

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094640 (CHEMBL140640 | N-(4-Amino-2-propyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094650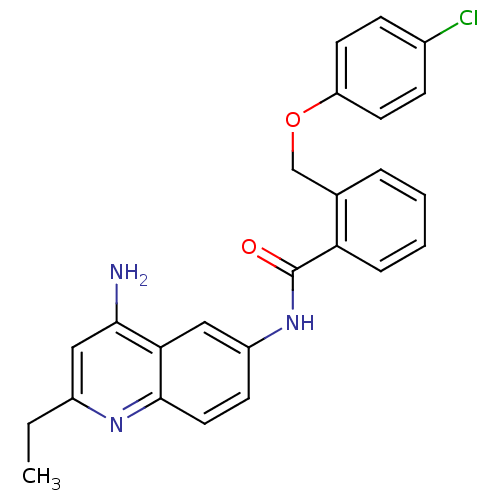 (CHEMBL434060 | N-(4-Amino-2-ethyl-quinolin-6-yl)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094648 (CHEMBL139776 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094651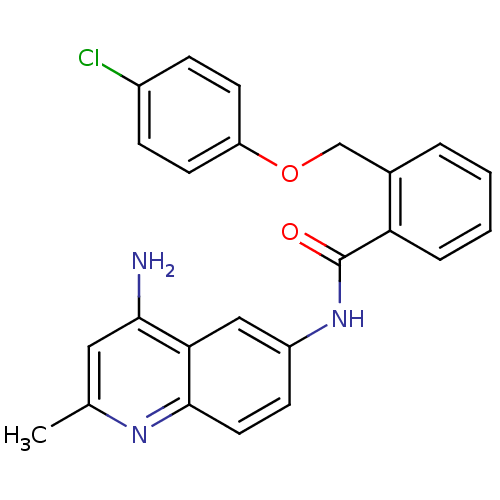 (CHEMBL342580 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094638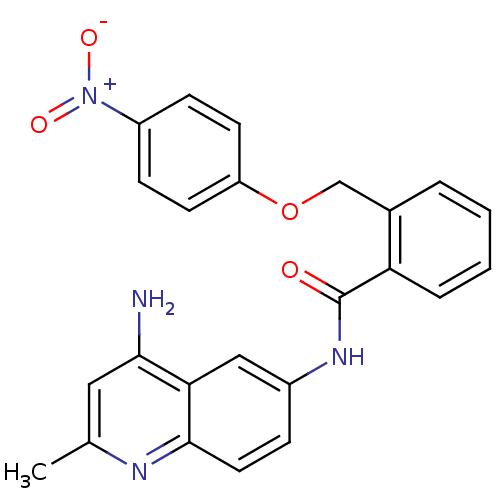 (CHEMBL337128 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094642 (CHEMBL142454 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094646 (CHEMBL139934 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094636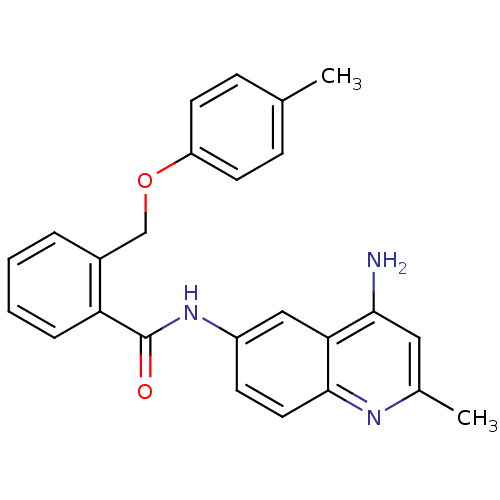 (CHEMBL140103 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094634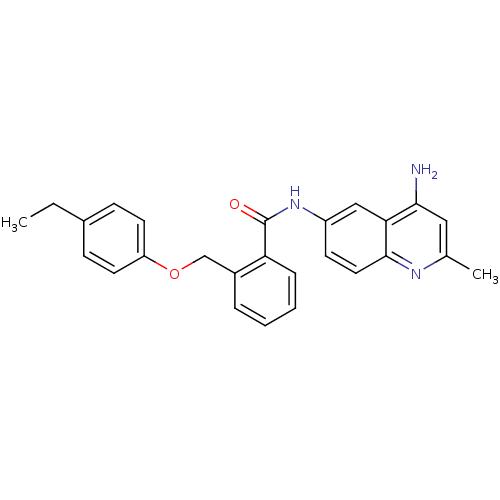 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM28681 (5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094644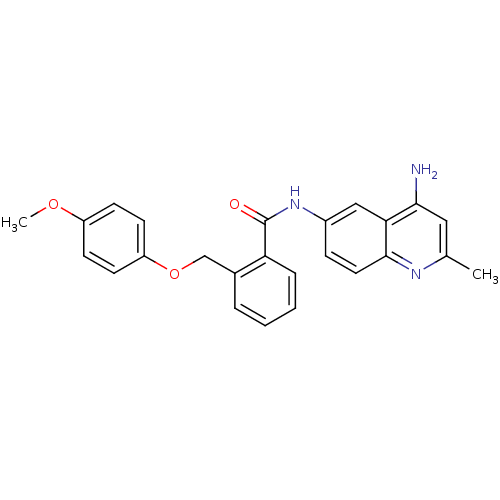 (CHEMBL140519 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094639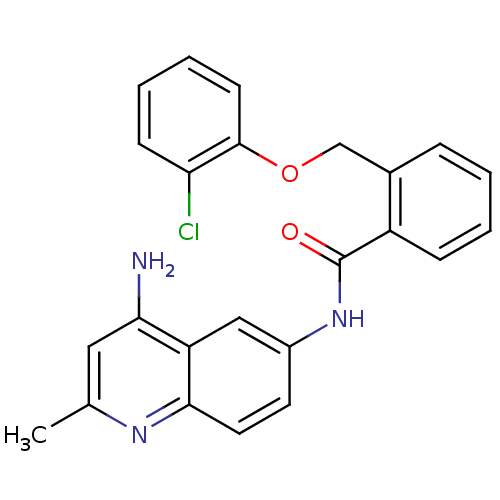 (CHEMBL142999 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094647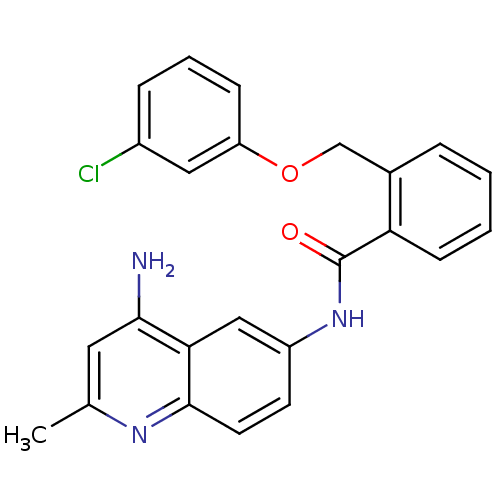 (CHEMBL143605 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094649 (CHEMBL140580 | N-(1-Amino-3-methyl-isoquinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094637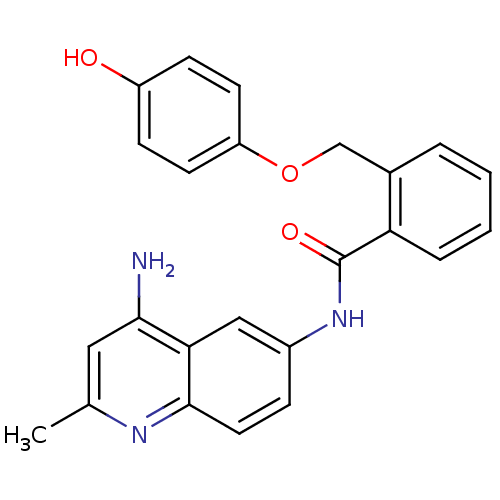 (CHEMBL139566 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094635 (CHEMBL336238 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094643 (CHEMBL358306 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]-nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094641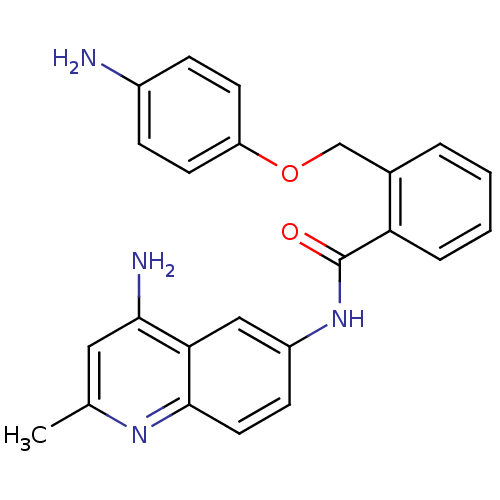 (CHEMBL422641 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094645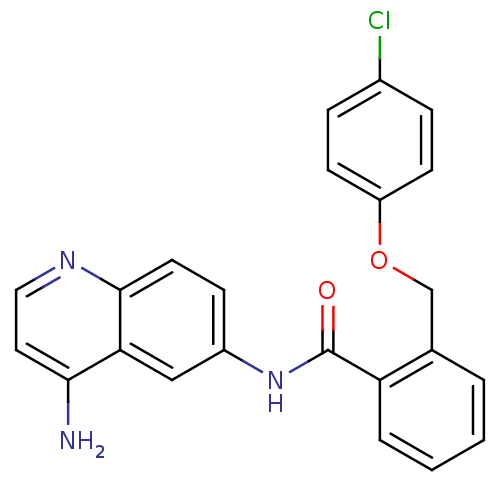 (CHEMBL143243 | N-(4-Amino-quinolin-6-yl)-2-(4-chlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094652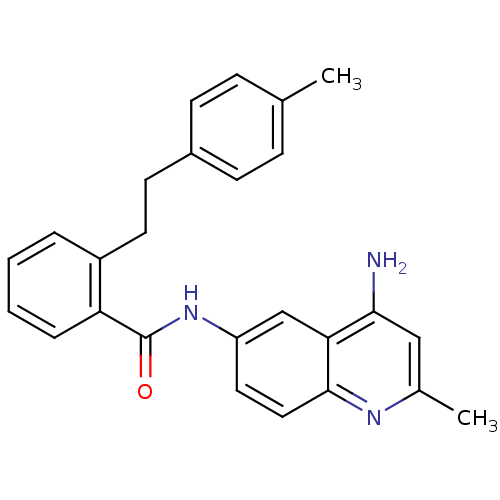 (CHEMBL343424 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]diprenorphine (0.33 nM) binding from human Opioid receptor mu 1 expressed in CHO-K1 