

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor 3 (RAT) | BDBM50289498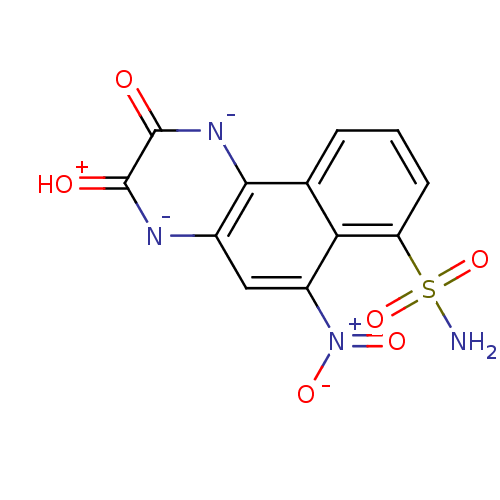 (3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM84949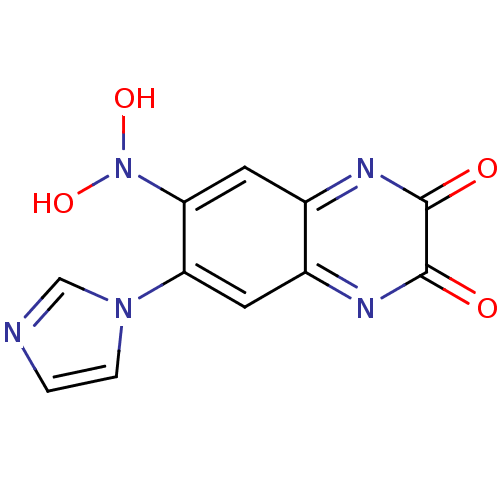 (CAS_3035216 | NSC_3035216 | YM90k) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50050449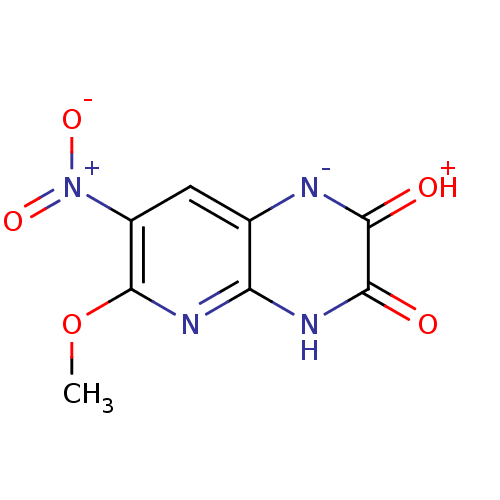 (6-Methoxy-7-nitro-1,4-dihydro-pyrido[2,3-b]pyrazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of the specific binding of [3H]-Gly to N-methyl-D-aspartate glutamate receptor 1 in rat whole brain m... | J Med Chem 39: 1331-8 (1996) Article DOI: 10.1021/jm950304+ BindingDB Entry DOI: 10.7270/Q2RN36XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50289504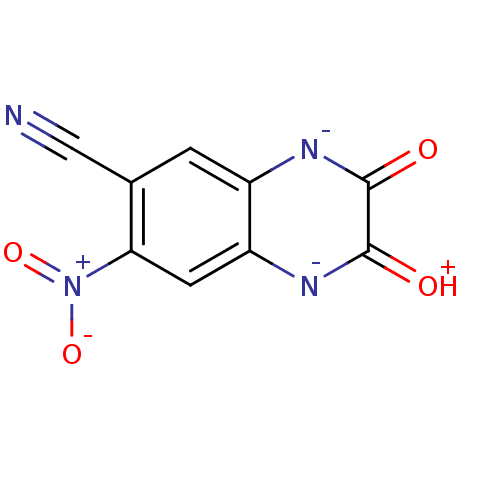 (7-Cyano-3-hydroxy-6-nitro-quinoxalin-2-ol anion | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM84949 (CAS_3035216 | NSC_3035216 | YM90k) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50131270 (2-methyl-N-[4-nitro-3-(trifluoromethyl)phenyl]prop...) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kanazawa University Curated by ChEMBL | Assay Description Inhibition of human recombinant CES2 assessed as compound hydrolysis | Drug Metab Dispos 40: 1080-4 (2012) Article DOI: 10.1124/dmd.112.044537 BindingDB Entry DOI: 10.7270/Q2B56MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50289498 (3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50053571 (1-Hydroxy-7-imidazol-1-yl-6-nitro-1,4-dihydro-quin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound