 Found 2107 hits with Last Name = 'shih' and Initial = 'n'
Found 2107 hits with Last Name = 'shih' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186527

((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...)Show SMILES C[C@@H](OCC1(CC(N)C1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,(-2.49,.49,;-2.49,-1.05,;-1.15,-1.81,;.18,-1.04,;1.52,-1.8,;.45,-2.91,;1.56,-3.98,;1.59,-5.52,;2.63,-2.87,;2.86,-1.02,;4.18,-1.79,;5.51,-1.03,;5.51,.51,;4.18,1.27,;2.85,.51,;-3.82,-1.82,;-5.15,-1.06,;-6.48,-1.83,;-6.48,-3.37,;-5.15,-4.14,;-3.81,-3.37,;-5.15,-5.68,;-6.7,-5.67,;-3.62,-5.68,;-5.14,-7.22,;-7.82,-1.06,;-7.05,.28,;-8.59,-2.39,;-9.15,-.29,)| Show InChI InChI=1S/C21H21F6NO/c1-13(14-7-16(20(22,23)24)9-17(8-14)21(25,26)27)29-12-19(10-18(28)11-19)15-5-3-2-4-6-15/h2-9,13,18H,10-12,28H2,1H3/t13-,18?,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26526
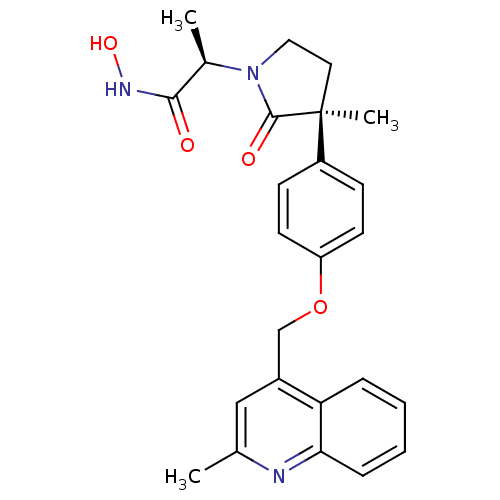
((2R)-N-hydroxy-2-[(3S)-3-methyl-3-{4-[(2-methylqui...)Show SMILES C[C@@H](N1CC[C@](C)(C1=O)c1ccc(OCc2cc(C)nc3ccccc23)cc1)C(=O)NO |r| Show InChI InChI=1S/C25H27N3O4/c1-16-14-18(21-6-4-5-7-22(21)26-16)15-32-20-10-8-19(9-11-20)25(3)12-13-28(24(25)30)17(2)23(29)27-31/h4-11,14,17,31H,12-13,15H2,1-3H3,(H,27,29)/t17-,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332270
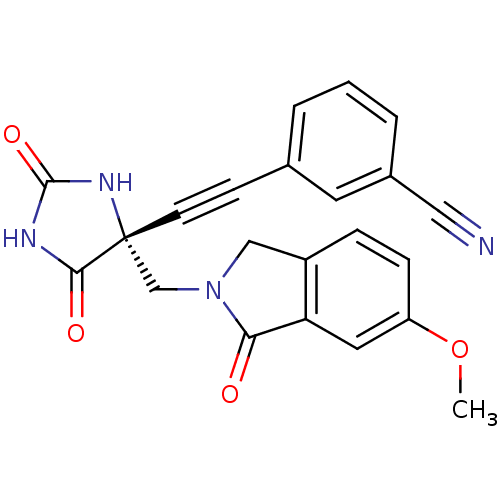
((R)-3-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(c3)C#N)C(=O)c2c1 |r| Show InChI InChI=1S/C22H16N4O4/c1-30-17-6-5-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)8-7-14-3-2-4-15(9-14)11-23/h2-6,9-10H,12-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332292
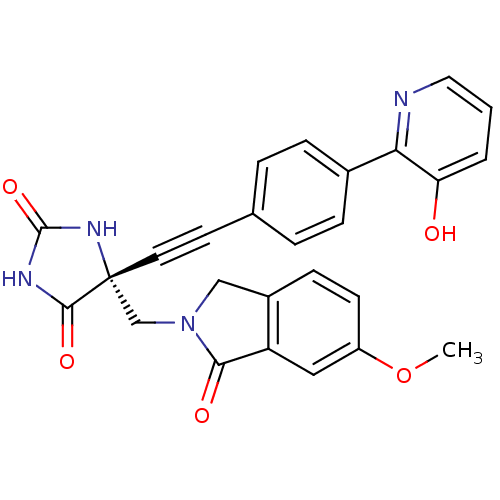
((R)-5-((4-(3-hydroxypyridin-2-yl)phenyl)ethynyl)-5...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)-c3ncccc3O)C(=O)c2c1 |r| Show InChI InChI=1S/C26H20N4O5/c1-35-19-9-8-18-14-30(23(32)20(18)13-19)15-26(24(33)28-25(34)29-26)11-10-16-4-6-17(7-5-16)22-21(31)3-2-12-27-22/h2-9,12-13,31H,14-15H2,1H3,(H2,28,29,33,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325003
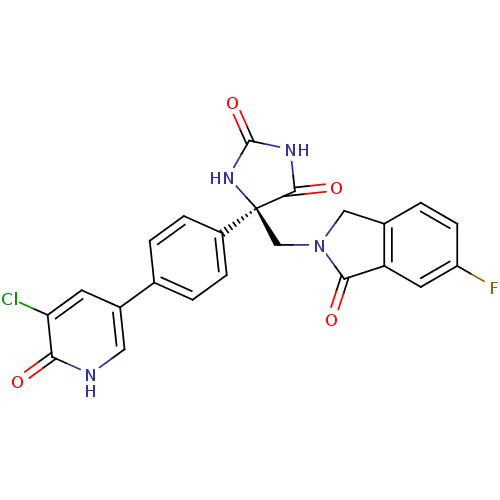
((R)-5-(4-(5-chloro-6-oxo-1,6-dihydropyridin-3-yl)p...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3c[nH]c(=O)c(Cl)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H16ClFN4O4/c24-18-7-14(9-26-19(18)30)12-1-4-15(5-2-12)23(21(32)27-22(33)28-23)11-29-10-13-3-6-16(25)8-17(13)20(29)31/h1-9H,10-11H2,(H,26,30)(H2,27,28,32,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50264895
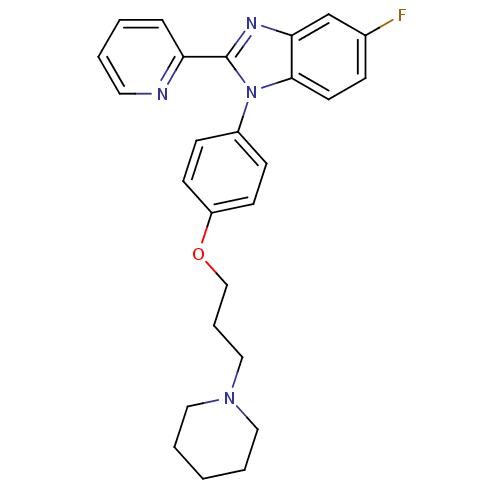
(5-fluoro-1-(4-(3-(piperidin-1-yl)propoxy)phenyl)-2...)Show SMILES Fc1ccc2n(c(nc2c1)-c1ccccn1)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C26H27FN4O/c27-20-8-13-25-24(19-20)29-26(23-7-2-3-14-28-23)31(25)21-9-11-22(12-10-21)32-18-6-17-30-15-4-1-5-16-30/h2-3,7-14,19H,1,4-6,15-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in HEK293 cells assessed as reversal of N-alpha-methylhistamine-induced inhibition of fo... |
Bioorg Med Chem Lett 18: 5032-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.008
BindingDB Entry DOI: 10.7270/Q2CZ370Q |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186522
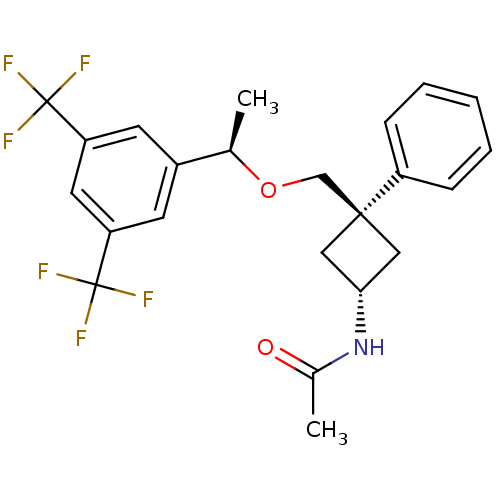
