531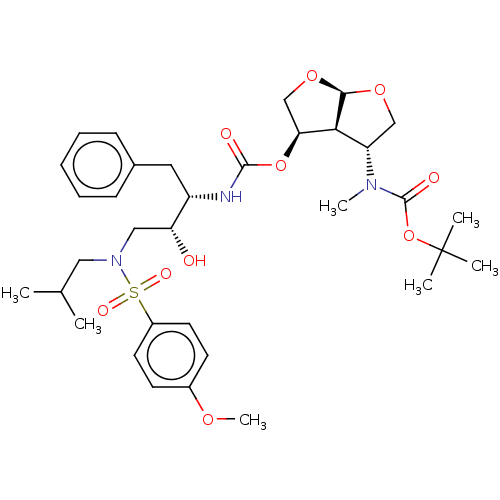 (CHEMBL3605642) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50118470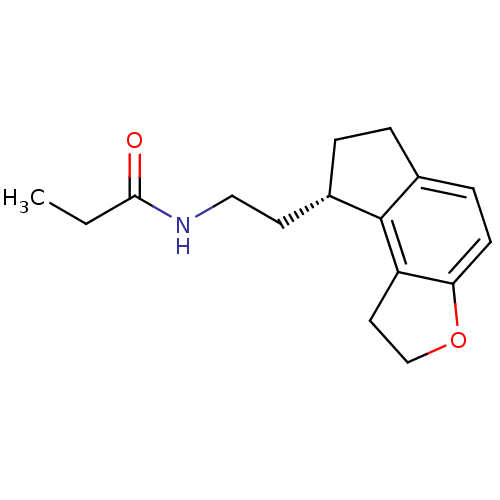 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.0138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Binding affinity against human Melatonin receptor type 1A (MT1) | J Med Chem 45: 4222-39 (2002) BindingDB Entry DOI: 10.7270/Q2D799S6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498530 (CHEMBL3605637) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498526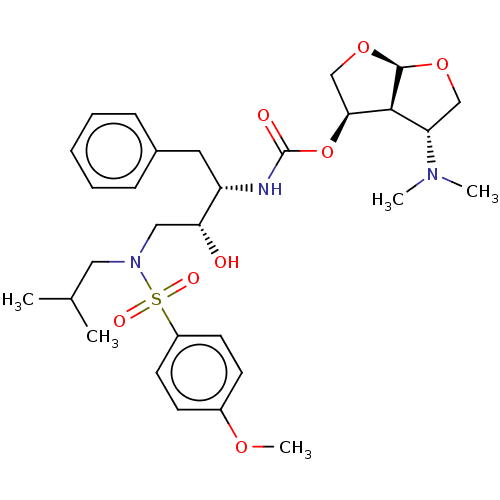 (CHEMBL3605636) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498527 (CHEMBL3605640) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953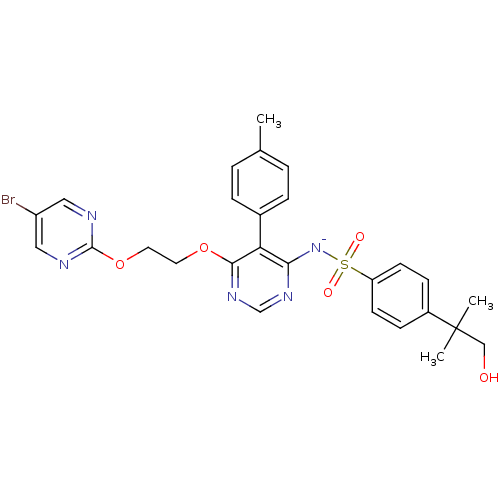 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50369953 (CHEMBL1627022) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd. Curated by ChEMBL | Assay Description Ability to inhibit [125I]ET1 binding to human cloned endothelin A receptor expressed on CHO cells | J Med Chem 44: 3369-77 (2001) BindingDB Entry DOI: 10.7270/Q27M08P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469407 (CHEMBL4286714) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469413 (CHEMBL4286231) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50083552 (1,9-di{4-[5-amino(imino)methylbenzo[b]furan-2-ylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD. Curated by ChEMBL | Assay Description Inhibition of tryptase activity | Bioorg Med Chem Lett 9: 3285-90 (2000) BindingDB Entry DOI: 10.7270/Q2TX3DK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498532 (CHEMBL3605641) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523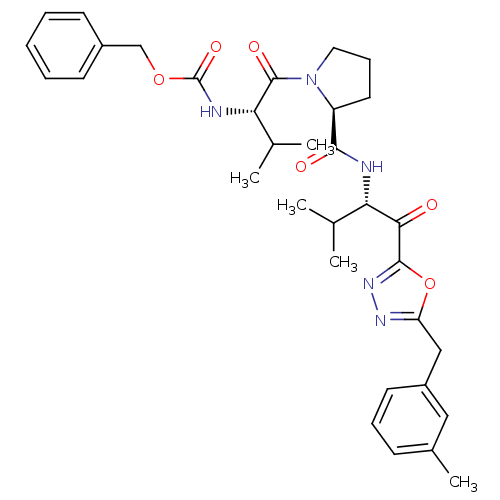 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado State University Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase HNE, hydrolysis of MeO-Suc-Ala-Ala-Pro-Val-pNa | J Med Chem 43: 4927-9 (2001) BindingDB Entry DOI: 10.7270/Q25D8R3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cortech, Inc. Curated by ChEMBL | Assay Description Binding constant derived from inhibition of elastase catalyzed hydrolysis of synthetic substrate | J Med Chem 44: 1268-85 (2001) BindingDB Entry DOI: 10.7270/Q2B858VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50076227 ((1S,7S)-7-Amino-7-benzyl-hexahydro-pyrazolo[1,2-a]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against trypsin. | J Med Chem 42: 1367-75 (1999) Article DOI: 10.1021/jm980354p BindingDB Entry DOI: 10.7270/Q20Z72G1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498533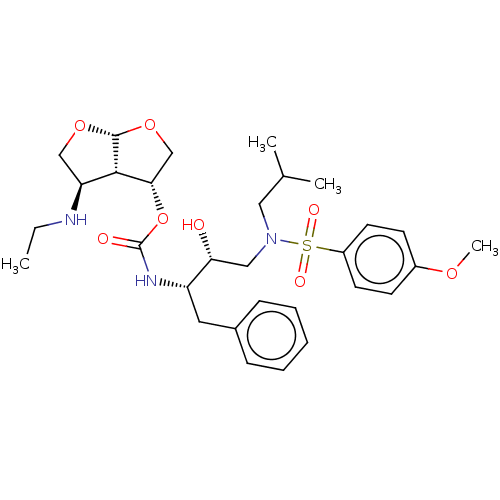 (CHEMBL3605646) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50083561 (1-{4-[5-amino(imino)methylbenzo[b]thiophen-2-ylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD. Curated by ChEMBL | Assay Description Inhibition of tryptase activity | Bioorg Med Chem Lett 9: 3285-90 (2000) BindingDB Entry DOI: 10.7270/Q2TX3DK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50083556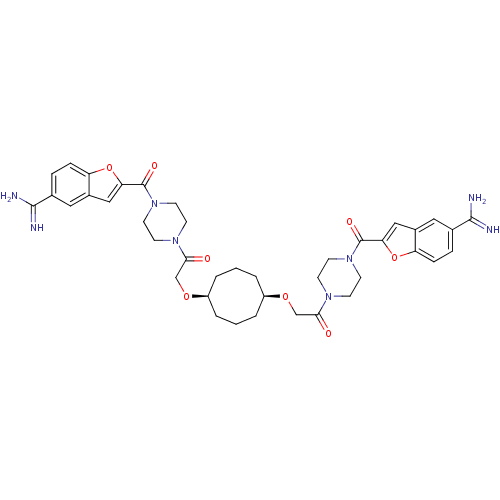 (1-{4-[5-amino(imino)methylbenzo[b]furan-2-ylcarbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yoshitomi Pharmaceutical Industries, LTD. Curated by ChEMBL | Assay