towards glycine binding site on NMDA receptor was determined in rat whole brain membrane using strychnine-insensitiv... | J Med Chem 39: 3971-9 (1996) Article DOI: 10.1021/jm960387+ BindingDB Entry DOI: 10.7270/Q2BZ655Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50289504 (7-Cyano-3-hydroxy-6-nitro-quinoxalin-2-ol anion | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50050450 (6,7-Dinitro-1,4-dihydro-pyrido[2,3-b]pyrazine-2,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of the specific binding of [3H]-Gly to N-methyl-D-aspartate glutamate receptor 1 in rat whole brain m... | J Med Chem 39: 1331-8 (1996) Article DOI: 10.1021/jm950304+ BindingDB Entry DOI: 10.7270/Q2RN36XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM84949 (CAS_3035216 | NSC_3035216 | YM90k) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM84949 (CAS_3035216 | NSC_3035216 | YM90k) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50289498 (3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50289504 (7-Cyano-3-hydroxy-6-nitro-quinoxalin-2-ol anion | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50289498 (3-Hydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxalin-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 276: 84-92 (1996) BindingDB Entry DOI: 10.7270/Q2BV7F5M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50030674 (6-(1H-1-imidazolyl)-7-nitro-1,2,3,4-tetrahydro-2,3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound towards glycine