

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197875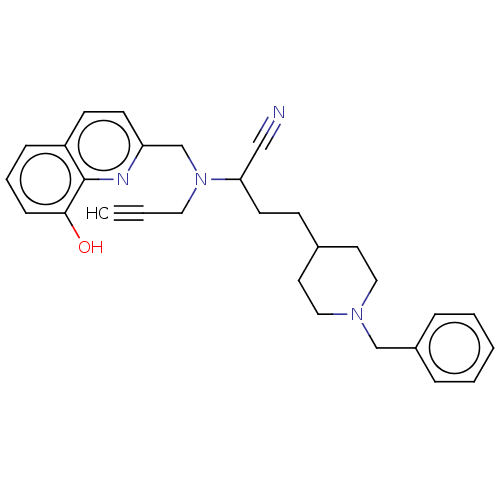 (CHEMBL3910142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Mixed-type inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins follow... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Mixed-type inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins fo... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197879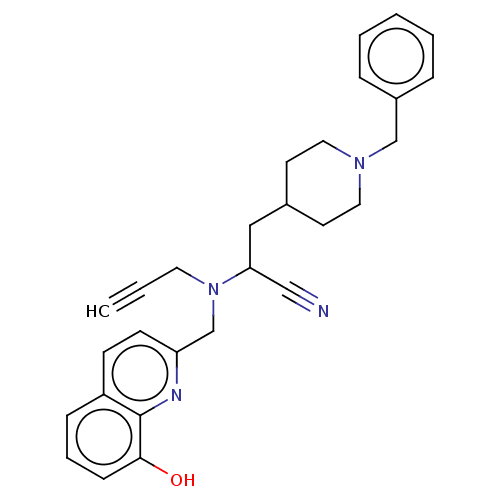 (CHEMBL3972214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197878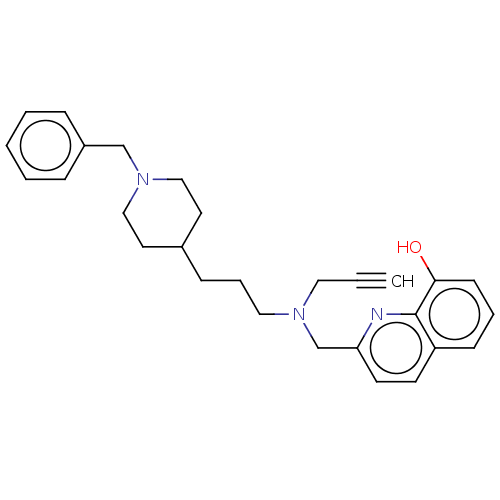 (CHEMBL3961103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197876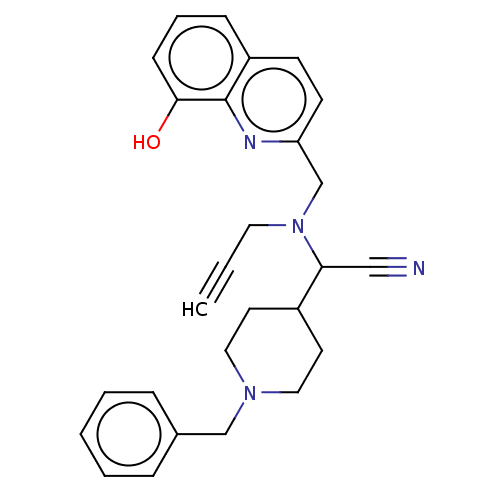 (CHEMBL3901082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197880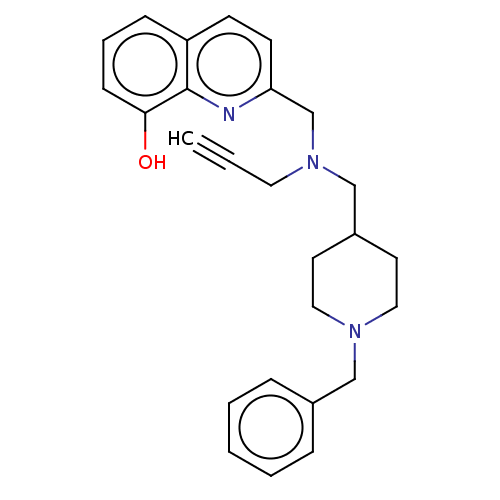 (CHEMBL3931599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50197877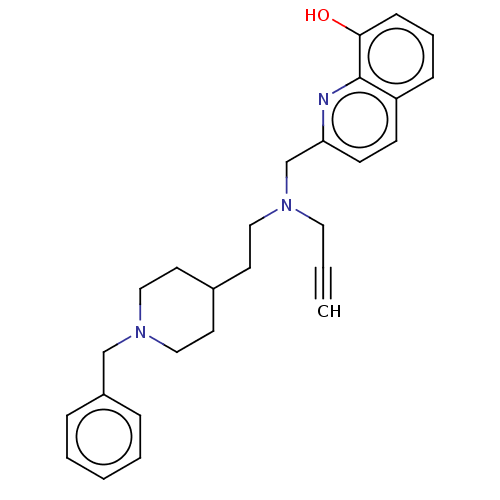 (CHEMBL3892118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50018671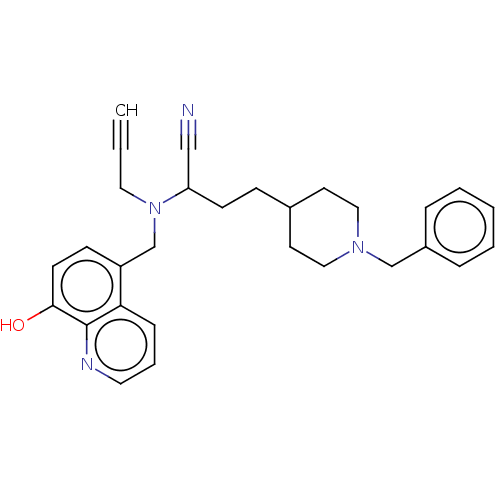 (CHEMBL3291019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 240 mins followed by substrate addition measured for 1 hr by hors... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197878 (CHEMBL3961103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 180 mins followed by substrate addition measured for 1 hr by hors... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 120 mins followed by substrate addition measured for 1 hr by hors... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50018671 (CHEMBL3291019) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197879 (CHEMBL3972214) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 60 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197877 (CHEMBL3892118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50018671 (CHEMBL3291019) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197880 (CHEMBL3931599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197876 (CHEMBL3901082) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50018671 (CHEMBL3291019) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197879 (CHEMBL3972214) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 15 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197877 (CHEMBL3892118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197878 (CHEMBL3961103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197880 (CHEMBL3931599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50197876 (CHEMBL3901082) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50197877 (CHEMBL3892118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50197880 (CHEMBL3931599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50197879 (CHEMBL3972214) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50197878 (CHEMBL3961103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50197876 (CHEMBL3901082) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50197875 (CHEMBL3910142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate measured for 1 hr by horse-radish peroxidase/amplex red-based fluorometric method | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant BuChE expressed in HEK293 cells using S-butyrylthiocholine iodide as substrate preincubated for 30 mins followed by s... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate preincubated for 30 mins followed by subst... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate preincubated for 30 mins followed by substrate addition measured for 1 hr by horse... | Eur J Med Chem 121: 864-879 (2016) Article DOI: 10.1016/j.ejmech.2015.10.001 BindingDB Entry DOI: 10.7270/Q2B27X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||