

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118462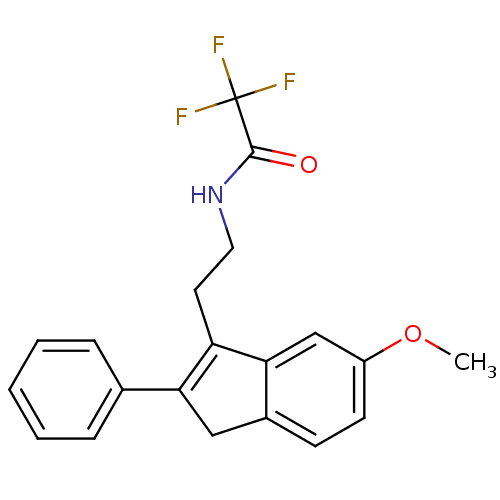 (2,2,2-Trifluoro-N-[2-(6-methoxy-2-phenyl-3H-inden-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00602 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50343599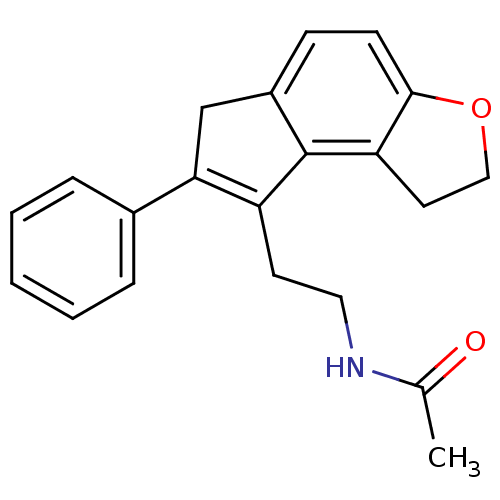 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT2 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50343599 (CHEMBL1774522 | N-[2-(7-Phenyl-1,6-dihydro-2H-inde...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118435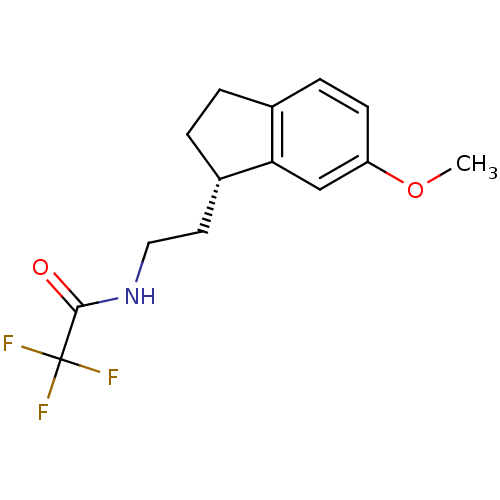 (2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50118470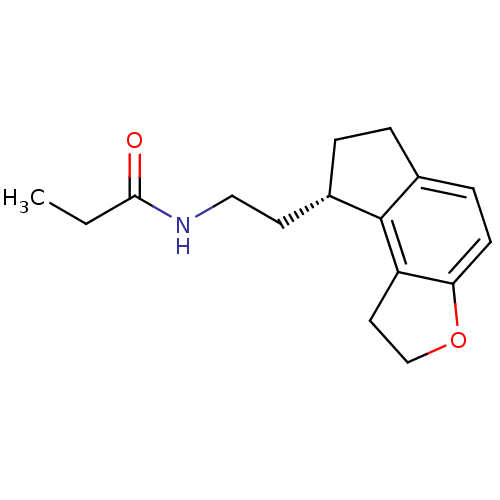 (CHEMBL1218 | N-[2-(1,6,7,8-Tetrahydro-2H-3-oxa-as-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human MT1 expressed in CHO cells | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495071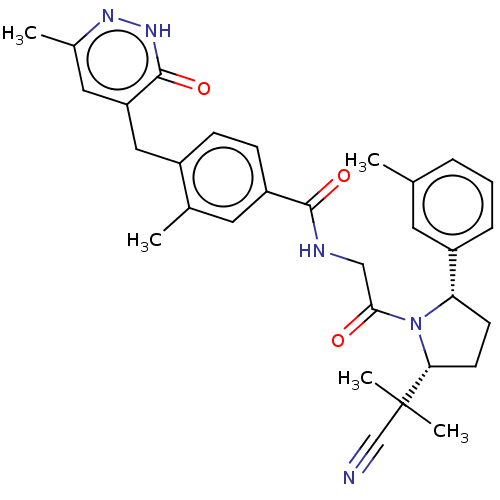 (US10995084, Ex. No. 3A-8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118446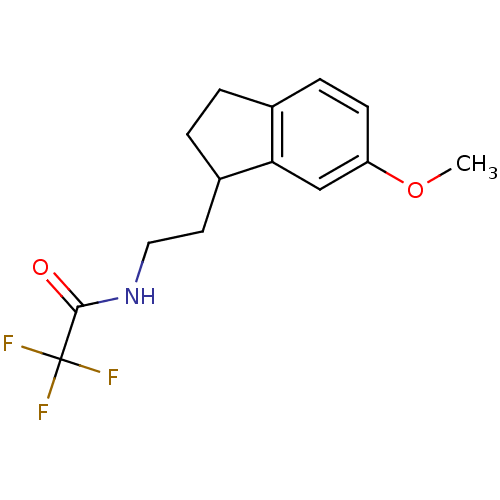 (2,2,2-Trifluoro-N-[2-(6-methoxy-indan-1-yl)-ethyl]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118456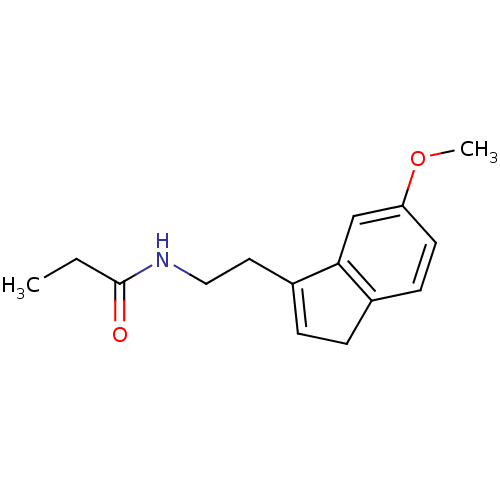 (CHEMBL334645 | N-[2-(6-Methoxy-3H-inden-1-yl)-ethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50419354 (GR205171A | VOFOPITANT DIHYDROCHLORIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description Displacement of [125I]BH-SP from NK1 receptor in human IM9 cells after 30 mins by scintillation counting | Bioorg Med Chem 19: 6430-46 (2011) Article DOI: 10.1016/j.bmc.2011.08.070 BindingDB Entry DOI: 10.7270/Q228081R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347590 (CHEMBL1802028) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50575683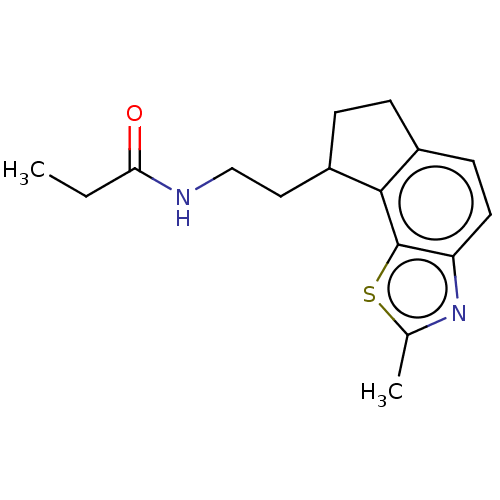 (CHEMBL4868554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50143784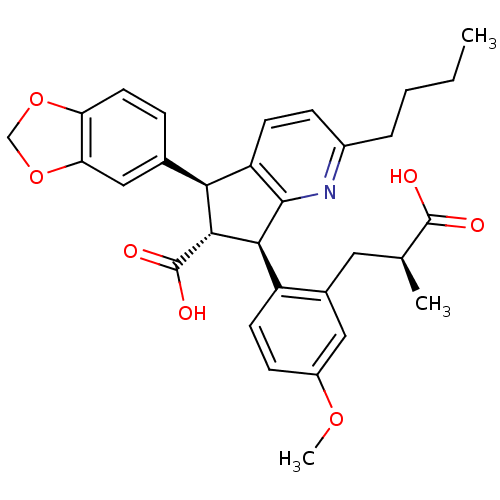 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-butyl-7-[2-((...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 289: 1262-70 (1999) BindingDB Entry DOI: 10.7270/Q2Q52N5Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495037 (US10995084, Ex. No. 1A-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103443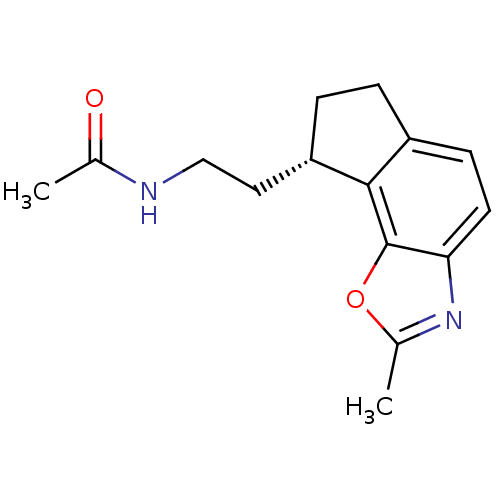 (US8552037, 90) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118453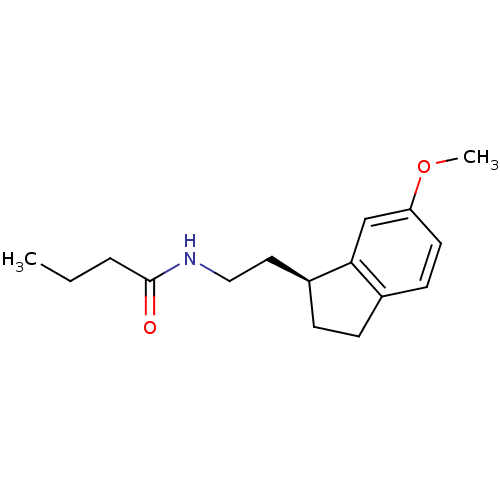 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-butyramide ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0321 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103449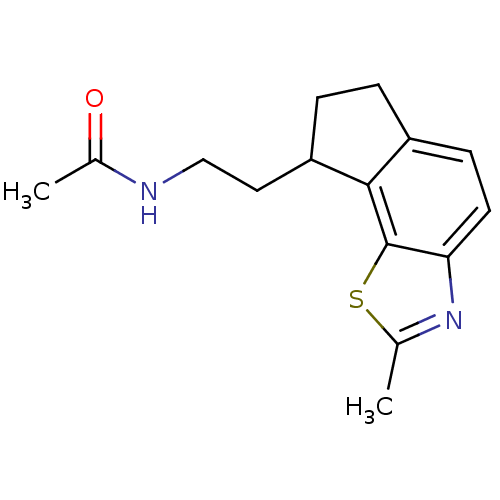 (US8552037, 97) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50420815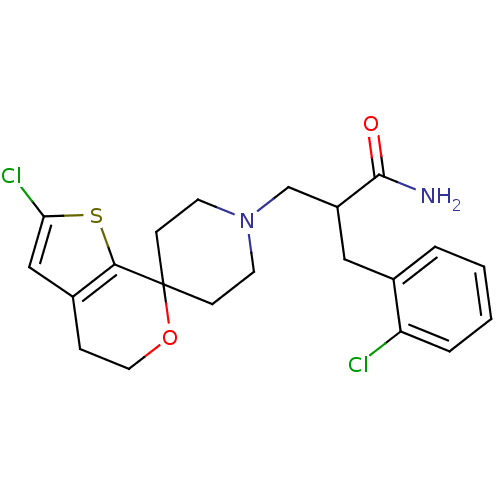 (CHEMBL2088054) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Agonist activity at human cloned NOP receptor expressed in CHO cell membranes after 30 mins by [35S]GTPgammaS binding assay | J Med Chem 55: 4955-67 (2012) Article DOI: 10.1021/jm201629q BindingDB Entry DOI: 10.7270/Q2TQ62SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347593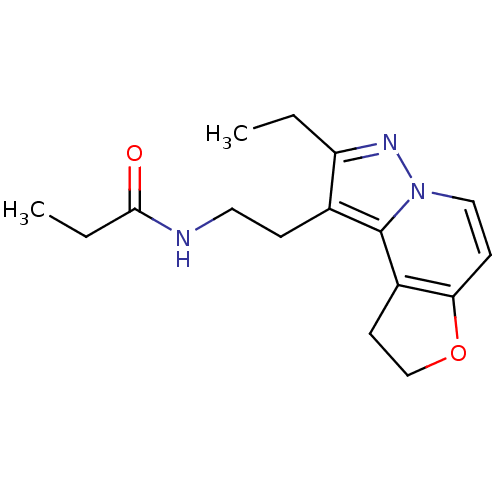 (CHEMBL1802026) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495155 (US10995084, Ex. No. 5C-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495051 (US10995084, Ex. No. 2B-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495100 (US10995084, Ex. No. 