cells. | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094653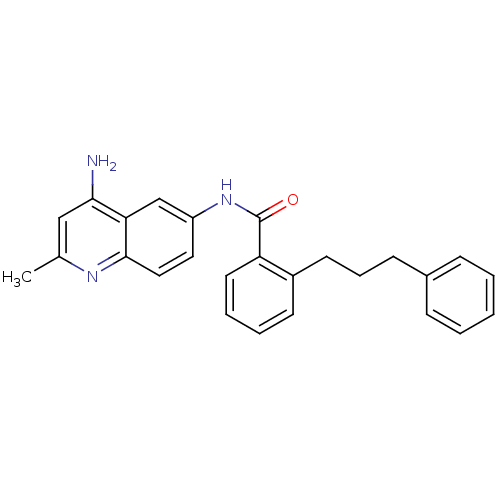 (CHEMBL141078 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50094654 (Biphenyl-2-carboxylic acid (4-amino-2-methyl-quino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]nociceptin ( 0.5 nM ) binding from Opioid receptor like 1 expressed in HeLa cells | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor kappa 1 | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50094634 (CHEMBL140979 | N-(4-Amino-2-methyl-quinolin-6-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 8.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]naltrindole (0.55 nM) binding from human Opioid receptor delta 1 expressed in CHO-K1 cells. | J Med Chem 43: 4667-77 (2001) BindingDB Entry DOI: 10.7270/Q2NK3D9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50428877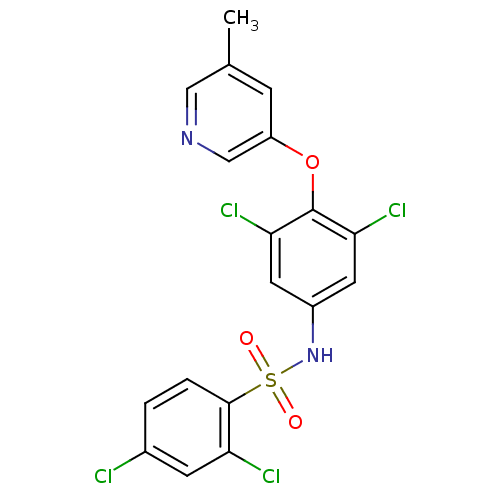 (CHEMBL2338480) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183273 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183275 ((S)-6-(3-chloro-2-fluorobenzyl)-1-(1-hydroxy-3-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183274 (6-(3-Chloro-2-fluorobenzyl)-1-(2-hydroxyethyl)-7-m...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
