

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305129 (6-(2-aminoethoxy)-2-(4-phenoxyphenyl)-9,10-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305140 (7-(2-aminoethoxy)-2-(4-phenoxyphenyl)-9,10-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305119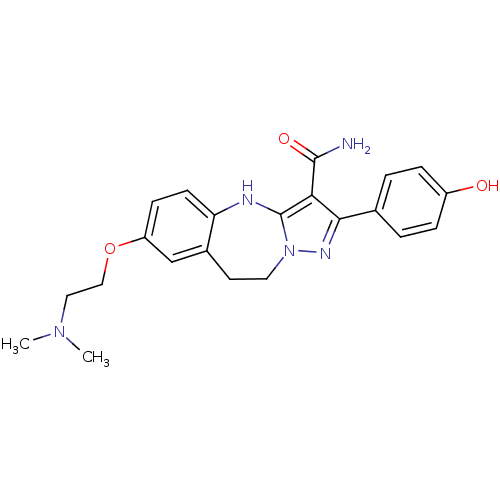 (7-(2-(dimethylamino)ethoxy)-2-(4-hydroxyphenyl)-9,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305131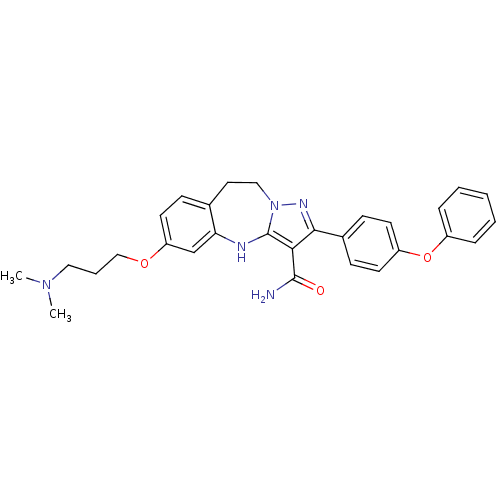 (6-(3-(dimethylamino)propoxy)-2-(4-phenoxyphenyl)-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305139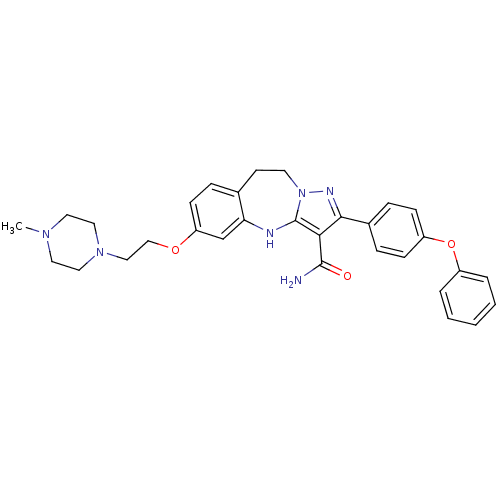 (6-(2-(4-methylpiperazin-1-yl)ethoxy)-2-(4-phenoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305117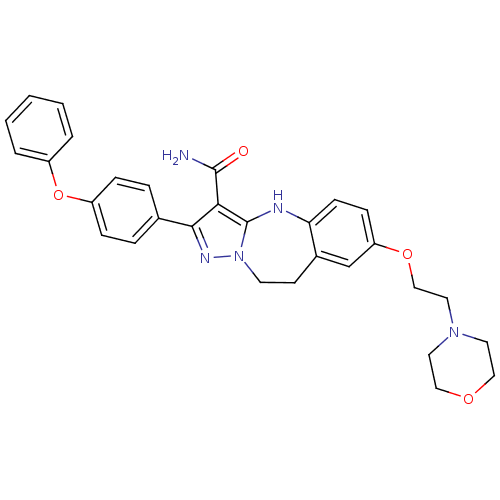 (7-(2-morpholinoethoxy)-2-(4-phenoxyphenyl)-9,10-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50305126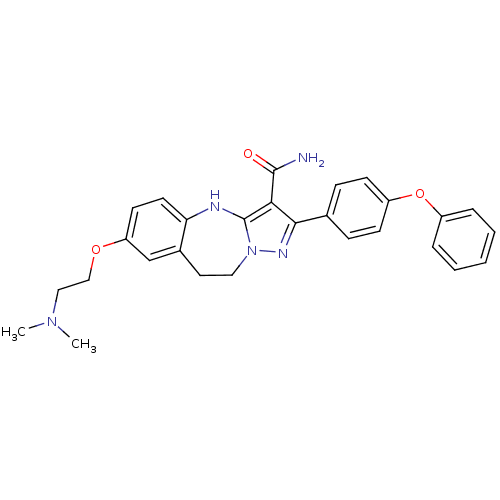 (7-(2-(dimethylamino)ethoxy)-2-(4-phenoxyphenyl)-9,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305126 (7-(2-(dimethylamino)ethoxy)-2-(4-phenoxyphenyl)-9,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305142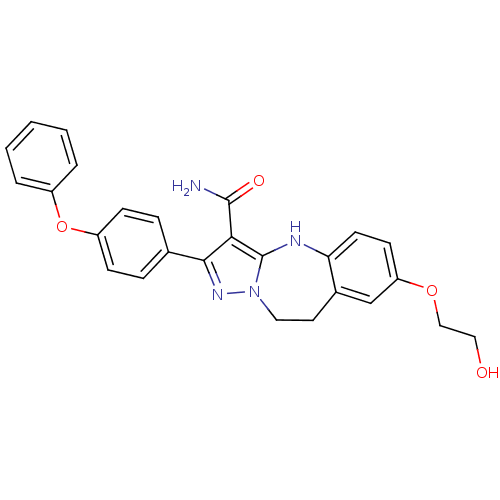 (7-(2-hydroxyethoxy)-2-(4-phenoxyphenyl)-9,10-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305138 (6-(2-morpholinoethoxy)-2-(4-phenoxyphenyl)-9,10-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305121 (7-(2-(dimethylamino)ethoxy)-2-(4-(phenylamino)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305125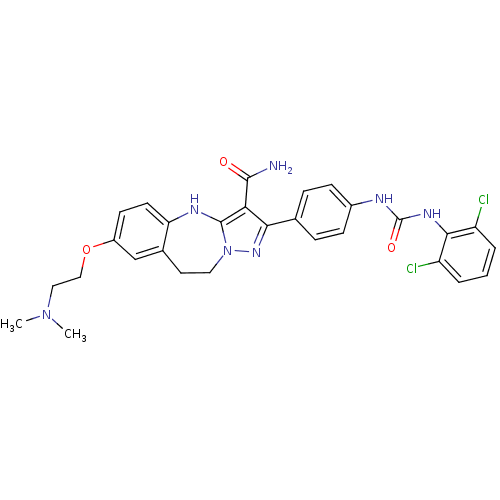 (2-(4-(3-(2,6-dichlorophenyl)ureido)phenyl)-7-(2-(d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305141 (7-(3-(dimethylamino)propoxy)-2-(4-phenoxyphenyl)-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305135 (2-(3-carbamoyl-2-(4-phenoxyphenyl)-9,10-dihydro-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305122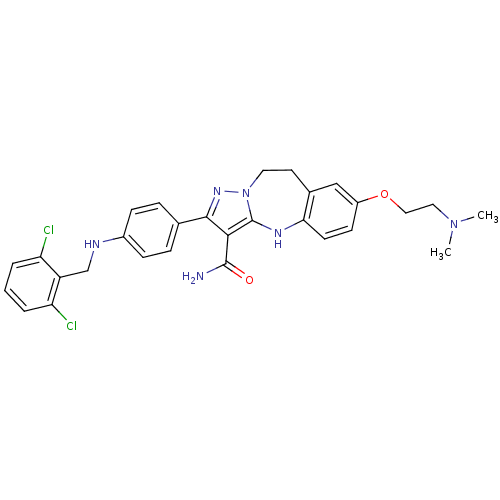 (2-(4-(2,6-dichlorobenzylamino)phenyl)-7-(2-(dimeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305118 (7-(2-(4-methylpiperazin-1-yl)ethoxy)-2-(4-phenoxyp...