(CHEMBL379072 | trans-N-{3-[(R)-1-(3,5-bis-trifluor...)Show SMILES C[C@@H](OC[C@]1(C[C@@H](C1)NC(C)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,6.8,wD:4.3,(.79,-3.02,;.8,-4.56,;2.13,-5.33,;3.46,-4.55,;4.8,-5.32,;3.73,-6.43,;4.84,-7.49,;5.91,-6.38,;4.88,-9.03,;3.56,-9.83,;3.59,-11.37,;2.21,-9.09,;6.13,-4.54,;7.46,-5.31,;8.79,-4.54,;8.78,-2.99,;7.44,-2.23,;6.11,-3.01,;-.54,-5.34,;-1.87,-4.57,;-3.2,-5.34,;-3.2,-6.89,;-1.87,-7.66,;-.53,-6.89,;-1.87,-9.2,;-3.41,-9.19,;-.33,-9.19,;-1.85,-10.74,;-4.53,-4.57,;-3.77,-3.24,;-5.31,-5.9,;-5.87,-3.8,)| Show InChI InChI=1S/C23H23F6NO2/c1-14(16-8-18(22(24,25)26)10-19(9-16)23(27,28)29)32-13-21(17-6-4-3-5-7-17)11-20(12-21)30-15(2)31/h3-10,14,20H,11-13H2,1-2H3,(H,30,31)/t14-,20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM102669
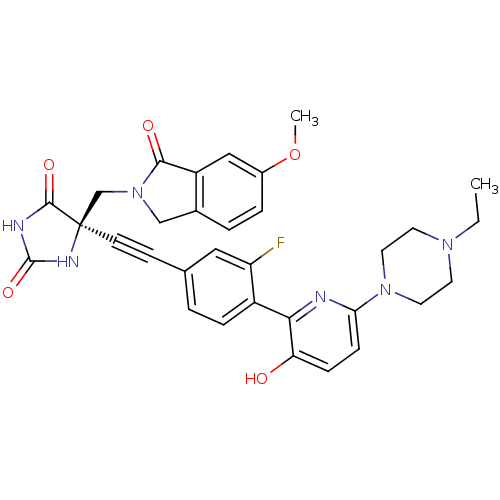
(CHEMBL1288726 | US8541572, 976)Show SMILES CCN1CCN(CC1)c1ccc(O)c(n1)-c1ccc(cc1F)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r| Show InChI InChI=1S/C32H31FN6O5/c1-3-37-12-14-38(15-13-37)27-9-8-26(40)28(34-27)23-7-4-20(16-25(23)33)10-11-32(30(42)35-31(43)36-32)19-39-18-21-5-6-22(44-2)17-24(21)29(39)41/h4-9,16-17,40H,3,12-15,18-19H2,1-2H3,(H2,35,36,42,43)/t32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332265

((R)-5-((2-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3F)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-15-7-6-14-11-25(18(26)16(14)10-15)12-21(19(27)23-20(28)24-21)9-8-13-4-2-3-5-17(13)22/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26524

((1R,2S)-1-({3-fluoro-4-[(2-phenylquinolin-4-yl)met...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)c(F)c2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H24FN3O4/c29-22-12-17(14-28(27(30)34)15-21(28)26(33)32-35)10-11-25(22)36-16-19-13-24(18-6-2-1-3-7-18)31-23-9-5-4-8-20(19)23/h1-13,21,35H,14-16H2,(H2,30,34)(H,32,33)/t21-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332262
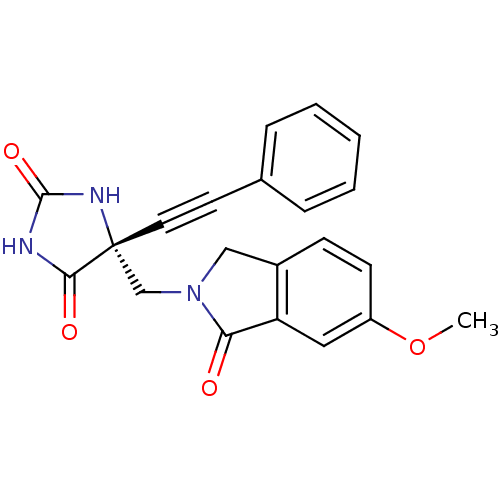
((R)-5-((6-methoxy-1-oxoisoindolin-2-yl)methyl)-5-(...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccccc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O4/c1-28-16-8-7-15-12-24(18(25)17(15)11-16)13-21(19(26)22-20(27)23-21)10-9-14-5-3-2-4-6-14/h2-8,11H,12-13H2,1H3,(H2,22,23,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328970
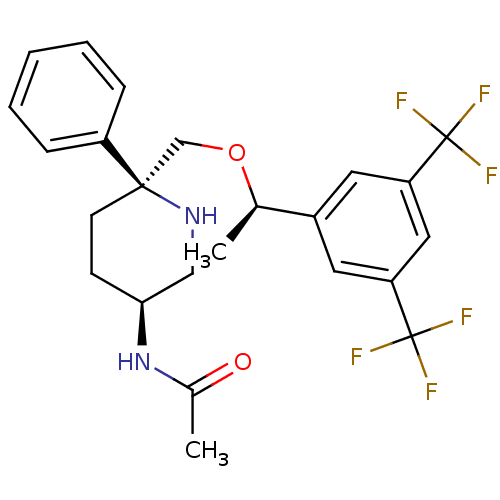
(CHEMBL1270066 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...)Show SMILES C[C@@H](OC[C@]1(CC[C@@H](CN1)NC(C)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C24H26F6N2O2/c1-15(17-10-19(23(25,26)27)12-20(11-17)24(28,29)30)34-14-22(18-6-4-3-5-7-18)9-8-21(13-31-22)32-16(2)33/h3-7,10-12,15,21,31H,8-9,13-14H2,1-2H3,(H,32,33)/t15-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332289

((R)-5-((4-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cc1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C28H30N6O5/c1-3-32-12-14-33(15-13-32)24(31-38)20-6-4-19(5-7-20)10-11-28(26(36)29-27(37)30-28)18-34-17-21-8-9-22(39-2)16-23(21)25(34)35/h4-9,16,38H,3,12-15,17-18H2,1-2H3,(H2,29,30,36,37)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332288
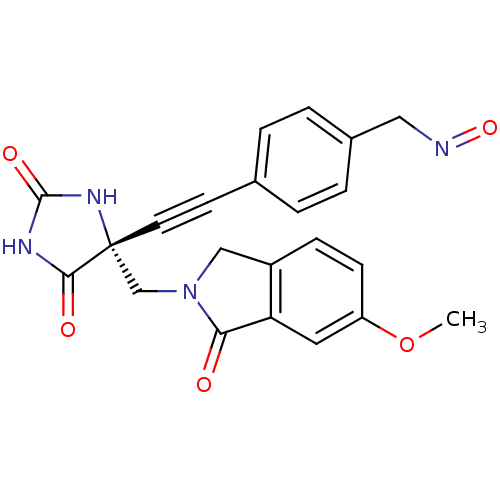
((R)-4-((4-((6-methoxy-1-oxoisoindolin-2-yl)methyl)...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(CN=O)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C22H18N4O5/c1-31-17-7-6-16-12-26(19(27)18(16)10-17)13-22(20(28)24-21(29)25-22)9-8-14-2-4-15(5-3-14)11-23-30/h2-7,10H,11-13H2,1H3,(H2,24,25,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332278
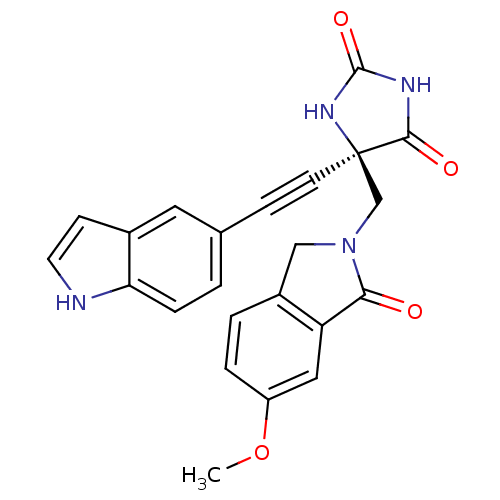