binding site on NMDA receptor was determined in rat whole brain membrane using strychnine-insensitiv... | J Med Chem 39: 3971-9 (1996) Article DOI: 10.1021/jm960387+ BindingDB Entry DOI: 10.7270/Q2BZ655Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1 (RAT) | BDBM50207594 (2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Binding affinity of the compound towards glycine binding site on NMDA receptor was determined in rat whole brain membrane using strychnine-insensitiv... | J Med Chem 39: 3971-9 (1996) Article DOI: 10.1021/jm960387+ BindingDB Entry DOI: 10.7270/Q2BZ655Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Parathyroid hormone/parathyroid hormone-related peptide receptor (Homo sapiens (Human)) | BDBM50318885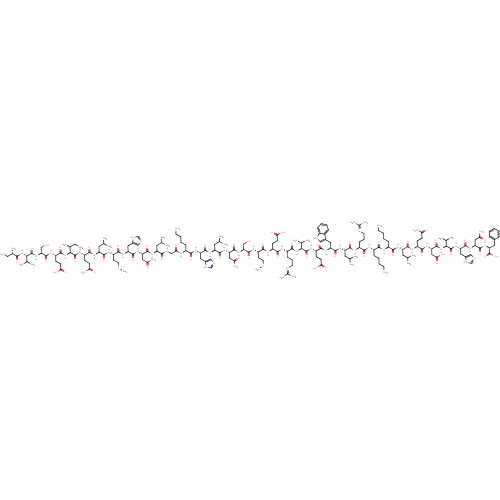 (CHEMBL525610 | teriparatide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of 125I-PTH (1 to 15 residues) from human PTHR1 expressed in African green monkey COS7 cell membranes at 300 uM after 90 mins by gamma c... | J Med Chem 61: 5949-5962 (2018) Article DOI: 10.1021/acs.jmedchem.8b00182 BindingDB Entry DOI: 10.7270/Q2ZC85GG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327443 (N-{3-[(1R,5S)-6-ethyl-3-azabicyclo[3.1.0]hexan-6-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327428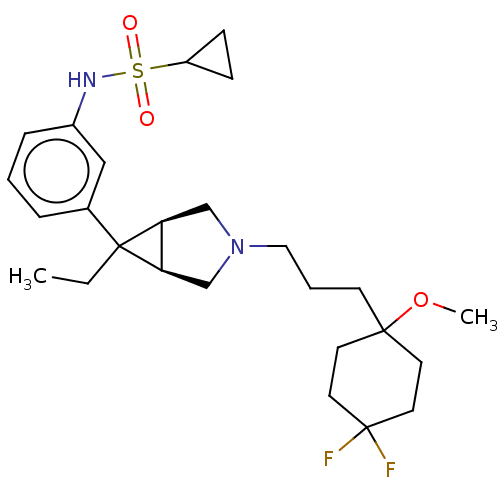 ( N-(3-{(1R,5S,6r)-3-[3-(4,4-difluoro-1-methoxycycl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327436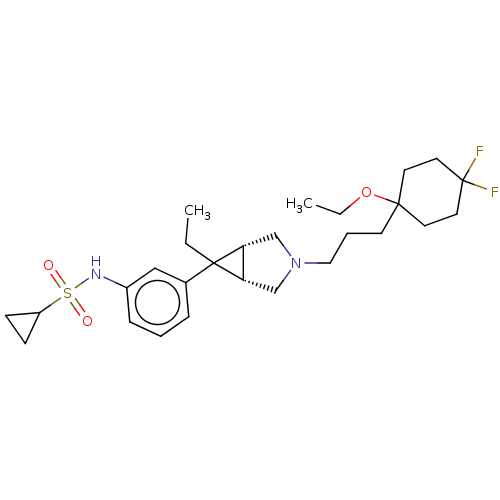 (N-(3-{(1R,5S,6r)-3-[3-(1-ethoxy-4,4-difluorocycloh...