Description Inhibition of tryptase activity | Bioorg Med Chem Lett 9: 3285-90 (2000) BindingDB Entry DOI: 10.7270/Q2TX3DK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469410 (CHEMBL4277886) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458528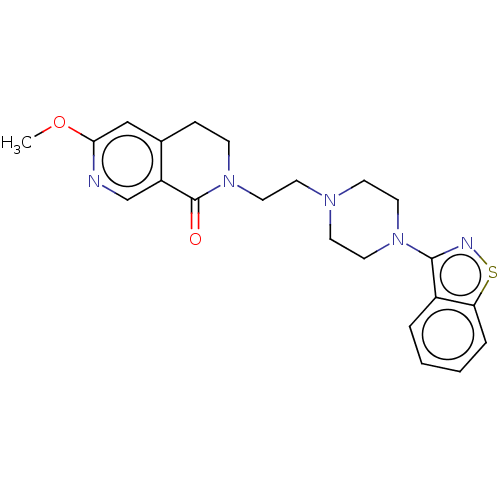 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458634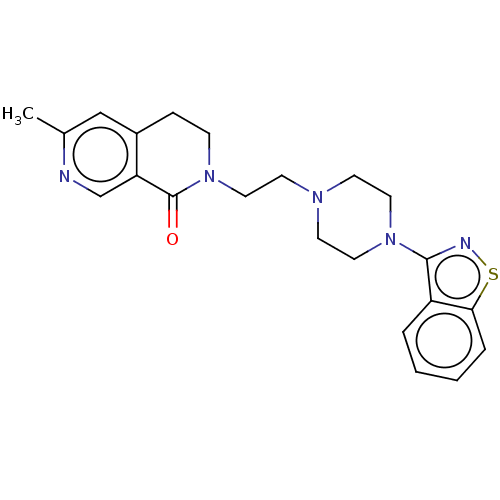 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50004276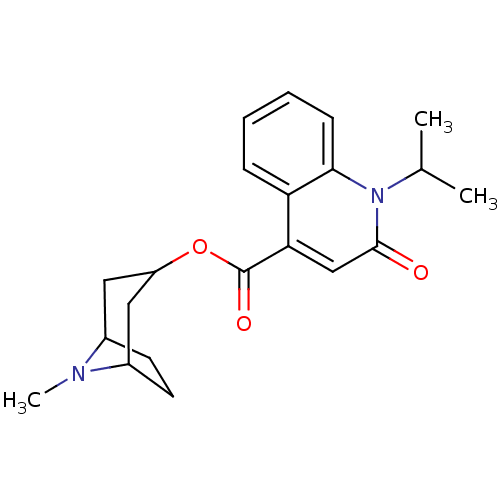 (1-Isopropyl-2-oxo-1,2-dihydro-quinoline-4-carboxyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Ability to displace [3H]quipazine binding to 5-hydroxytryptamine 3 receptor sites in NG 108-15. | J Med Chem 35: 4893-902 (1992) BindingDB Entry DOI: 10.7270/Q2BK1CZ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50295693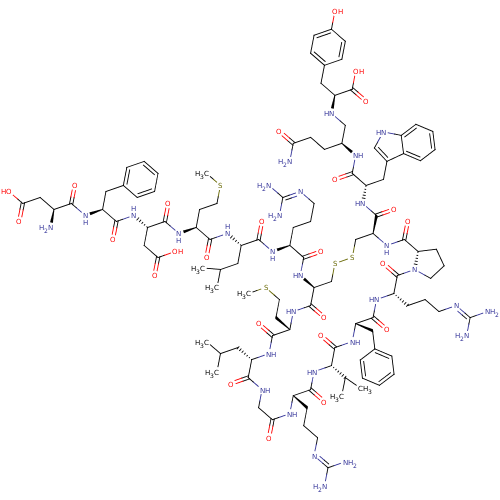 (CHEMBL557629) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [35S] 4-(1-(3,4-difluorophenyl)-2-(ethyl(3-(6-fluoro-3H-spiro[isobenzofuran-1,4'-piperidine]-1'-yl)propyl)amino)-2-oxoethyl)-3-oxopip... | Bioorg Med Chem Lett 19: 2835-9 (2009) Article DOI: 10.1016/j.bmcl.2009.03.102 BindingDB Entry DOI: 10.7270/Q2R49QS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580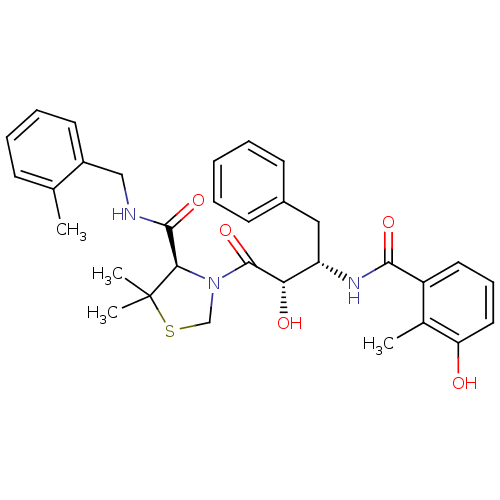 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071565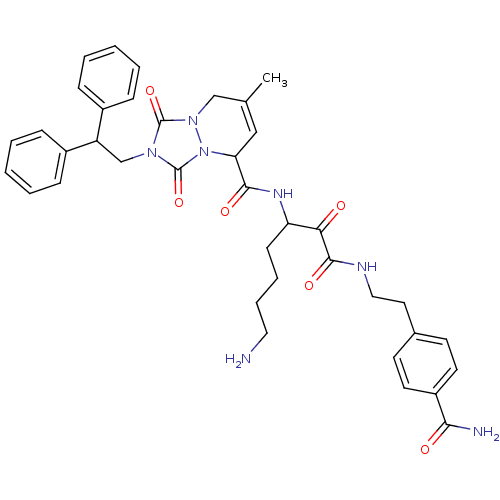 (2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to the thrombin | Bioorg Med Chem Lett 8: 2321-6 (1999) BindingDB Entry DOI: 10.7270/Q29C6WKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 32144 total ) | Next | Last >> |