((R)-5-((1H-indol-5-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4[nH]ccc4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-4-3-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-5-19-15(10-14)7-9-24-19/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17 [215-477,S266A,N452Q]
(Homo sapiens (Human)) | BDBM26525
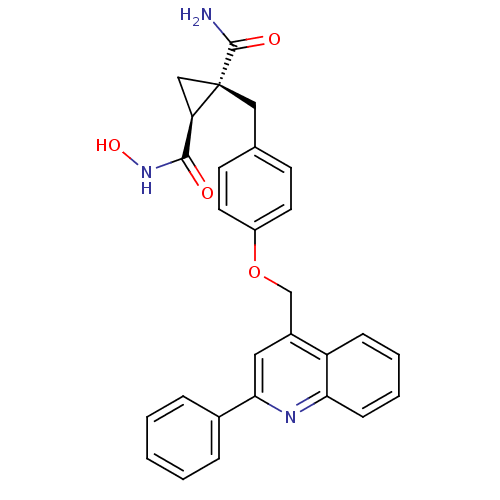
((1S,2R)-1-N-hydroxy-2-({4-[(2-phenylquinolin-4-yl)...)Show SMILES NC(=O)[C@@]1(Cc2ccc(OCc3cc(nc4ccccc34)-c3ccccc3)cc2)C[C@@H]1C(=O)NO |r| Show InChI InChI=1S/C28H25N3O4/c29-27(33)28(16-23(28)26(32)31-34)15-18-10-12-21(13-11-18)35-17-20-14-25(19-6-2-1-3-7-19)30-24-9-5-4-8-22(20)24/h1-14,23,34H,15-17H2,(H2,29,33)(H,31,32)/t23-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.180 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
Schering-Plough Research Institute
| Assay Description
Enzyme activity was determined by a kinetic assay measuring the rate of increase in fluorescent intensity generated by the cleavage of an internally ... |
Bioorg Med Chem Lett 19: 54-7 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.034
BindingDB Entry DOI: 10.7270/Q2JH3JH7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332290
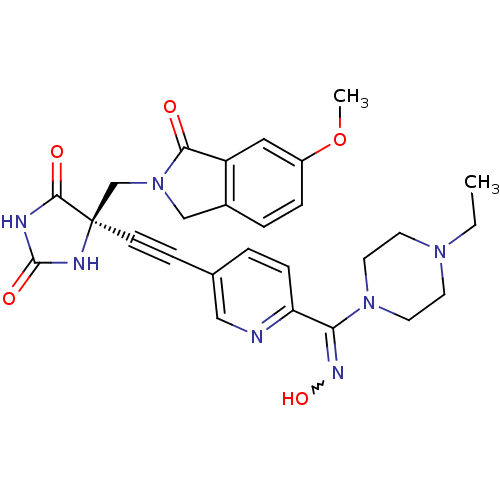
((R)-5-((6-((4-ethylpiperazin-1-yl)(hydroxyimino)me...)Show SMILES CCN1CCN(CC1)C(=NO)c1ccc(cn1)C#C[C@]1(CN2Cc3ccc(OC)cc3C2=O)NC(=O)NC1=O |r,w:9.10| Show InChI InChI=1S/C27H29N7O5/c1-3-32-10-12-33(13-11-32)23(31-38)22-7-4-18(15-28-22)8-9-27(25(36)29-26(37)30-27)17-34-16-19-5-6-20(39-2)14-21(19)24(34)35/h4-7,14-15,38H,3,10-13,16-17H2,1-2H3,(H2,29,30,36,37)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332279
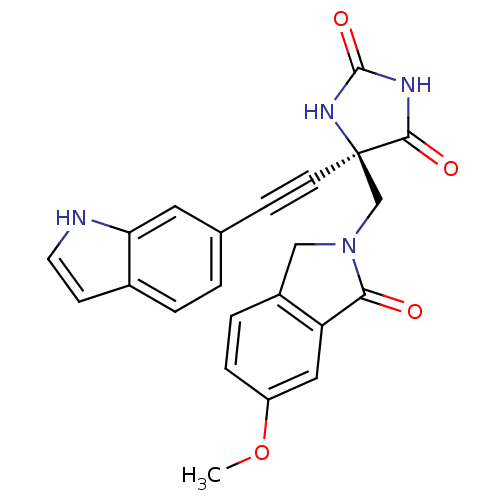
((R)-5-((1H-indol-6-yl)ethynyl)-5-((6-methoxy-1-oxo...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc4cc[nH]c4c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N4O4/c1-31-17-5-4-16-12-27(20(28)18(16)11-17)13-23(21(29)25-22(30)26-23)8-6-14-2-3-15-7-9-24-19(15)10-14/h2-5,7,9-11,24H,12-13H2,1H3,(H2,25,26,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328981
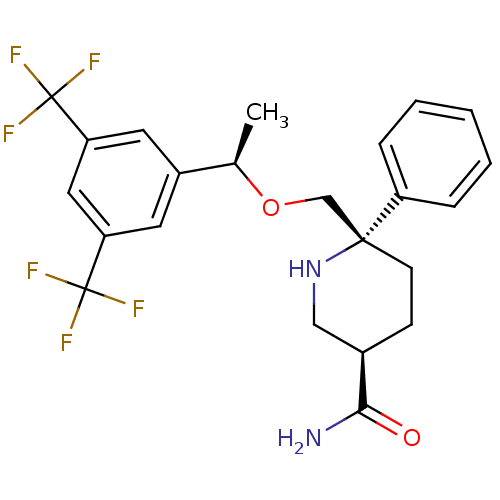
((3R,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...)Show SMILES C[C@@H](OC[C@]1(CC[C@H](CN1)C(N)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F6N2O2/c1-14(16-9-18(22(24,25)26)11-19(10-16)23(27,28)29)33-13-21(17-5-3-2-4-6-17)8-7-15(12-31-21)20(30)32/h2-6,9-11,14-15,31H,7-8,12-13H2,1H3,(H2,30,32)/t14-,15-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50343977
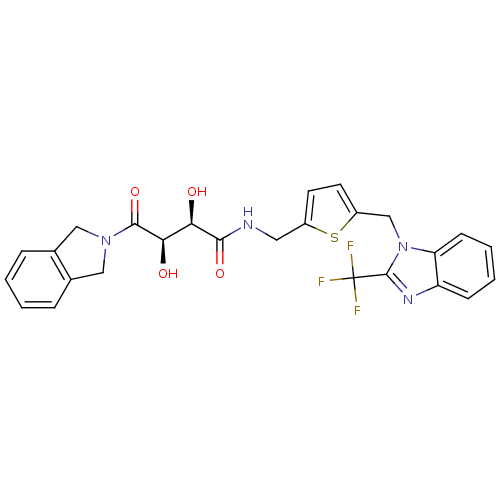
((2R,3R)-2,3-dihydroxy-4-(isoindolin-2-yl)-4-oxo-N-...)Show SMILES O[C@H]([C@@H](O)C(=O)N1Cc2ccccc2C1)C(=O)NCc1ccc(Cn2c(nc3ccccc23)C(F)(F)F)s1 |r| Show InChI InChI=1S/C26H23F3N4O4S/c27-26(28,29)25-31-19-7-3-4-8-20(19)33(25)14-18-10-9-17(38-18)11-30-23(36)21(34)22(35)24(37)32-12-15-5-1-2-6-16(15)13-32/h1-10,21-22,34-35H,11-14H2,(H,30,36)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 4812-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.104
BindingDB Entry DOI: 10.7270/Q2GX4BWK |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50116083
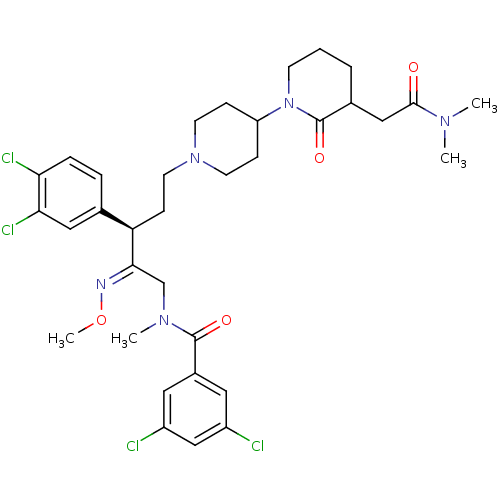
(3,5-Dichloro-N-[3-(3,4-dichloro-phenyl)-5-(3-dimet...)Show SMILES CO\N=C(/CN(C)C(=O)c1cc(Cl)cc(Cl)c1)[C@H](CCN1CCC(CC1)N1CCCC(CC(=O)N(C)C)C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H43Cl4N5O4/c1-40(2)32(44)19-23-6-5-12-43(34(23)46)27-9-13-42(14-10-27)15-11-28(22-7-8-29(37)30(38)18-22)31(39-47-4)21-41(3)33(45)24-16-25(35)20-26(36)17-24/h7-8,16-18,20,23,27-28H,5-6,9-15,19,21H2,1-4H3/b39-31+/t23?,28-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding to Tachykinin receptor 2 |