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327429 (N-(3-{(1R,5S,6r)-3-[3-(4,4-difluoropiperidin-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288232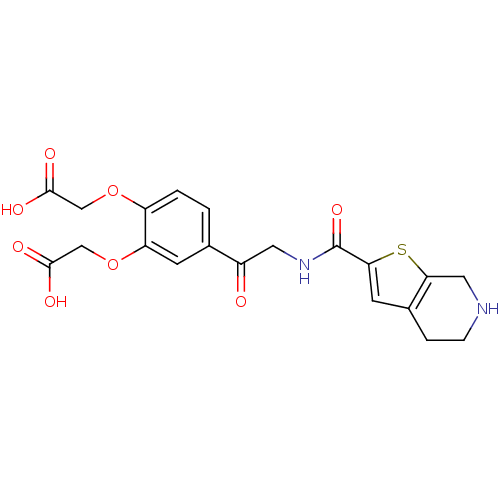 (2-[2-(carboxymethoxy)-4-(2-{4H,5H,6H,7H-thieno[2,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Vitronectin binding to GPIIb/IIIIa Vitronectin receptor | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327423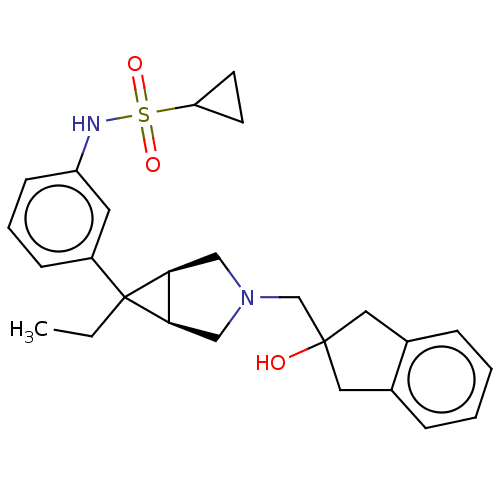 ( N-(3-{(1R,5S,6r)-6-ethyl-3-[(2-hydroxy-2,3-dihydr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327426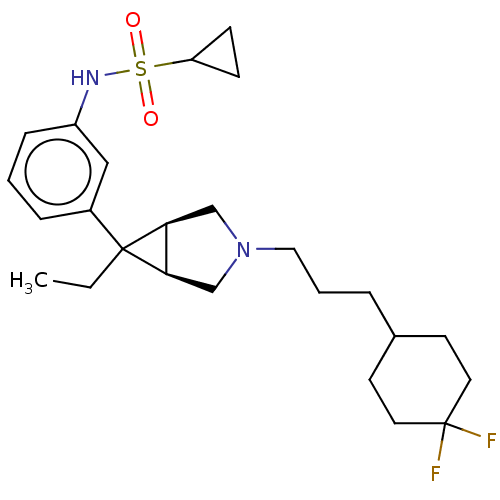 (N-(3-{(1R,5S,6r)-3-[3-(4,4-difluorocyclohexyl)prop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327427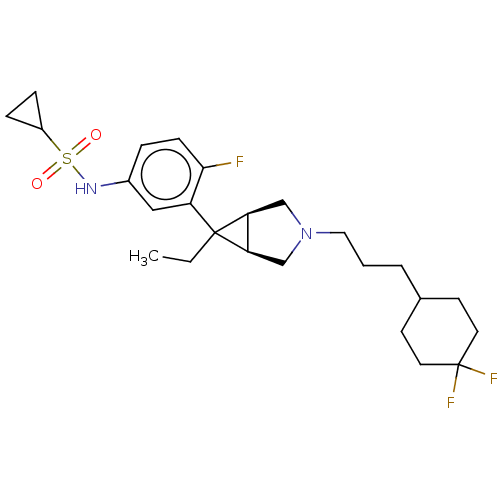 (N-(3-{(1R,5S,6r)-3-[3-(4,4-difluorocyclohexyl)prop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327437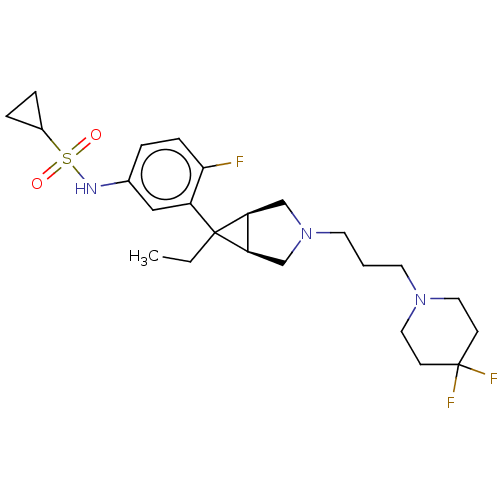 (N-(3-{(1R,5S,6r)-3-[3-(4,4-difluoropiperidin-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327424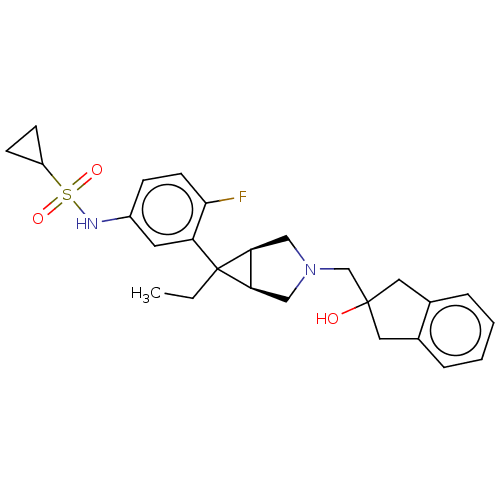 (N-(3-{(1R,5S,6r)-6-ethyl-3-[(2-hydroxy-2,3-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327431 (N-(3-{(1R,5S,6r)-3-[3-(3,3-difluoropyrrolidin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327433 (N-(3-{(1R,5S,6r)-6-ethyl-3-[(2-methoxy-2,3-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327430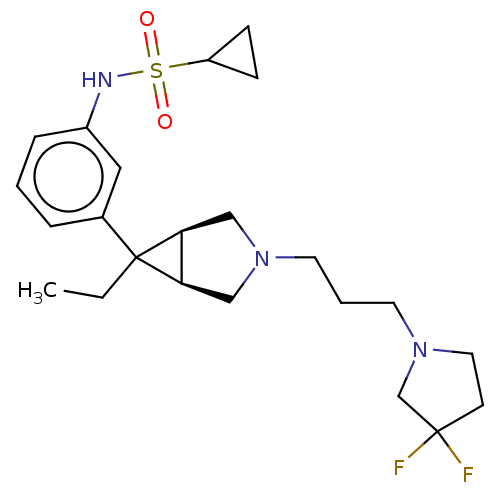 (N-(3-{(1R,5S,6r)-3-[3-(3,3-difluoropyrrolidin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM50292705 ((25R)-25-Adamantyl-1alpha,25-dihydroxy-2-methylene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at VDR expressed in COS7 cells assessed as inhibition of 1,25-Dihydroxyvitamin D3-induced response by transient transcription ass... | J Med Chem 51: 5320-9 (2008) Article DOI: 10.1021/jm8004477 BindingDB Entry DOI: 10.7270/Q28C9W2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327438 (N-(3-{(1R,5S,6r)-3-[3-(3,3-difluoropiperidin-1-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327432 (N-(3-{(1R,5S,6r)-6-ethyl-3-[(2-hydroxy-2,3-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327425 (N-(3-{(1R,5S,6r)-6-ethyl-3-[(2-methoxy-2,3-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327435 (N-(3-{(1R,5S,6r)-3-[(2-ethoxy-2,3-dihydro-1H-inden...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327442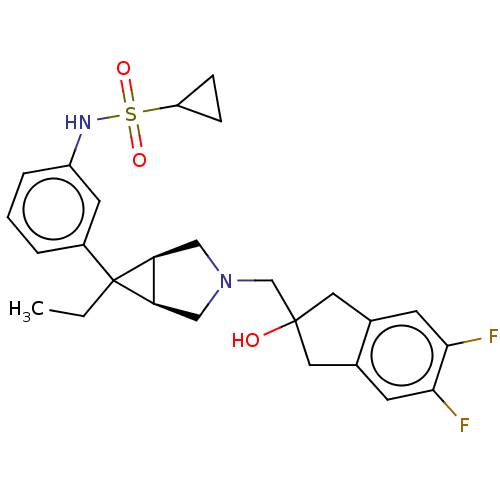 (N-(3-{(1R,5S,6r)-3-[(5,6-difluoro-2-hydroxy-2,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466366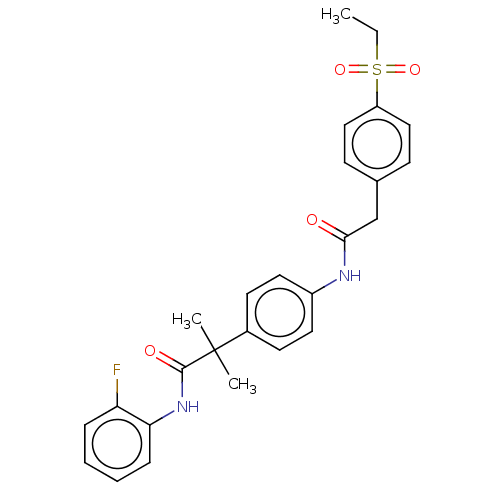 (CHEMBL4286190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 28: 3549-3553 (2018) Article DOI: 10.1016/j.bmcl.2018.09.032 BindingDB Entry DOI: 10.7270/Q2H997XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327441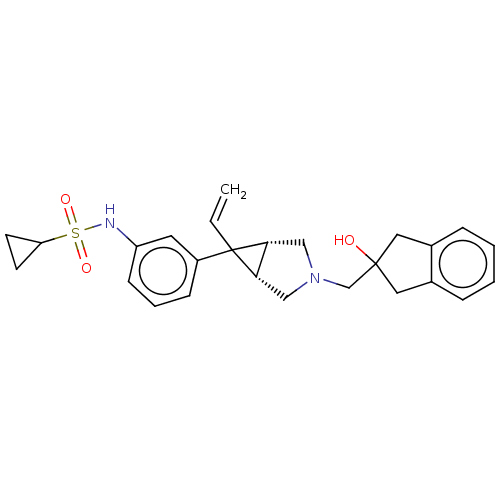 (N-(3-{(1R,5S,6r)-3-[(2-hydroxy-2,3-dihydro-1H-inde...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288220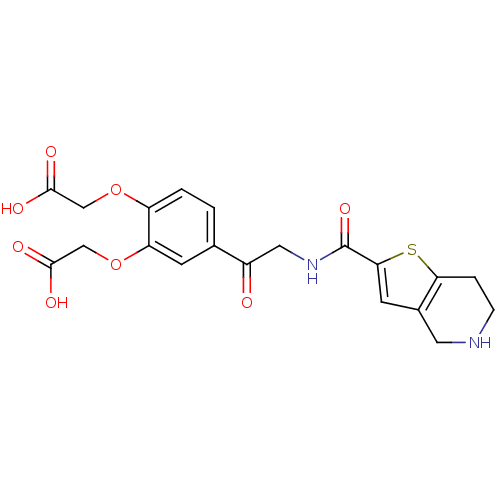 ((2-Carboxymethoxy-5-{2-[(4,5,6,7-tetrahydro-thieno...