4B-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118440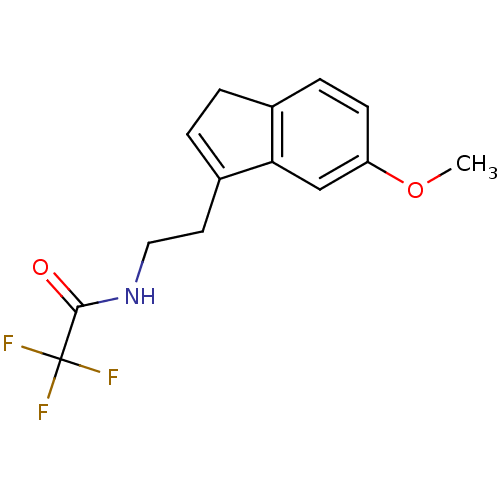 (2,2,2-Trifluoro-N-[2-(6-methoxy-3H-inden-1-yl)-eth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0408 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118430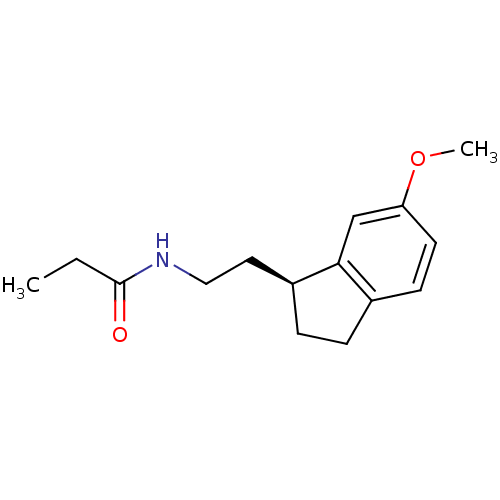 ((S) N-[2-(6-Methoxy-indan-1-yl)-ethyl]-propionamid...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347589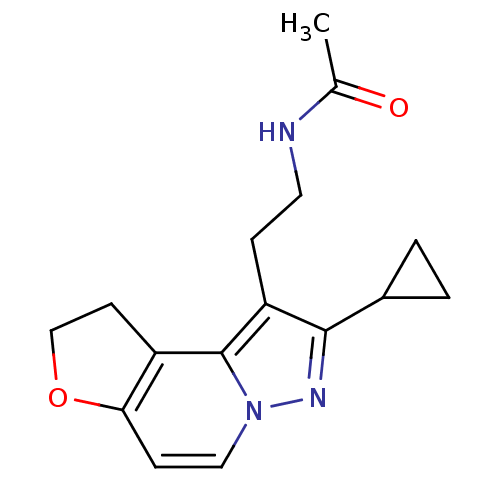 (CHEMBL1802027) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118463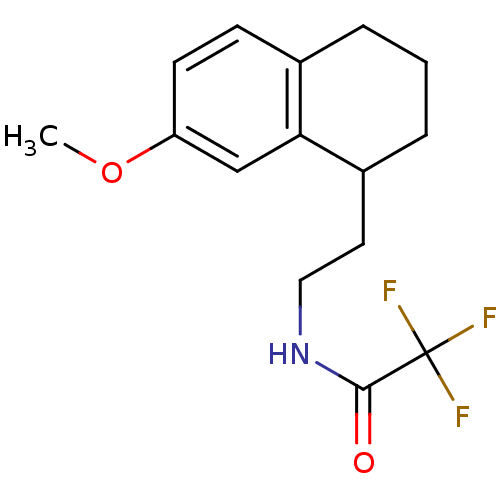 (2,2,2-Trifluoro-N-[2-(7-methoxy-1,2,3,4-tetrahydro...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0469 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495145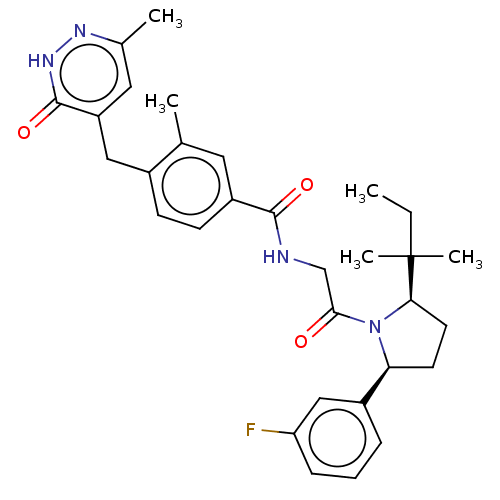 (US10995084, Ex. No. 5A-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495072 (US10995084, Ex. No. 3A-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495101 (US10995084, Ex. No. 4B-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495102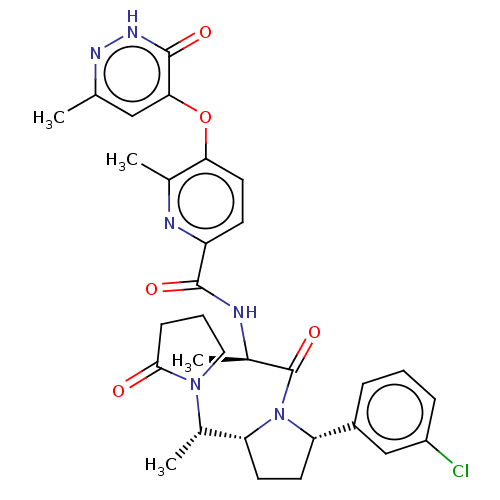 (US10995084, Ex. No. 4B-8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495135 (US10995084, Ex. No. 4B-41) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495127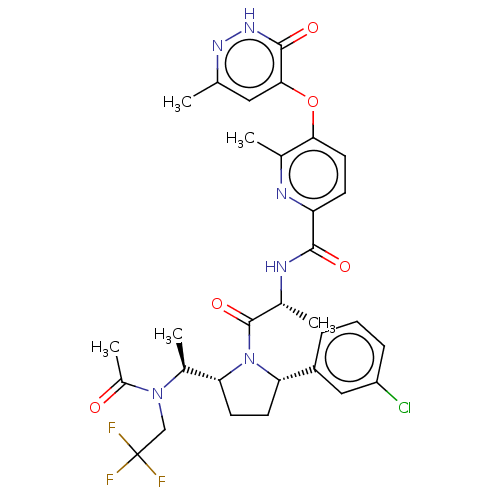 (US10995084, Ex. No. 4B-33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495041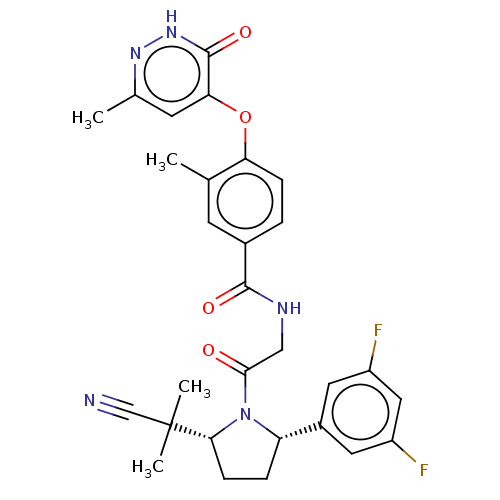 (US10995084, Ex. No. 2A-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495042 (US10995084, Ex. No. 2B-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495054 (US10995084, Ex. No. 2B-12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A/1B (Homo sapiens (Human)) | BDBM50118450 (CHEMBL335437 | N-[2-(6-Methoxy-indan-1-yl)-ethyl]-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0553 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd. Curated by ChEMBL | Assay Description Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells. | J Med Chem 45: 4212-21 (2002) BindingDB Entry DOI: 10.7270/Q2J102HG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50575683 (CHEMBL4868554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT2 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495036 (US10995084, Ex. No. 1A-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495125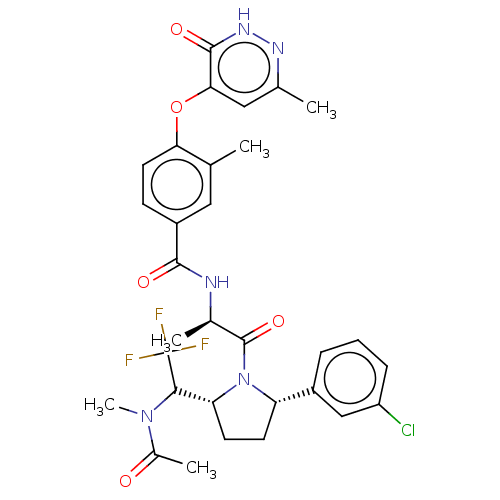 (US10995084, Ex. No. 4B-31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495124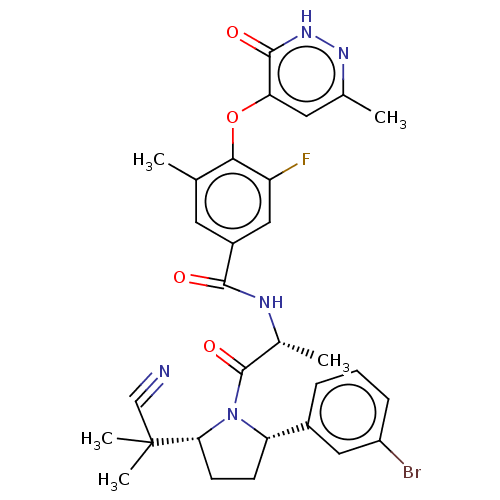 (US10995084, Ex. No. 4B-30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495119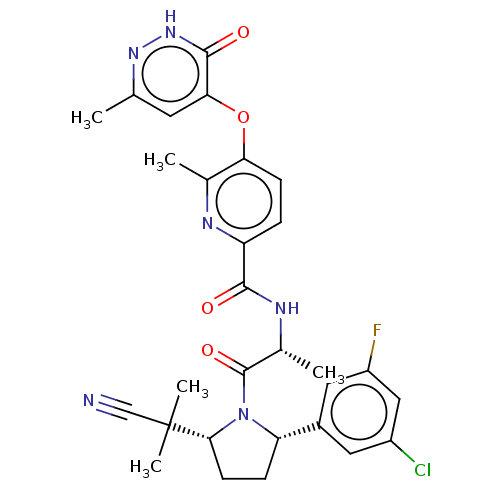 (US10995084, Ex. No. 4B-25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495126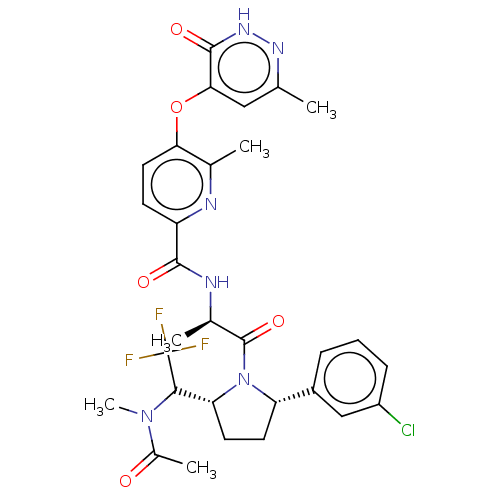 (US10995084, Ex. No. 4B-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495092 (US10995084, Ex. No. 4A-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495099 (US10995084, Ex. No. 4B-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM495153 (US10995084, Ex. No. 5B-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The binding affinity assay of compounds for human CGRP receptor was carried out by inhibition of radiolabeled ligand [125I]-CGRP binding in human neu... | US Patent US10995084 (2021) BindingDB Entry DOI: 10.7270/Q24T6NH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50347588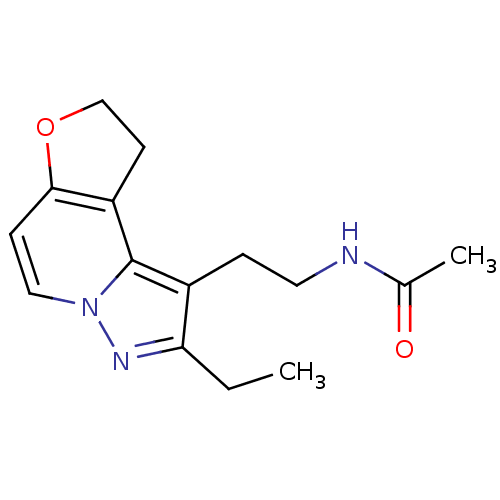 (CHEMBL1802025) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Displacement