Bioorg Med Chem Lett 12: 2125-8 (2002)
BindingDB Entry DOI: 10.7270/Q2JQ11JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50243045
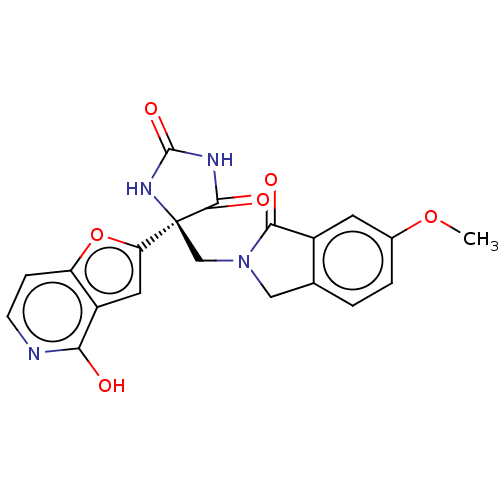
(CHEMBL4095412)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3cc4c(O)nccc4o3)C(=O)c2c1 |r| Show InChI InChI=1S/C20H16N4O6/c1-29-11-3-2-10-8-24(17(26)12(10)6-11)9-20(18(27)22-19(28)23-20)15-7-13-14(30-15)4-5-21-16(13)25/h2-7H,8-9H2,1H3,(H,21,25)(H2,22,23,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain TACE (unknown origin) by FRET assay |
Bioorg Med Chem Lett 27: 3037-3042 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.062
BindingDB Entry DOI: 10.7270/Q2319Z8G |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50243056
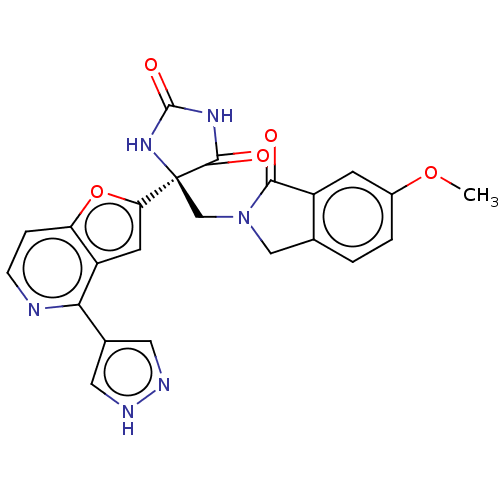
(CHEMBL4085232)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3cc4c(nccc4o3)-c3cn[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H18N6O5/c1-33-14-3-2-12-10-29(20(30)15(12)6-14)11-23(21(31)27-22(32)28-23)18-7-16-17(34-18)4-5-24-19(16)13-8-25-26-9-13/h2-9H,10-11H2,1H3,(H,25,26)(H2,27,28,31,32)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain TACE (unknown origin) by FRET assay |
Bioorg Med Chem Lett 27: 3037-3042 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.062
BindingDB Entry DOI: 10.7270/Q2319Z8G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329148
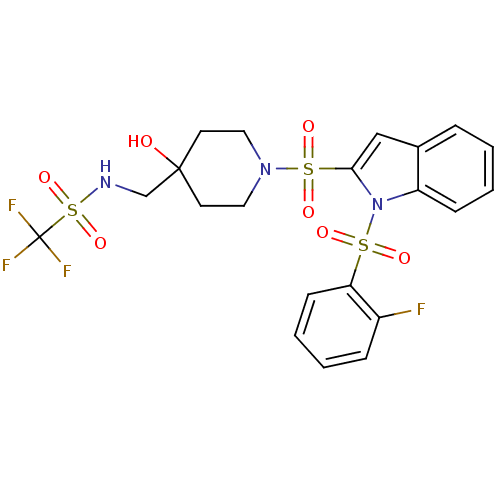
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES OC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H21F4N3O7S3/c22-16-6-2-4-8-18(16)36(30,31)28-17-7-3-1-5-15(17)13-19(28)37(32,33)27-11-9-20(29,10-12-27)14-26-38(34,35)21(23,24)25/h1-8,13,26,29H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50325002
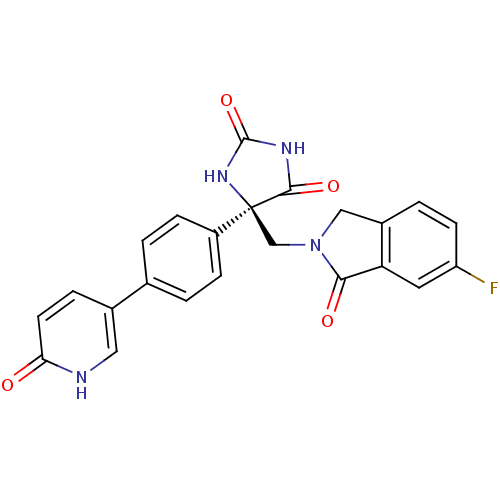
((R)-5-((6-fluoro-1-oxoisoindolin-2-yl)methyl)-5-(4...)Show SMILES Fc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3ccc(cc3)-c3ccc(=O)[nH]c3)C(=O)c2c1 |r| Show InChI InChI=1S/C23H17FN4O4/c24-17-7-3-15-11-28(20(30)18(15)9-17)12-23(21(31)26-22(32)27-23)16-5-1-13(2-6-16)14-4-8-19(29)25-10-14/h1-10H,11-12H2,(H,25,29)(H2,26,27,31,32)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE assessed as inhibition of pro-TNFalpha peptide cleavage |
Bioorg Med Chem Lett 20: 5286-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.134
BindingDB Entry DOI: 10.7270/Q26W9B8K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50305990
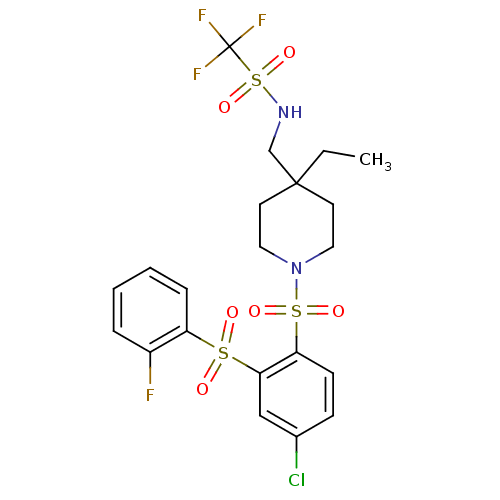
(CHEMBL596388 | N-((1-(4-chloro-2-(2-fluorophenylsu...)Show SMILES CCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H23ClF4N2O6S3/c1-2-20(14-27-37(33,34)21(24,25)26)9-11-28(12-10-20)36(31,32)18-8-7-15(22)13-19(18)35(29,30)17-6-4-3-5-16(17)23/h3-8,13,27H,2,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50116745
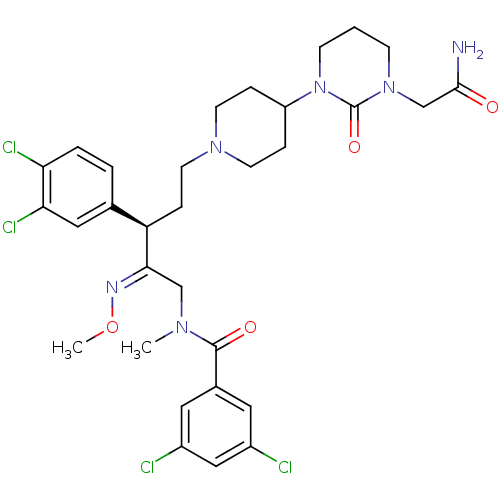
(CHEMBL78284 | N-{(R)-5-[4-(3-Carbamoylmethyl-2-oxo...)Show SMILES CO\N=C(/CN(C)C(=O)c1cc(Cl)cc(Cl)c1)[C@H](CCN1CCC(CC1)N1CCCN(CC(N)=O)C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H38Cl4N6O4/c1-38(30(43)21-14-22(32)17-23(33)15-21)18-28(37-45-2)25(20-4-5-26(34)27(35)16-20)8-13-39-11-6-24(7-12-39)41-10-3-9-40(31(41)44)19-29(36)42/h4-5,14-17,24-25H,3,6-13,18-19H2,1-2H3,(H2,36,42)/b37-28+/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human tachykinin receptor 2 in CHO cells using [3H]-NKA as radioligand |