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of biotinylated fibrinogen binding to alphaIIb-beta3 integrin | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288220 ((2-Carboxymethoxy-5-{2-[(4,5,6,7-tetrahydro-thieno...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Biotinylated fibrinogen binding to alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of ACE by fluorometric assay | J Nat Prod 51: 357-359 (1988) Article DOI: 10.1021/np50056a033 BindingDB Entry DOI: 10.7270/Q2XW4M1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327439 (N-(3-{(1R,5S,6r)-3-[(2-hydroxy-2,3-dihydro-1H-inde...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288227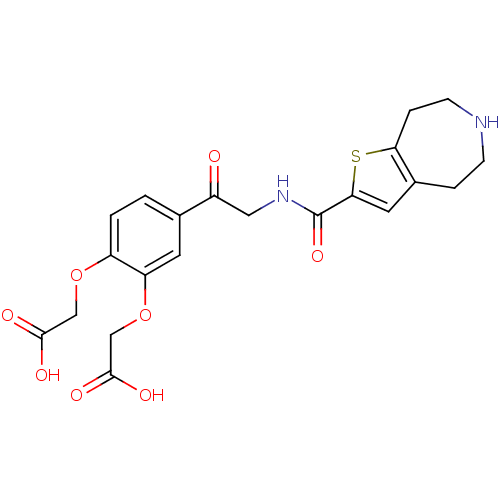 ((2-Carboxymethoxy-5-{2-[(5,6,7,8-tetrahydro-4H-thi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of vWF binding to alphaIIb-beta3 integrin | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466386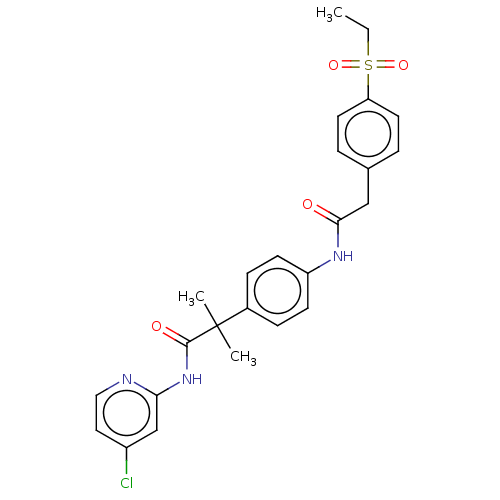 (CHEMBL4286603) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 28: 3549-3553 (2018) Article DOI: 10.1016/j.bmcl.2018.09.032 BindingDB Entry DOI: 10.7270/Q2H997XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288218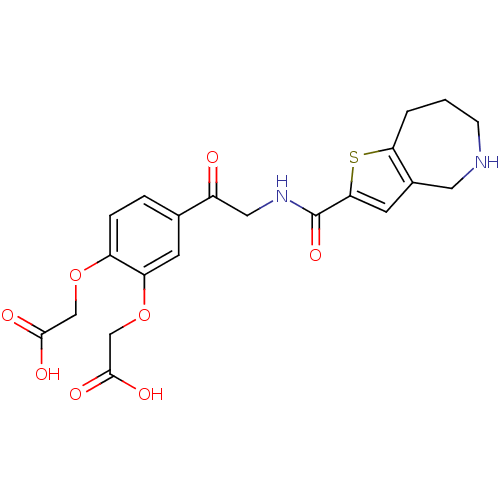 ((2-Carboxymethoxy-5-{2-[(5,6,7,8-tetrahydro-4H-thi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of biotinylated fibrinogen binding to alphaIIb-beta3 integrin | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288230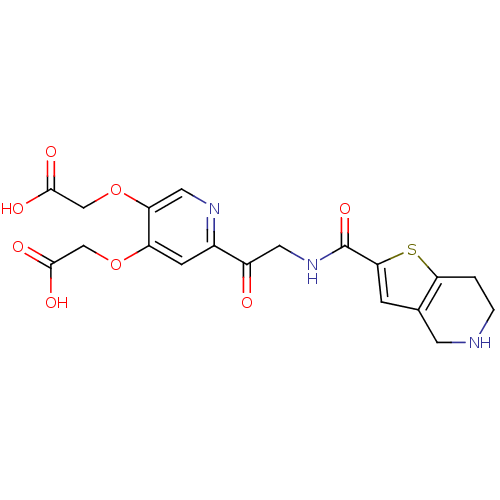 ((4-Carboxymethoxy-6-{2-[(4,5,6,7-tetrahydro-thieno...