of 2-[125I]iodomelatonin from human MT1 receptor expressed in CHO cells | J Med Chem 54: 4207-18 (2011) Article DOI: 10.1021/jm200385u BindingDB Entry DOI: 10.7270/Q2HT2PN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004193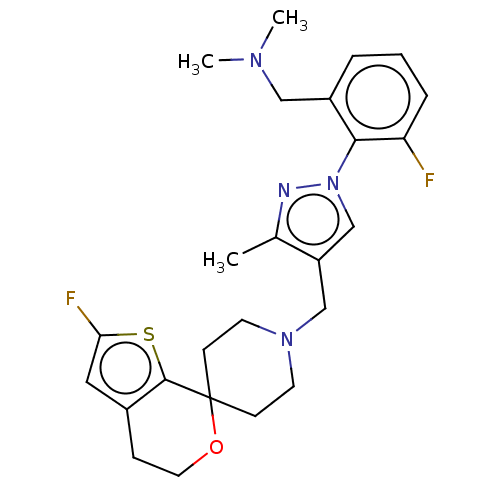 (CHEMBL3236476) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0648 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centro de Investigaci�n Lilly Curated by ChEMBL | Assay Description Displacement of [3H]-nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by scintillation counting | J Med Chem 57: 3418-29 (2014) Article DOI: 10.1021/jm500117r BindingDB Entry DOI: 10.7270/Q26Q1ZS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM103445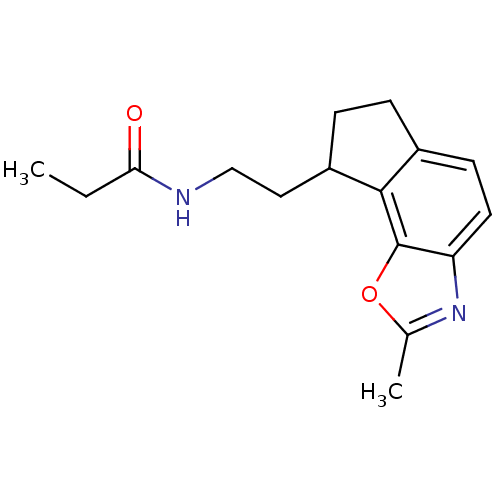 (US8552037, 92) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of 2-[125l]-lodomelatonin from human MT1 expressed in CHO cell membrane incubated for 150 mins by radioligand binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01836 BindingDB Entry DOI: 10.7270/Q25H7M2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 29821 total ) | Next | Last >> |