Bioorg Med Chem Lett 12: 2355-8 (2002)
BindingDB Entry DOI: 10.7270/Q26T0KXW |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50175828
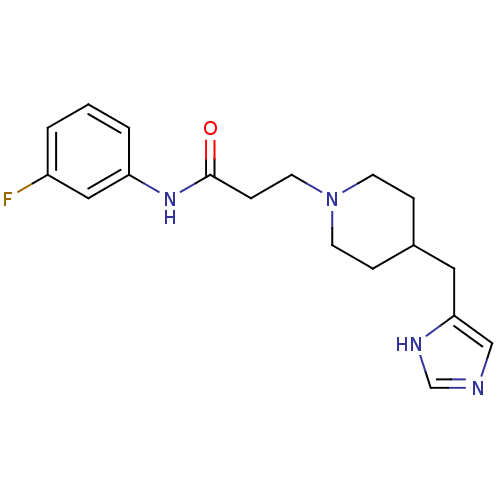
(3-(4-((1H-imidazol-4-yl)methyl)piperidin-1-yl)-N-(...)Show InChI InChI=1S/C18H23FN4O/c19-15-2-1-3-16(11-15)22-18(24)6-9-23-7-4-14(5-8-23)10-17-12-20-13-21-17/h1-3,11-14H,4-10H2,(H,20,21)(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in guinea pig brain |
Bioorg Med Chem Lett 16: 395-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.076
BindingDB Entry DOI: 10.7270/Q29S1QK1 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM50175843
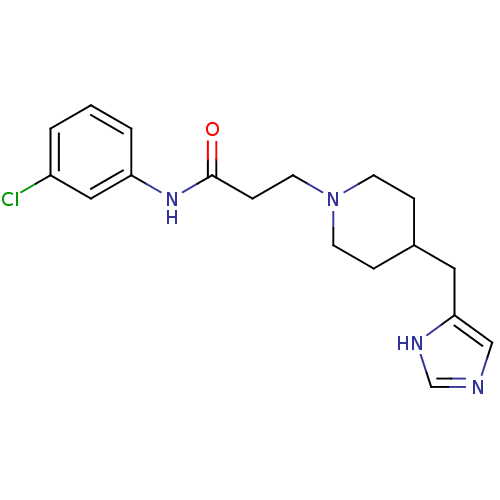
(3-(4-((1H-imidazol-4-yl)methyl)piperidin-1-yl)-N-(...)Show InChI InChI=1S/C18H23ClN4O/c19-15-2-1-3-16(11-15)22-18(24)6-9-23-7-4-14(5-8-23)10-17-12-20-13-21-17/h1-3,11-14H,4-10H2,(H,20,21)(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in guinea pig brain |
Bioorg Med Chem Lett 16: 395-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.076
BindingDB Entry DOI: 10.7270/Q29S1QK1 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50243428
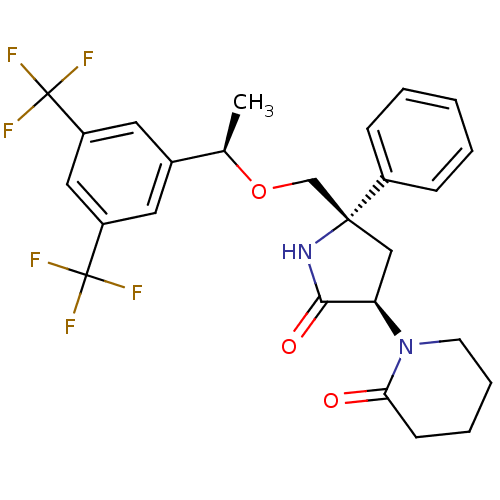
(1-((3R,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](OC[C@]1(C[C@@H](N2CCCCC2=O)C(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H26F6N2O3/c1-16(17-11-19(25(27,28)29)13-20(12-17)26(30,31)32)37-15-24(18-7-3-2-4-8-18)14-21(23(36)33-24)34-10-6-5-9-22(34)35/h2-4,7-8,11-13,16,21H,5-6,9-10,14-15H2,1H3,(H,33,36)/t16-,21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 4168-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.082
BindingDB Entry DOI: 10.7270/Q2D79B7F |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50243427
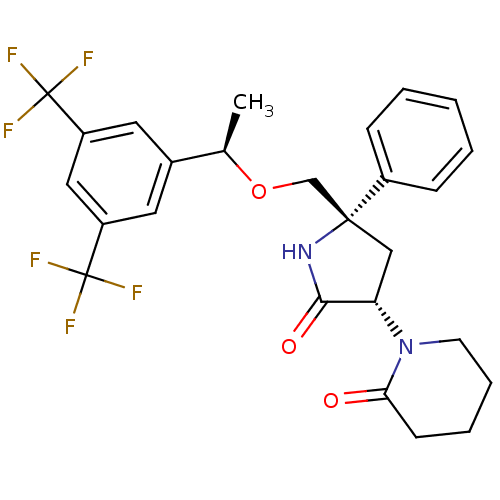
(1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](OC[C@]1(C[C@H](N2CCCCC2=O)C(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H26F6N2O3/c1-16(17-11-19(25(27,28)29)13-20(12-17)26(30,31)32)37-15-24(18-7-3-2-4-8-18)14-21(23(36)33-24)34-10-6-5-9-22(34)35/h2-4,7-8,11-13,16,21H,5-6,9-10,14-15H2,1H3,(H,33,36)/t16-,21+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 4168-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.082
BindingDB Entry DOI: 10.7270/Q2D79B7F |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50243425
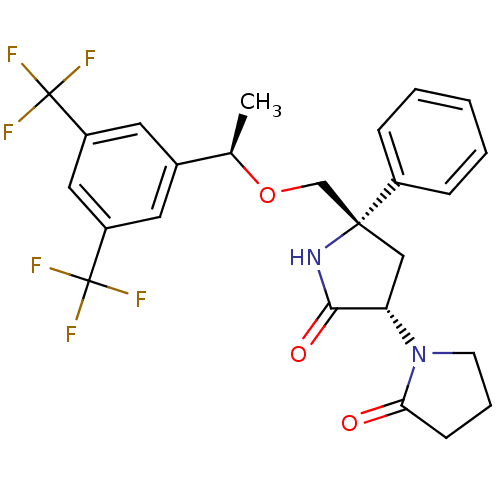
(1-((3S,5S)-5-(((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](OC[C@]1(C[C@H](N2CCCC2=O)C(=O)N1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C25H24F6N2O3/c1-15(16-10-18(24(26,27)28)12-19(11-16)25(29,30)31)36-14-23(17-6-3-2-4-7-17)13-20(22(35)32-23)33-9-5-8-21(33)34/h2-4,6-7,10-12,15,20H,5,8-9,13-14H2,1H3,(H,32,35)/t15-,20+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 18: 4168-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.082
BindingDB Entry DOI: 10.7270/Q2D79B7F |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332264
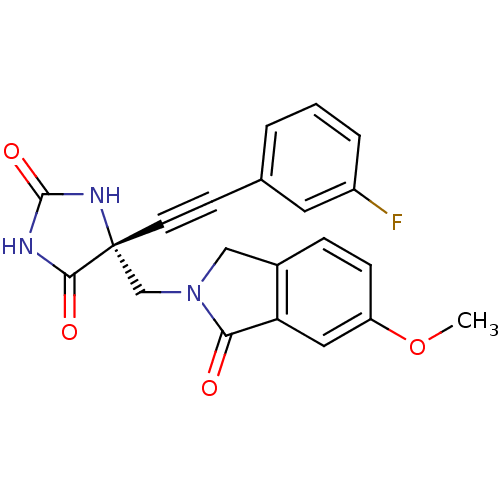
((R)-5-((3-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3cccc(F)c3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-16-6-5-14-11-25(18(26)17(14)10-16)12-21(19(27)23-20(28)24-21)8-7-13-3-2-4-15(22)9-13/h2-6,9-10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332291
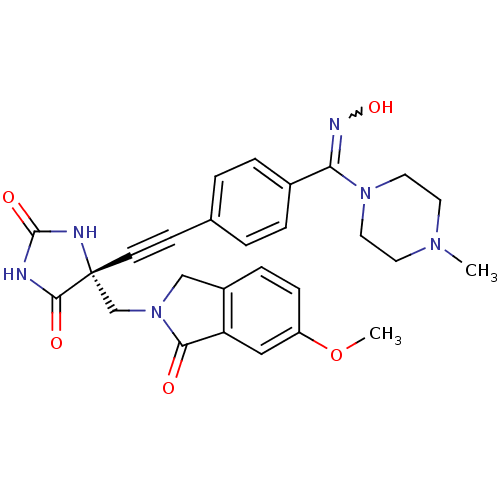