JT Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | J Med Chem 52: 4869-82 (2009) Article DOI: 10.1021/jm900460z BindingDB Entry DOI: 10.7270/Q2GT5R07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50183274 (6-(3-Chloro-2-fluorobenzyl)-1-(2-hydroxyethyl)-7-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant HIV1 integrase strand transfer activity | J Med Chem 49: 1506-8 (2006) Article DOI: 10.1021/jm0600139 BindingDB Entry DOI: 10.7270/Q2H131NP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50428878 (CHEMBL2338479) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50428881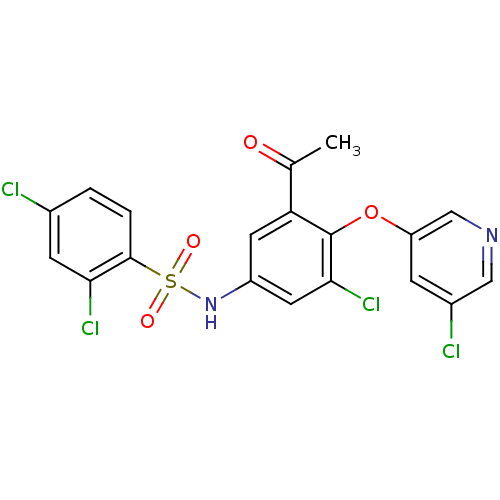 (CHEMBL2331773) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480416 (CHEMBL549549) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
JT Inc. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | J Med Chem 52: 4869-82 (2009) Article DOI: 10.1021/jm900460z BindingDB Entry DOI: 10.7270/Q2GT5R07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM138031 (US8871934, 624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50428885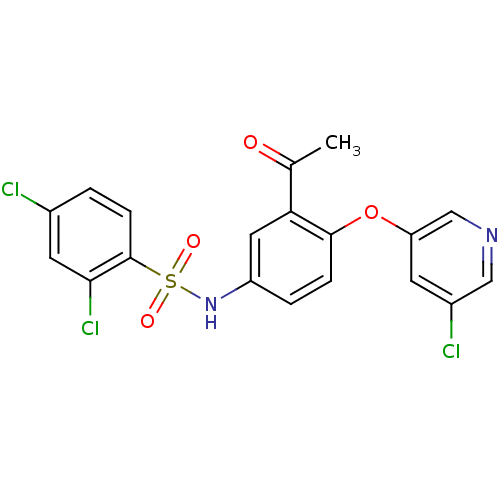 (CHEMBL2331781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50428884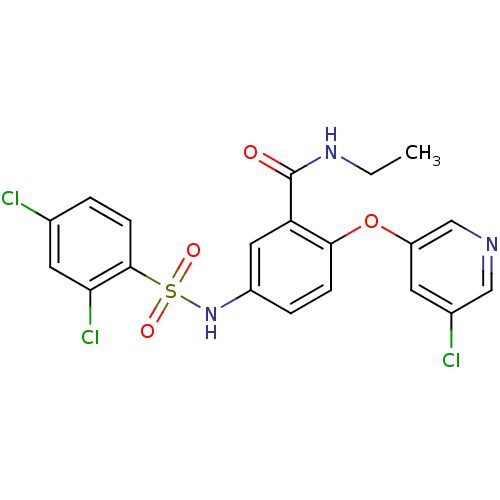 (CHEMBL2331786) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM138071 (US8871934, 664) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM138069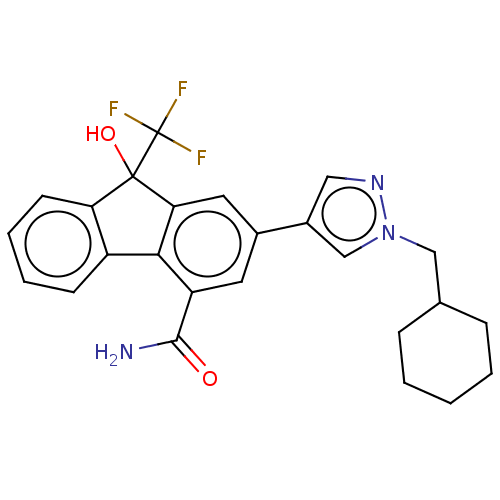 (US8871934, 662 | US8871934, 667) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137919 (US8871934, 512 | US8871934, 533) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137948 (US8871934, 541) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137949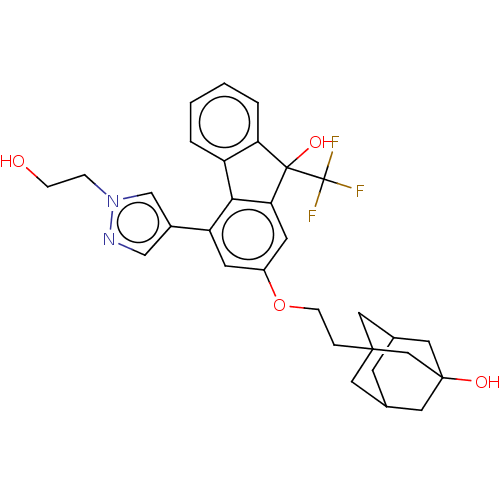 (US8871934, 542) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137953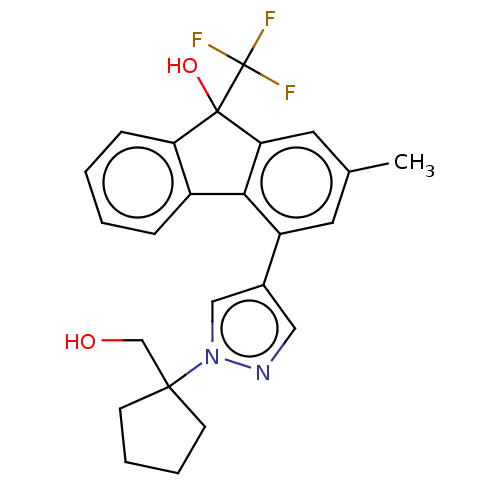 (US8871934, 546) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM138053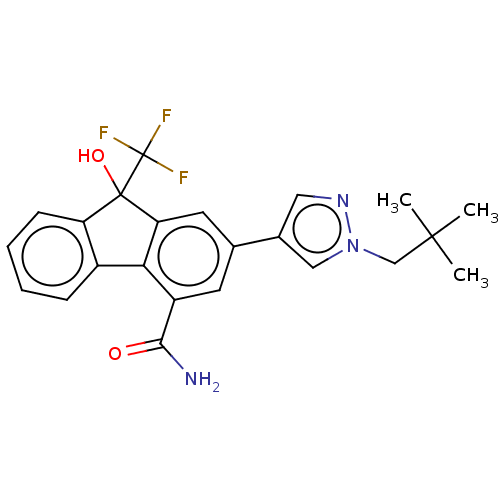 (US8871934, 646 | US8871934, 659) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137914 (US8871934, 507 | US8871934, 532) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137922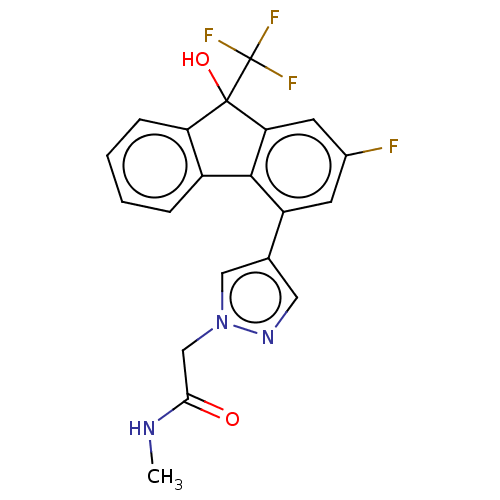 (US8871934, 515 | US8871934, 534) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137947 (US8871934, 540) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137955 (US8871934, 548) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50428854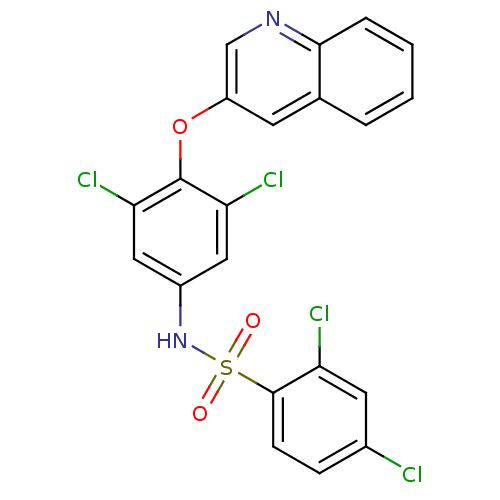 (CHEMBL1236924) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay | Bioorg Med Chem 21: 979-92 (2013) Article DOI: 10.1016/j.bmc.2012.11.058 BindingDB Entry DOI: 10.7270/Q23F4R13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM137693 (US8871934, 286) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| [Pyruvate dehydrogenase (acetyl-transferring)] kinase isozyme 2, mitochondrial (Homo sapiens (Human)) | BDBM138057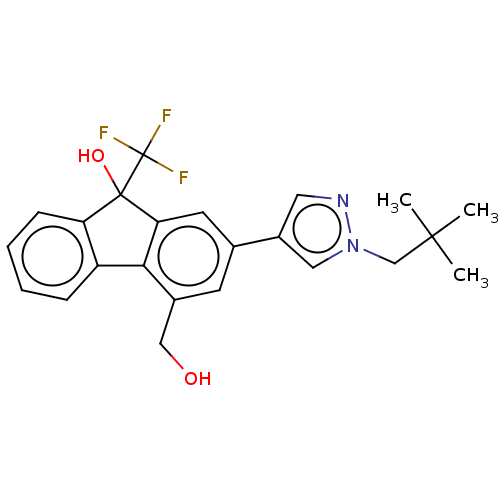 (US8871934, 650 | US8871934, 660) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Japan Tobacco Inc. US Patent | Assay Description The inhibitory action of PDHK activity was indirectly evaluated by performing a kinase reaction in the presence of a test compound and measuring the ... | US Patent US8871934 (2014) BindingDB Entry DOI: 10.7270/Q2JQ0ZQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 899 total ) | Next | Last >> |