

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478141 (CHEMBL403526) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719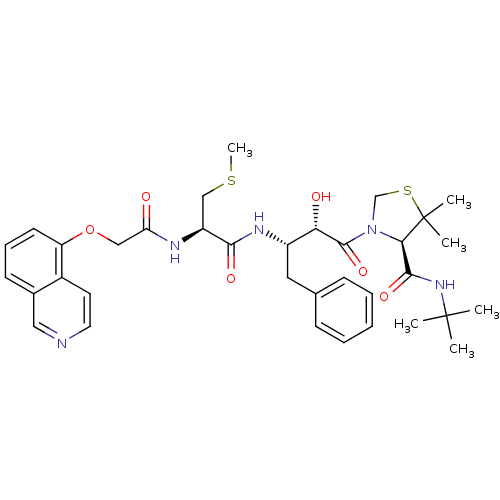 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0880 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50088504 (A-84538 | ABBOTT-84538 | CHEBI:45409 | Norvir | Ri...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052442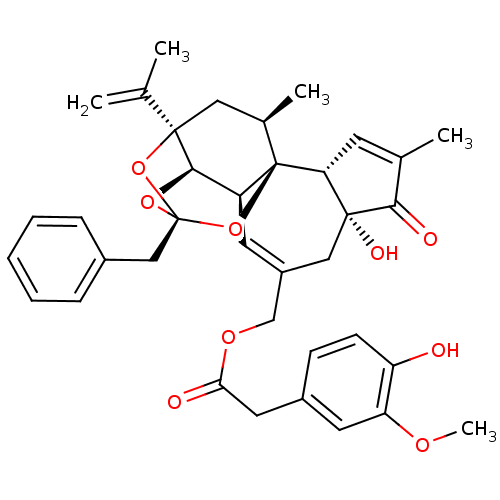 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid (2R,3S,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding to Rat Vanilloid receptor 1 (VR1) expressing CHO cells compared to capsacin | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478138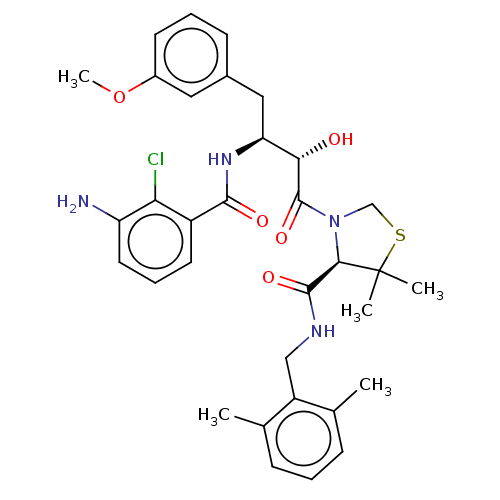 (CHEMBL256934 | SM-309515) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021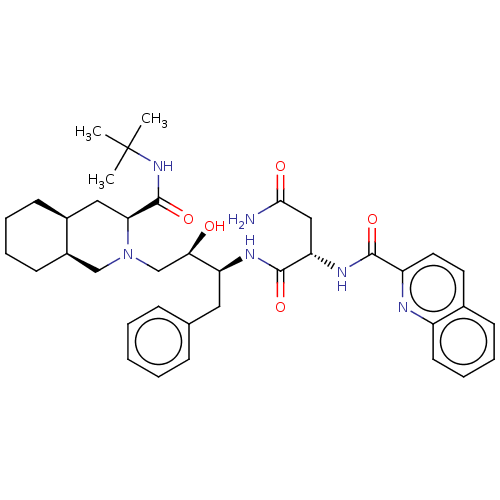 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478135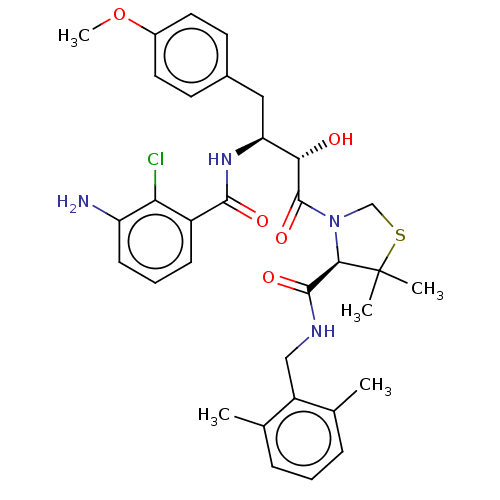 (CHEMBL409007) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478136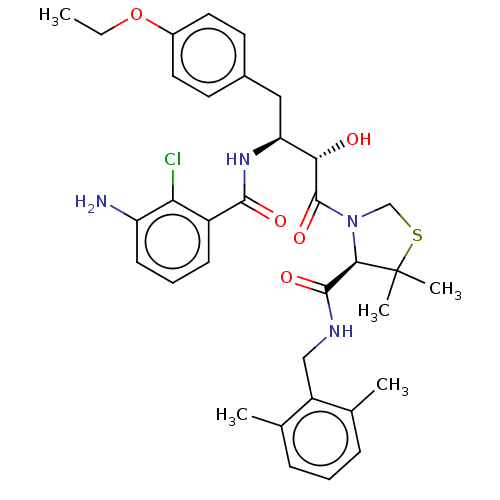 (CHEMBL257257) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM718 ((4R)-3-[(2S,3S)-3-[(2-ethyl-3-hydroxyphenyl)formam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478137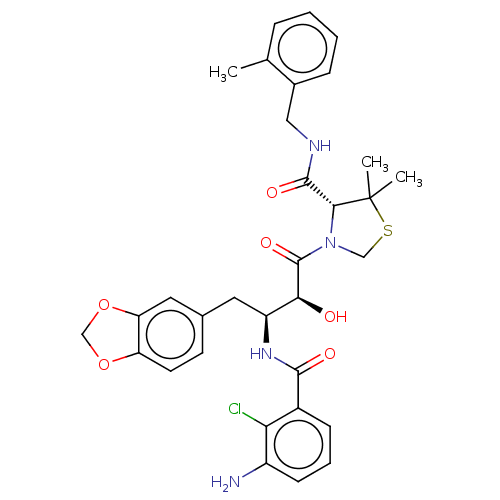 (CHEMBL271391) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478143 (CHEMBL269904) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM717 ((4R)-N-[(2-chlorophenyl)methyl]-3-[(2S,3S)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478144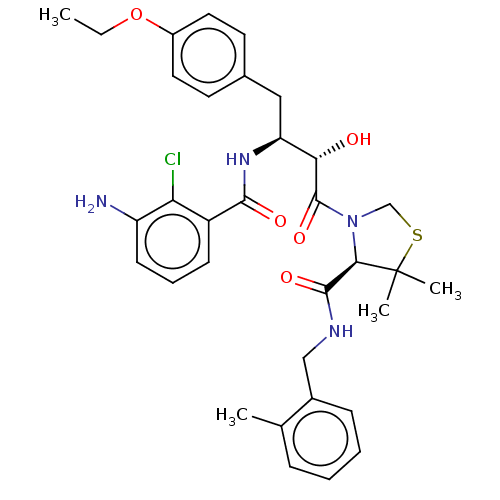 (CHEMBL272025) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478142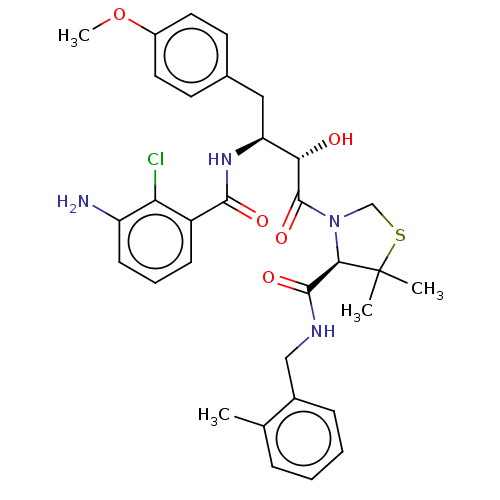 (CHEMBL272797) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.311 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | -56.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478140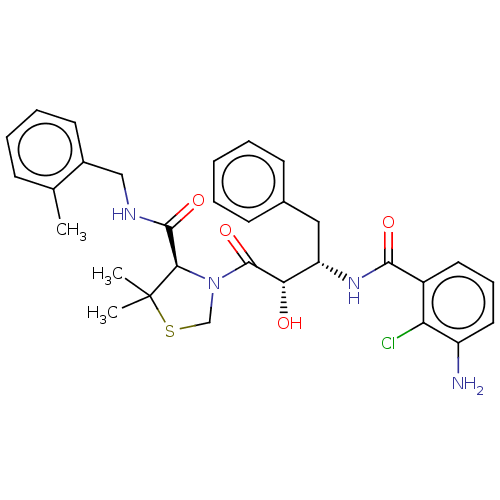 (CHEMBL404154) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.359 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553757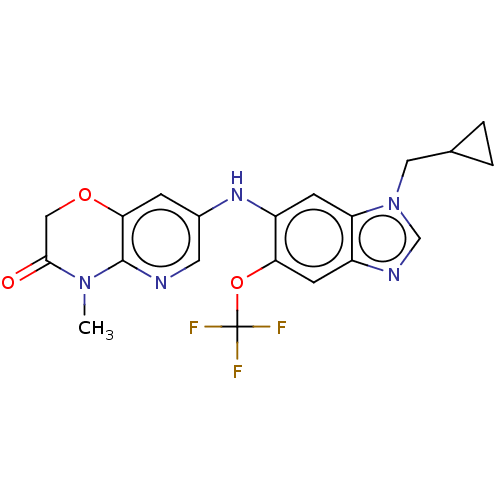 (CHEMBL4746471) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593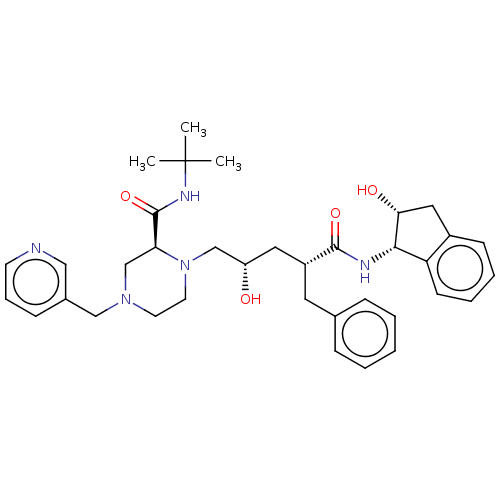 (CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.739 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.740 | -54.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478133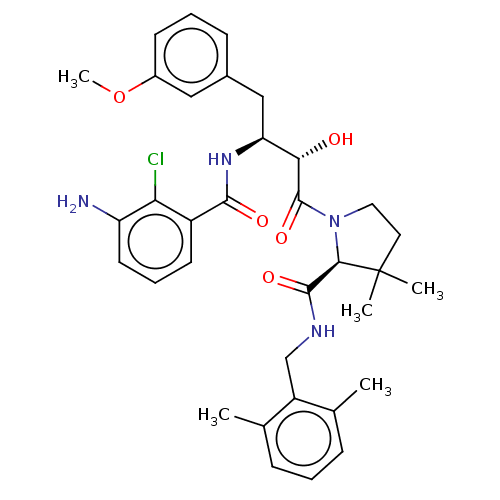 (CHEMBL272796) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478134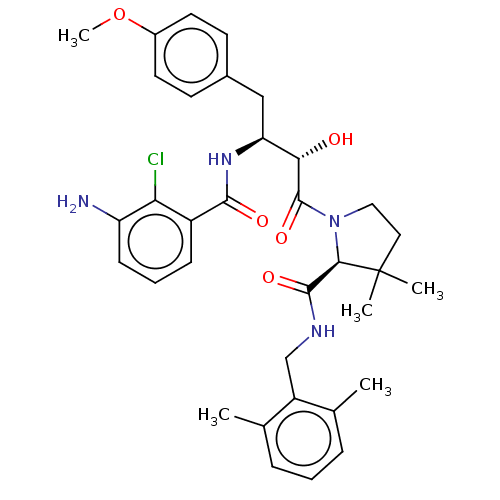 (CHEMBL408620) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.804 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50478139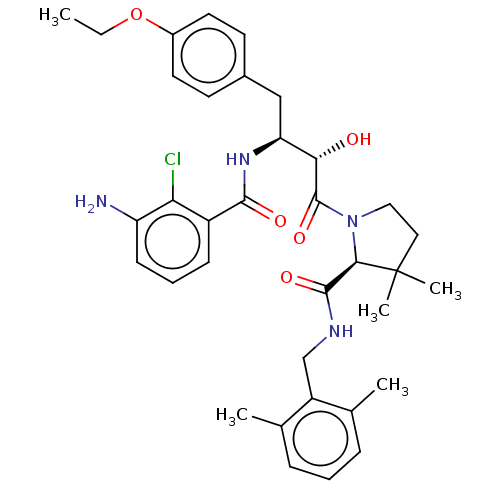 (CHEMBL437457) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.861 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM403740 (6-Chloro-7-((3-(cyclopropylmethyl)-7-methyl-3H-imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50061306 ((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.931 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 16: 1299-308 (2008) Article DOI: 10.1016/j.bmc.2007.10.062 BindingDB Entry DOI: 10.7270/Q2RJ4N8X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553762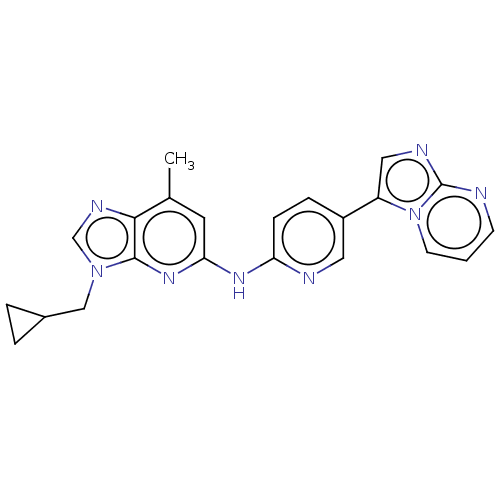 (CHEMBL4758608) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat brain membranes using [3H]CHA as radioligand | J Med Chem 35: 241-52 (1992) BindingDB Entry DOI: 10.7270/Q20C4WD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM712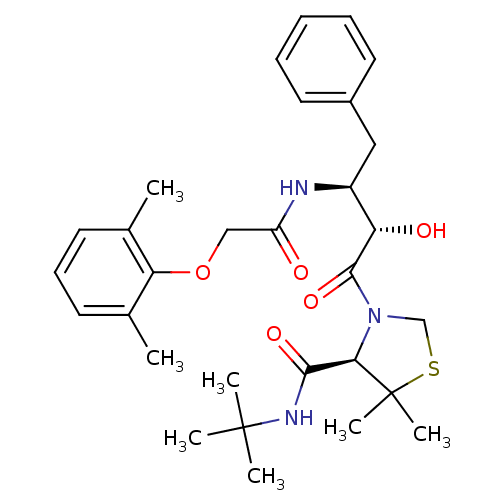 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553763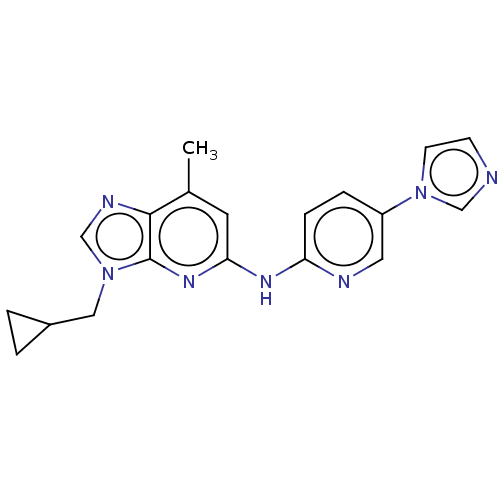 (CHEMBL4785208) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553754 (CHEMBL4746482) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553761 (CHEMBL4756857) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553764 (CHEMBL4749423) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM715 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[(2-ethyl-3-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.24 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50119168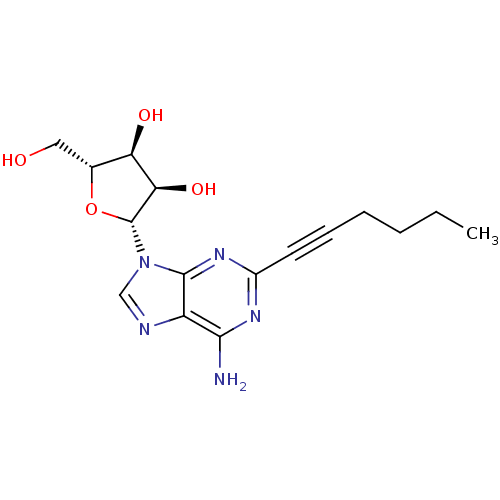 ((2R,3R,4S,5R)-2-(6-Amino-2-hex-1-ynyl-purin-9-yl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity against adenosine A2 receptor in rat striatal membranes using [3H]NECA as radioligand | J Med Chem 35: 241-52 (1992) BindingDB Entry DOI: 10.7270/Q20C4WD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330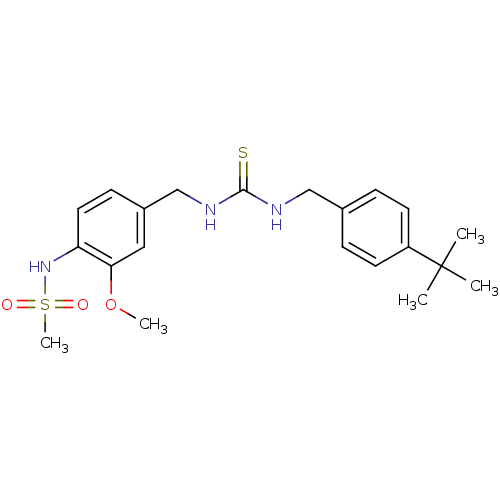 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity towards rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4143-50 (2005) Article DOI: 10.1016/j.bmcl.2005.06.006 BindingDB Entry DOI: 10.7270/Q2JH3KQB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM20330 (3-[(4-tert-butylphenyl)methyl]-1-[(4-methanesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity for rat TRPV1 expressed in CHO cells | Bioorg Med Chem Lett 15: 4136-42 (2005) Article DOI: 10.1016/j.bmcl.2005.06.009 BindingDB Entry DOI: 10.7270/Q23B5ZN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255730 (CHEMBL4065067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255690 (CHEMBL4068735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255692 (CHEMBL4100475) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal Strep2-tagged SIRT5 expressed in Escherichia coli BL21 (DE3) using Abz-GVLK(glutaryl)AY(NO2)GV-NH2 as subs... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255693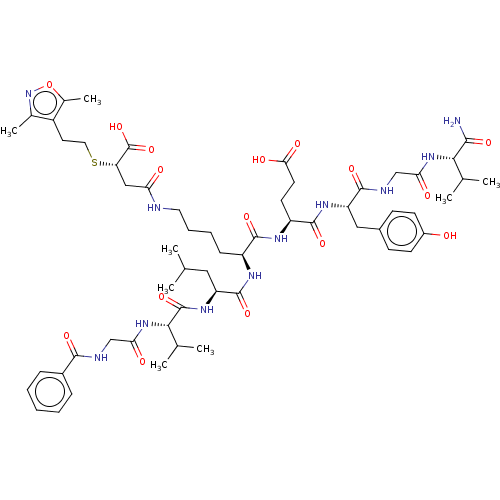 (CHEMBL4073655) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacylase sirtuin-5, mitochondrial (Homo sapiens (Human)) | BDBM50255691 (CHEMBL4092751) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Enzymology, Institute of Biochemistry and Biotechnology , Martin-Luther-University Halle-Wittenberg , 06120 Halle/Saale , Germany. Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His-tagged SIRT5 (34 to 269 residues) expressed in Escherichia coli Transetta(DE3) cells using Benzyl Lys(... | J Med Chem 61: 2460-2471 (2018) Article DOI: 10.1021/acs.jmedchem.7b01648 BindingDB Entry DOI: 10.7270/Q2WW7M4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553760 (CHEMBL4757188) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553753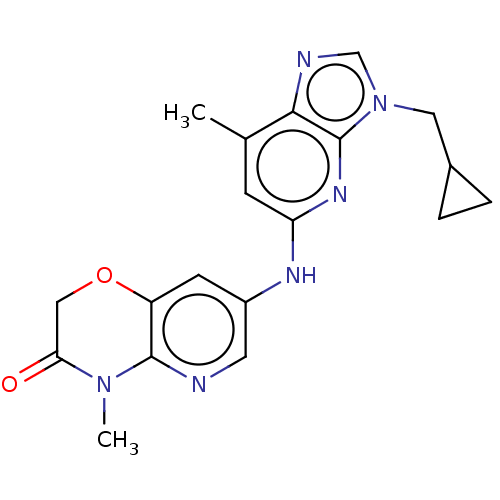 (CHEMBL4799496) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM400239 (7-((1-(2-Cyclopropylethyl)-5-fluoro-1H-benzo[d]imi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd US Patent | Assay Description The GABAAα5/β3/γ2 protein used for the Scintillation Proximity Assay was derived from membranes produced from HEK293 GABAA α5/ ... | US Patent US10016439 (2018) BindingDB Entry DOI: 10.7270/Q2NC63J0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM714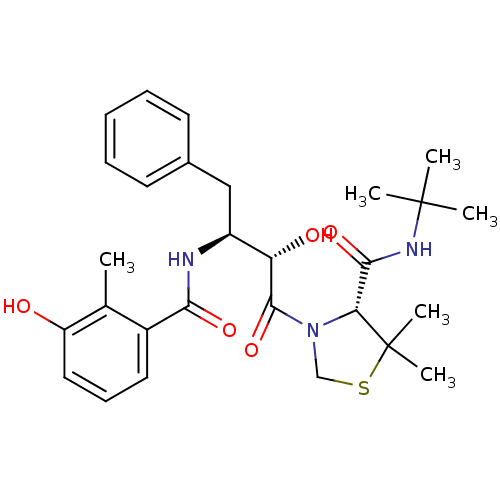 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.14 | -49.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50453223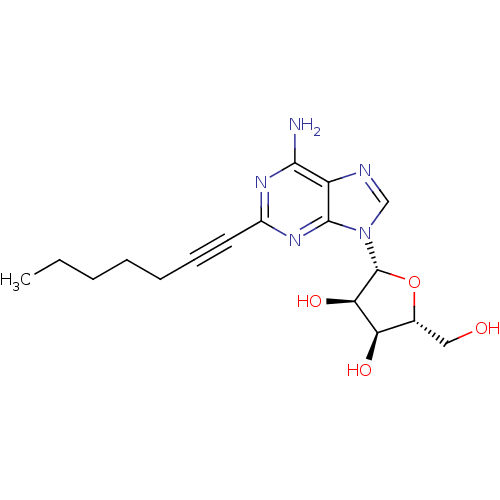 (CHEMBL2113507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Binding affinity against adenosine A2 receptor in rat striatal membranes using [3H]NECA as radioligand | J Med Chem 35: 241-52 (1992) BindingDB Entry DOI: 10.7270/Q20C4WD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50196343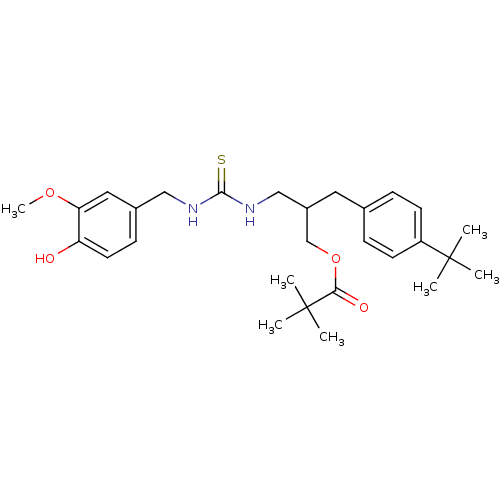 (2,2-Dimethyl-propionic acid 2-(4-tert-butyl-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description In vitro binding affinity towards Rat Vanilloid receptor 1 (VR1) by [3H]-RTX displacement. | J Med Chem 46: 3116-26 (2003) Article DOI: 10.1021/jm030089u BindingDB Entry DOI: 10.7270/Q2SB4551 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2271 total ) | Next | Last >> |