((R)-5-((4-((hydroxyimino)(4-methylpiperazin-1-yl)m...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(cc3)C(=NO)N3CCN(C)CC3)C(=O)c2c1 |r,w:25.27| Show InChI InChI=1S/C27H28N6O5/c1-31-11-13-32(14-12-31)23(30-37)19-5-3-18(4-6-19)9-10-27(25(35)28-26(36)29-27)17-33-16-20-7-8-21(38-2)15-22(20)24(33)34/h3-8,15,37H,11-14,16-17H2,1-2H3,(H2,28,29,35,36)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328980
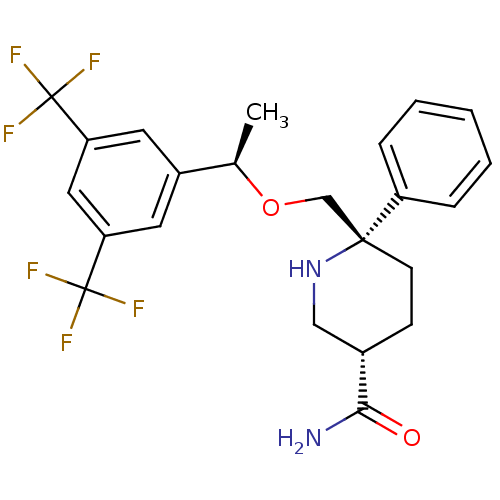
((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...)Show SMILES C[C@@H](OC[C@]1(CC[C@@H](CN1)C(N)=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H24F6N2O2/c1-14(16-9-18(22(24,25)26)11-19(10-16)23(27,28)29)33-13-21(17-5-3-2-4-6-17)8-7-15(12-31-21)20(30)32/h2-6,9-11,14-15,31H,7-8,12-13H2,1H3,(H2,30,32)/t14-,15+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328973
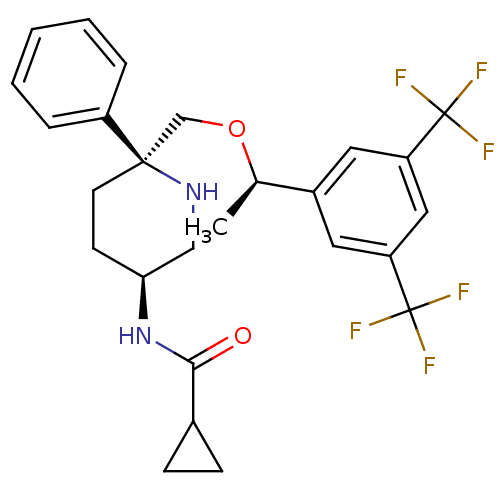
(CHEMBL1270175 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...)Show SMILES C[C@@H](OC[C@]1(CC[C@@H](CN1)NC(=O)C1CC1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H28F6N2O2/c1-16(18-11-20(25(27,28)29)13-21(12-18)26(30,31)32)36-15-24(19-5-3-2-4-6-19)10-9-22(14-33-24)34-23(35)17-7-8-17/h2-6,11-13,16-17,22,33H,7-10,14-15H2,1H3,(H,34,35)/t16-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328974
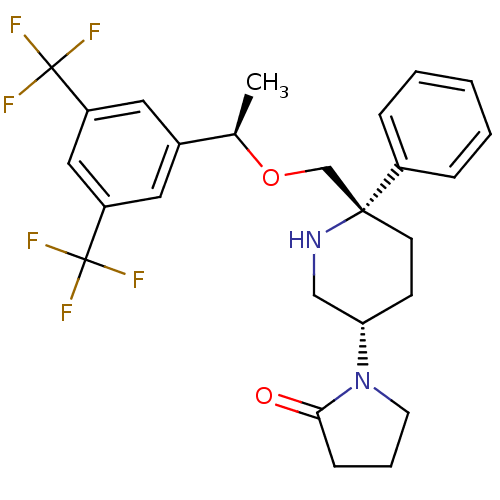
(1-((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phen...)Show SMILES C[C@@H](OC[C@]1(CC[C@@H](CN1)N1CCCC1=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C26H28F6N2O2/c1-17(18-12-20(25(27,28)29)14-21(13-18)26(30,31)32)36-16-24(19-6-3-2-4-7-19)10-9-22(15-33-24)34-11-5-8-23(34)35/h2-4,6-7,12-14,17,22,33H,5,8-11,15-16H2,1H3/t17-,22+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332263
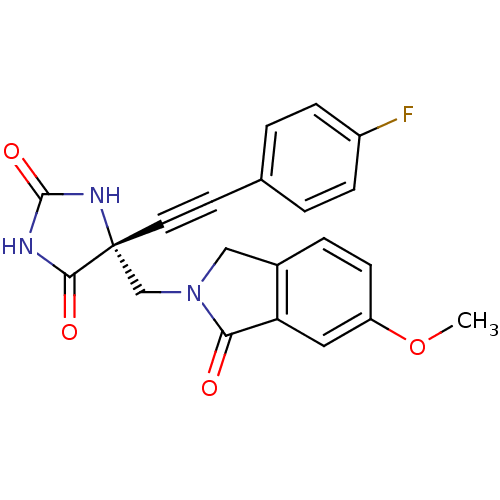
((R)-5-((4-fluorophenyl)ethynyl)-5-((6-methoxy-1-ox...)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)C#Cc3ccc(F)cc3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H16FN3O4/c1-29-16-7-4-14-11-25(18(26)17(14)10-16)12-21(19(27)23-20(28)24-21)9-8-13-2-5-15(22)6-3-13/h2-7,10H,11-12H2,1H3,(H2,23,24,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328982
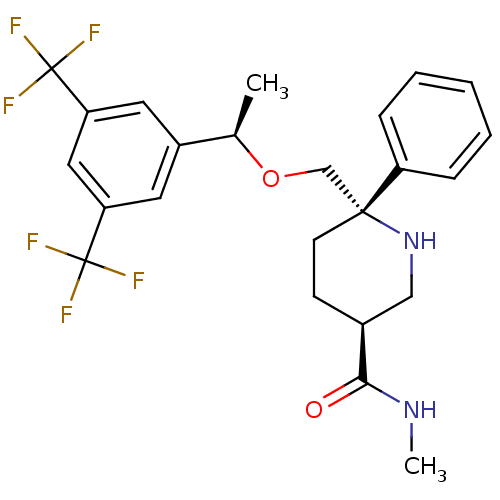
((3S,6S)-6-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)...)Show SMILES CNC(=O)[C@H]1CC[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(NC1)c1ccccc1 |r| Show InChI InChI=1S/C24H26F6N2O2/c1-15(17-10-19(23(25,26)27)12-20(11-17)24(28,29)30)34-14-22(18-6-4-3-5-7-18)9-8-16(13-32-22)21(33)31-2/h3-7,10-12,15-16,32H,8-9,13-14H2,1-2H3,(H,31,33)/t15-,16+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328979
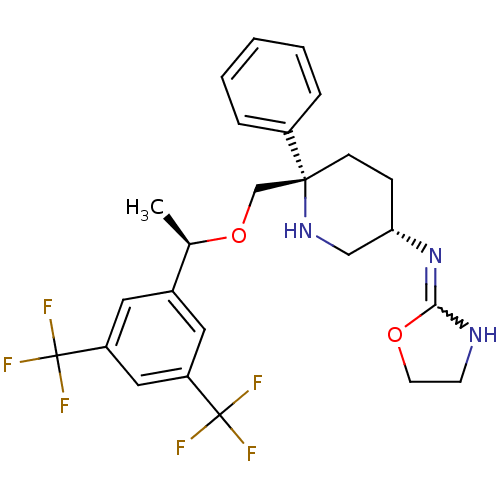
(CHEMBL1270465 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...)Show SMILES C[C@@H](OC[C@]1(CC[C@@H](CN1)N=C1NCCO1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,w:11.12| Show InChI InChI=1S/C25H27F6N3O2/c1-16(17-11-19(24(26,27)28)13-20(12-17)25(29,30)31)36-15-23(18-5-3-2-4-6-18)8-7-21(14-33-23)34-22-32-9-10-35-22/h2-6,11-13,16,21,33H,7-10,14-15H2,1H3,(H,32,34)/t16-,21+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50332285
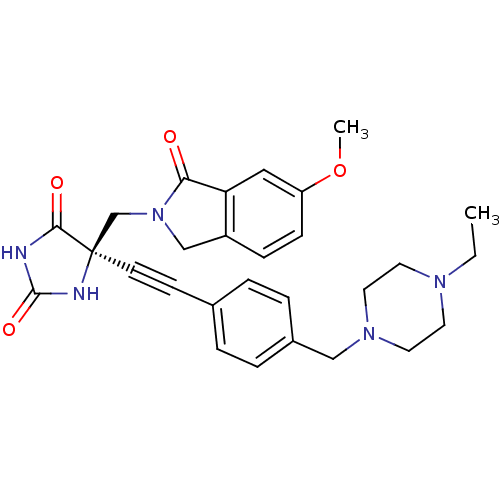
((R)-5-((4-((4-ethylpiperazin-1-yl)methyl)phenyl)et...)Show SMILES CCN1CCN(Cc2ccc(cc2)C#C[C@]2(CN3Cc4ccc(OC)cc4C3=O)NC(=O)NC2=O)CC1 |r| Show InChI InChI=1S/C28H31N5O4/c1-3-31-12-14-32(15-13-31)17-21-6-4-20(5-7-21)10-11-28(26(35)29-27(36)30-28)19-33-18-22-8-9-23(37-2)16-24(22)25(33)34/h4-9,16H,3,12-15,17-19H2,1-2H3,(H2,29,30,35,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of TACE |