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Biotinylated fibrinogen binding to alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2601-2606 (1996) Article DOI: 10.1016/0960-894X(96)00476-3 BindingDB Entry DOI: 10.7270/Q28C9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466381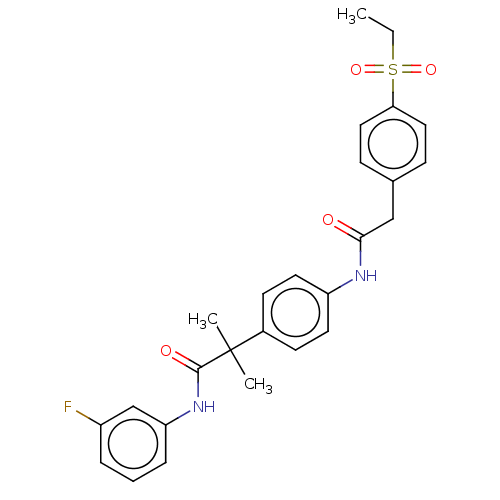 (CHEMBL4281750) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 28: 3549-3553 (2018) Article DOI: 10.1016/j.bmcl.2018.09.032 BindingDB Entry DOI: 10.7270/Q2H997XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466369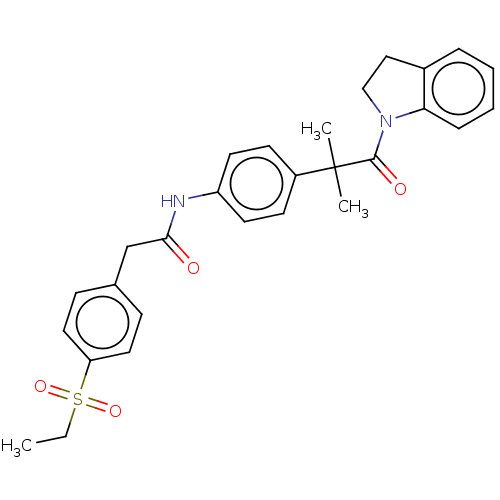 (CHEMBL4282801) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Transrepression of recombinant human GAL4-DBD fused RORgammat LBD expressed in HEK293 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 28: 3549-3553 (2018) Article DOI: 10.1016/j.bmcl.2018.09.032 BindingDB Entry DOI: 10.7270/Q2H997XS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM327434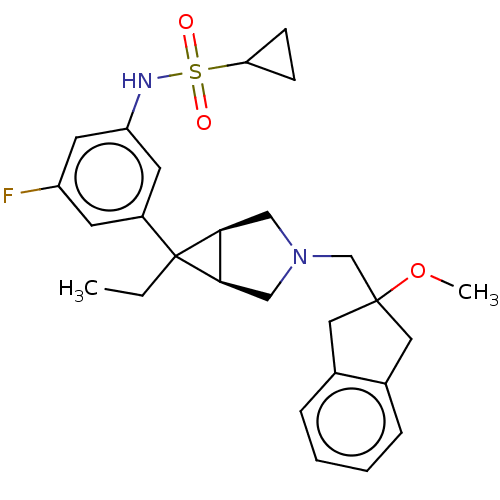 (N-(3-{(1R,5S,6r)-6-ethyl-3-[(2-methoxy-2,3-dihydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SANWA KAGAKU KENKYUSHO CO., LTD.; UBE INDUSTRIES, LTD. US Patent | Assay Description The inhibition ratio of the test substance at the respective concentrations were calculated with setting the reaction value of the well to which DMSO... | US Patent US9663463 (2017) BindingDB Entry DOI: 10.7270/Q27946RR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 309 total ) | Next | Last >> |