Bioorg Med Chem Lett 20: 7283-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.081
BindingDB Entry DOI: 10.7270/Q2Z89CP0 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328972
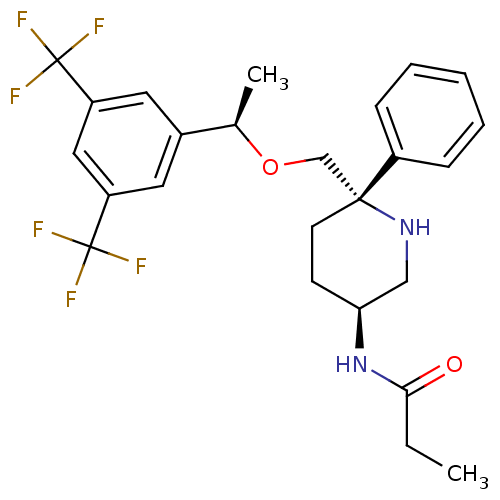
(CHEMBL1270174 | N-((3S,6S)-6-(((R)-1-(3,5-bis(trif...)Show SMILES CCC(=O)N[C@H]1CC[C@](CO[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)(NC1)c1ccccc1 |r| Show InChI InChI=1S/C25H28F6N2O2/c1-3-22(34)33-21-9-10-23(32-14-21,18-7-5-4-6-8-18)15-35-16(2)17-11-19(24(26,27)28)13-20(12-17)25(29,30)31/h4-8,11-13,16,21,32H,3,9-10,14-15H2,1-2H3,(H,33,34)/t16-,21+,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50116082
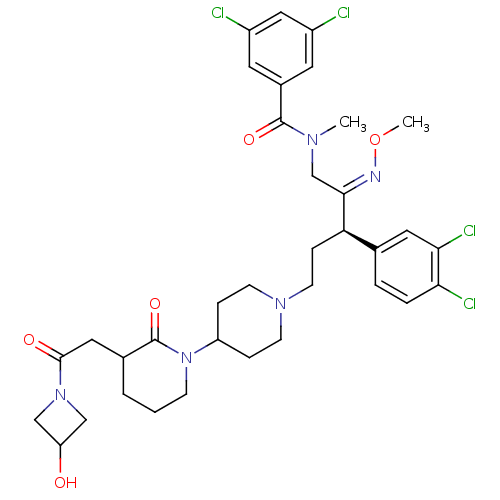
(3,5-Dichloro-N-(3-(3,4-dichloro-phenyl)-5-{3-[2-(2...)Show SMILES CO\N=C(/CN(C)C(=O)c1cc(Cl)cc(Cl)c1)[C@H](CCN1CCC(CC1)N1CCCC(CC(=O)N2CC(O)C2)C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C35H43Cl4N5O5/c1-41(34(47)24-14-25(36)18-26(37)15-24)21-32(40-49-2)29(22-5-6-30(38)31(39)16-22)9-13-42-11-7-27(8-12-42)44-10-3-4-23(35(44)48)17-33(46)43-19-28(45)20-43/h5-6,14-16,18,23,27-29,45H,3-4,7-13,17,19-21H2,1-2H3/b40-32+/t23?,29-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding to Tachykinin receptor 2 |
Bioorg Med Chem Lett 12: 2125-8 (2002)
BindingDB Entry DOI: 10.7270/Q2JQ11JS |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50116745

(CHEMBL78284 | N-{(R)-5-[4-(3-Carbamoylmethyl-2-oxo...)Show SMILES CO\N=C(/CN(C)C(=O)c1cc(Cl)cc(Cl)c1)[C@H](CCN1CCC(CC1)N1CCCN(CC(N)=O)C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C31H38Cl4N6O4/c1-38(30(43)21-14-22(32)17-23(33)15-21)18-28(37-45-2)25(20-4-5-26(34)27(35)16-20)8-13-39-11-6-24(7-12-39)41-10-3-9-40(31(41)44)19-29(36)42/h4-5,14-17,24-25H,3,6-13,18-19H2,1-2H3,(H2,36,42)/b37-28+/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human tachykinin receptor 1 in CHO cells using [3H]-Sar SP as radioligand |
Bioorg Med Chem Lett 12: 2355-8 (2002)
BindingDB Entry DOI: 10.7270/Q26T0KXW |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(GUINEA PIG) | BDBM50116091
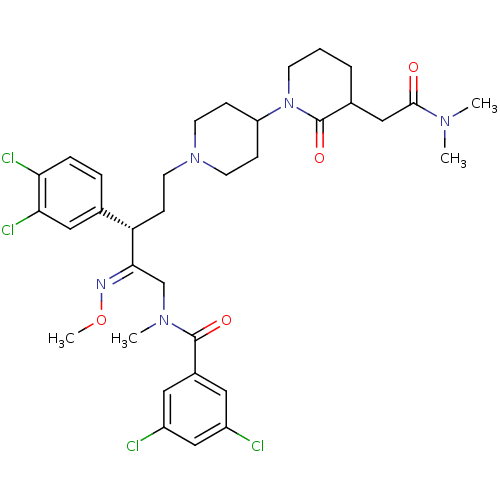
(3,5-Dichloro-N-[3-(3,4-dichloro-phenyl)-5-(3-dimet...)Show SMILES CO\N=C(/CN(C)C(=O)c1cc(Cl)cc(Cl)c1)[C@@H](CCN1CCC(CC1)N1CCCC(CC(=O)N(C)C)C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C34H43Cl4N5O4/c1-40(2)32(44)19-23-6-5-12-43(34(23)46)27-9-13-42(14-10-27)15-11-28(22-7-8-29(37)30(38)18-22)31(39-47-4)21-41(3)33(45)24-16-25(35)20-26(36)17-24/h7-8,16-18,20,23,27-28H,5-6,9-15,19,21H2,1-4H3/b39-31+/t23?,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
In vitro binding to Tachykinin receptor 2 |
Bioorg Med Chem Lett 12: 2125-8 (2002)
BindingDB Entry DOI: 10.7270/Q2JQ11JS |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50243039

(CHEMBL4089108)Show SMILES COc1ccc2CN(C[C@]3(NC(=O)NC3=O)c3cc4ccccc4o3)C(=O)c2c1 |r| Show InChI InChI=1S/C21H17N3O5/c1-28-14-7-6-13-10-24(18(25)15(13)9-14)11-21(19(26)22-20(27)23-21)17-8-12-4-2-3-5-16(12)29-17/h2-9H,10-11H2,1H3,(H2,22,23,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of catalytic domain TACE (unknown origin) by FRET assay |
Bioorg Med Chem Lett 27: 3037-3042 (2017)
Article DOI: 10.1016/j.bmcl.2017.05.062
BindingDB Entry DOI: 10.7270/Q2319Z8G |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50328988
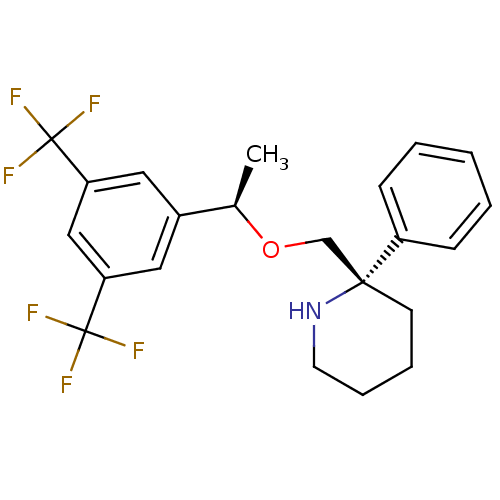
((S)-2-(((R)-1-(3,5-bis(trifluoromethyl)phenyl)etho...)Show SMILES C[C@@H](OC[C@]1(CCCCN1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C22H23F6NO/c1-15(16-11-18(21(23,24)25)13-19(12-16)22(26,27)28)30-14-20(9-5-6-10-29-20)17-7-3-2-4-8-17/h2-4,7-8,11-13,15,29H,5-6,9-10,14H2,1H3/t15-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SAr-Met from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 6313-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.059
BindingDB Entry DOI: 10.7270/Q23X86VK |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50116722
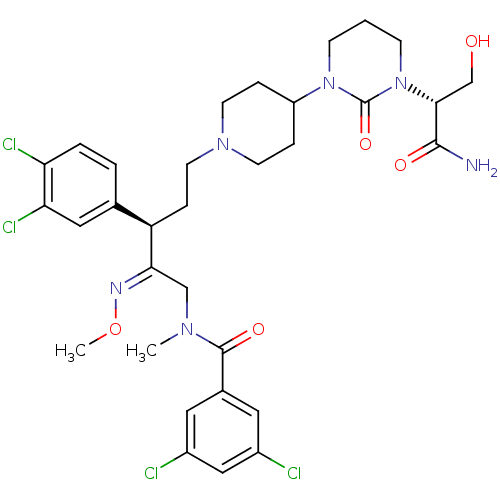
(CHEMBL263243 | N-{(R)-5-{4-[3-((R)-1-Carbamoyl-2-h...)Show SMILES CO\N=C(/CN(C)C(=O)c1cc(Cl)cc(Cl)c1)[C@H](CCN1CCC(CC1)N1CCCN([C@H](CO)C(N)=O)C1=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C32H40Cl4N6O5/c1-39(31(45)21-14-22(33)17-23(34)15-21)18-28(38-47-2)25(20-4-5-26(35)27(36)16-20)8-13-40-11-6-24(7-12-40)41-9-3-10-42(32(41)46)29(19-43)30(37)44/h4-5,14-17,24-25,29,43H,3,6-13,18-19H2,1-2H3,(H2,37,44)/b38-28+/t25-,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant human tachykinin receptor 3 in CHO cells using [125I]-[MePhe]-NKB as radioligand |
Bioorg Med Chem Lett 12: 2355-8 (2002)
BindingDB Entry DOI: 10.7270/Q26T0KXW |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186525
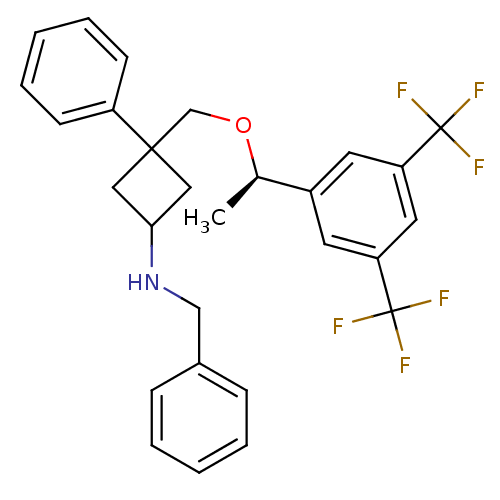
((R)-3-((1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)m...)Show SMILES C[C@@H](OCC1(CC(C1)NCc1ccccc1)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,(17.4,-36.54,;17.4,-38.08,;18.74,-38.84,;20.07,-38.07,;21.41,-38.83,;20.34,-39.94,;21.45,-41.01,;22.52,-39.9,;21.48,-42.55,;20.17,-43.35,;20.2,-44.89,;18.88,-45.68,;18.91,-47.22,;20.26,-47.96,;21.59,-47.15,;21.55,-45.62,;22.74,-38.06,;24.06,-38.82,;25.39,-38.05,;25.39,-36.51,;24.04,-35.74,;22.72,-36.52,;16.07,-38.85,;14.74,-38.09,;13.41,-38.86,;13.4,-40.4,;14.74,-41.17,;16.08,-40.4,;14.74,-42.71,;13.19,-42.7,;16.27,-42.71,;14.75,-44.25,;12.07,-38.09,;12.83,-36.75,;11.29,-39.42,;10.74,-37.32,)| Show InChI InChI=1S/C28H27F6NO/c1-19(21-12-23(27(29,30)31)14-24(13-21)28(32,33)34)36-18-26(22-10-6-3-7-11-22)15-25(16-26)35-17-20-8-4-2-5-9-20/h2-14,19,25,35H,15-18H2,1H3/t19-,25?,26?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50186528

(CHEMBL212112 | cis-1-{3-[(R)-1-(3,5-bis-trifluorom...)Show SMILES C[C@@H](OC[C@@]1(C[C@@H](C1)N1CCCC1=O)c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |wU:1.0,wD:6.8,4.3,(22.41,-39.62,;22.42,-41.16,;23.75,-41.93,;25.09,-41.15,;26.42,-41.92,;27.53,-42.98,;26.47,-44.1,;25.36,-43.03,;26.5,-45.64,;25.28,-46.57,;25.78,-48.02,;27.32,-47.99,;27.77,-46.52,;29.22,-46.01,;27.75,-41.14,;29.08,-41.91,;30.41,-41.14,;30.41,-39.6,;29.06,-38.83,;27.74,-39.61,;21.09,-41.94,;19.75,-41.17,;18.42,-41.95,;18.42,-43.49,;19.76,-44.26,;21.09,-43.49,;19.75,-45.8,;18.21,-45.79,;21.29,-45.8,;19.77,-47.34,;17.09,-41.18,;17.85,-39.84,;16.31,-42.51,;15.76,-40.4,)| Show InChI InChI=1S/C25H25F6NO2/c1-16(17-10-19(24(26,27)28)12-20(11-17)25(29,30)31)34-15-23(18-6-3-2-4-7-18)13-21(14-23)32-9-5-8-22(32)33/h2-4,6-7,10-12,16,21H,5,8-9,13-15H2,1H3/t16-,21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Sar-Met substance P from human recombinant NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 3859-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.031
BindingDB Entry DOI: 10.7270/Q2QV3M44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data