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305143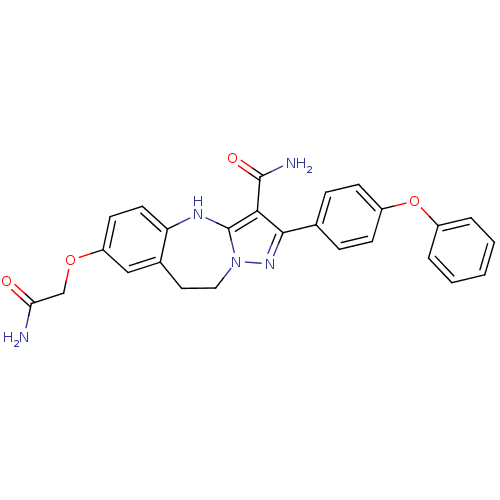 (7-(2-amino-2-oxoethoxy)-2-(4-phenoxyphenyl)-9,10-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305130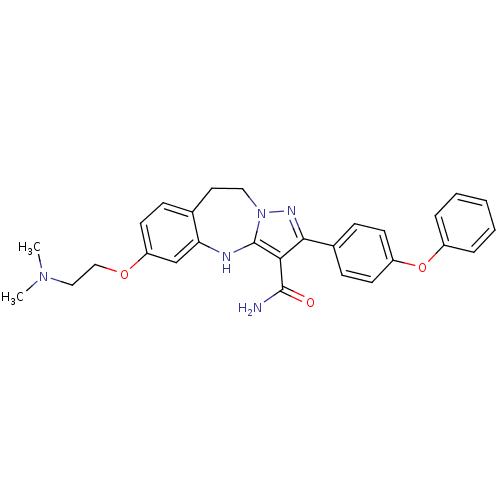 (6-(2-(dimethylamino)ethoxy)-2-(4-phenoxyphenyl)-9,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305132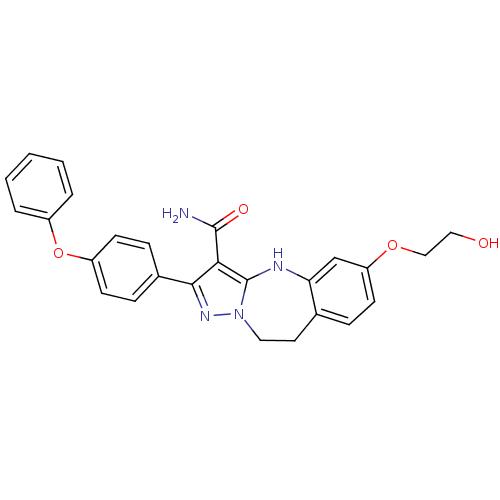 (6-(2-hydroxyethoxy)-2-(4-phenoxyphenyl)-9,10-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305134 (6-(2-amino-2-oxoethoxy)-2-(4-phenoxyphenyl)-9,10-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM50305126 (7-(2-(dimethylamino)ethoxy)-2-(4-phenoxyphenyl)-9,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of Csk | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305123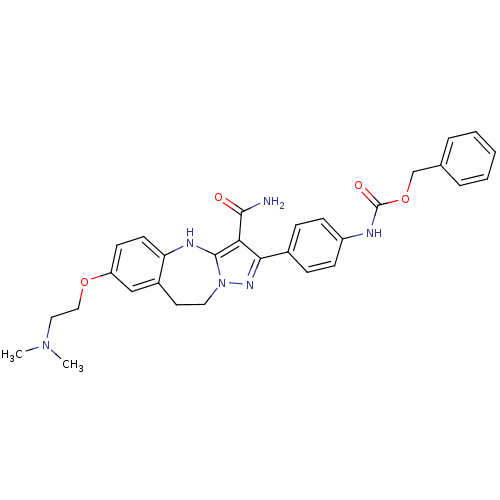 (CHEMBL595625 | benzyl 4-(3-carbamoyl-7-(2-(dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305124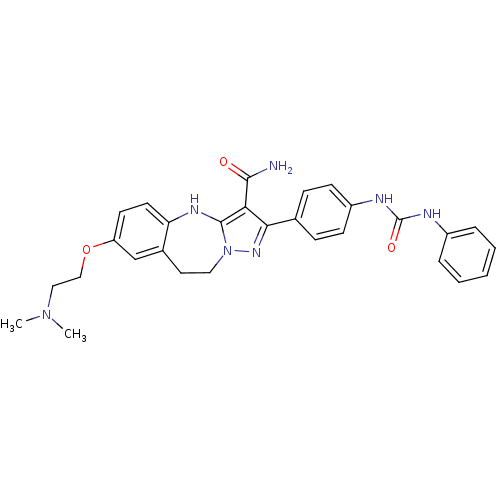 (7-(2-(dimethylamino)ethoxy)-2-(4-(3-phenylureido)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305128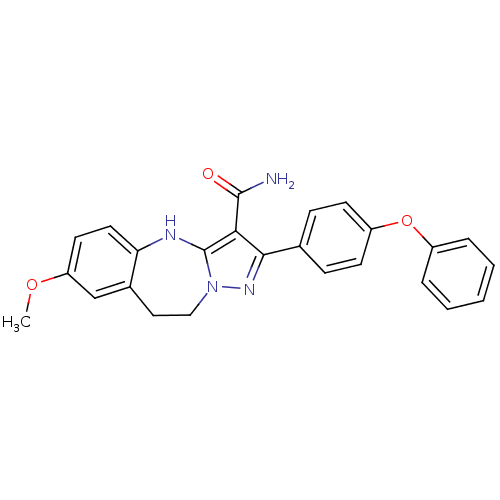 (7-methoxy-2-(4-phenoxyphenyl)-9,10-dihydro-4H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305145 (2-(4-phenoxyphenyl)-7-(pyridin-3-ylmethoxy)-9,10-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305136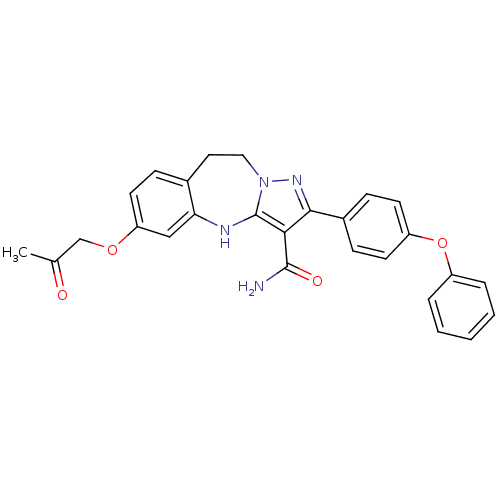 (6-(2-oxopropoxy)-2-(4-phenoxyphenyl)-9,10-dihydro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305120 (2-(4-aminophenyl)-7-(2-(dimethylamino)ethoxy)-9,10...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase CSK (Homo sapiens (Human)) | BDBM50305146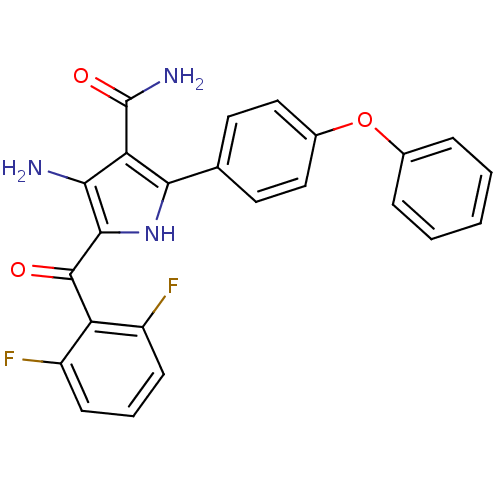 (4-amino-5-(2,6-difluorobenzoyl)-2-(4-phenoxyphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of Csk | Bioorg Med Chem Lett 20: 108-11 (2010) Article DOI: 10.1016/j.bmcl.2009.11.014 BindingDB Entry DOI: 10.7270/Q2Z60P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305127 (6-methoxy-2-(4-phenoxyphenyl)-9,10-dihydro-4H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305144 (2-(4-phenoxyphenyl)-7-(pyridin-2-ylmethoxy)-9,10-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50365553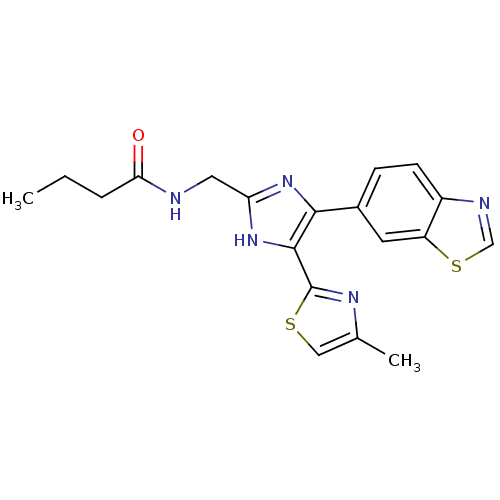 (CHEMBL1957675) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-fused ALK5 using TMB substrate after 30 mins by ELISA | Bioorg Med Chem Lett 22: 2024-9 (2012) Article DOI: 10.1016/j.bmcl.2012.01.066 BindingDB Entry DOI: 10.7270/Q2668DPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305137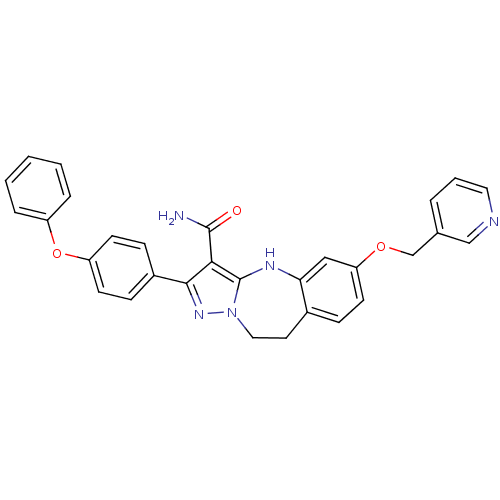 (2-(4-phenoxyphenyl)-6-(pyridin-3-ylmethoxy)-9,10-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50305116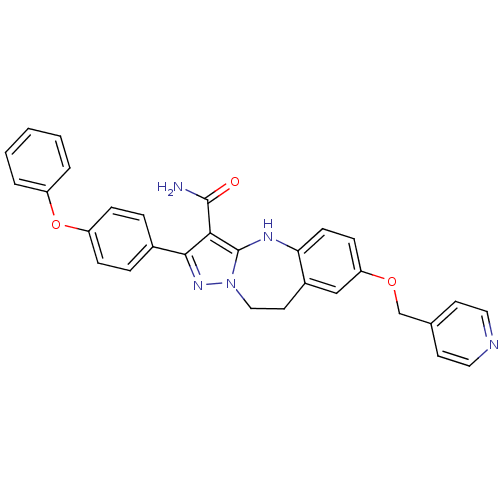 (2-(4-phenoxyphenyl)-7-(pyridin-4-ylmethoxy)-9,10-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of GST-tagged human LCK by scintillation counting | Bioorg Med Chem Lett 20: 112-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.013 BindingDB Entry DOI: 10.7270/Q22Z15NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503838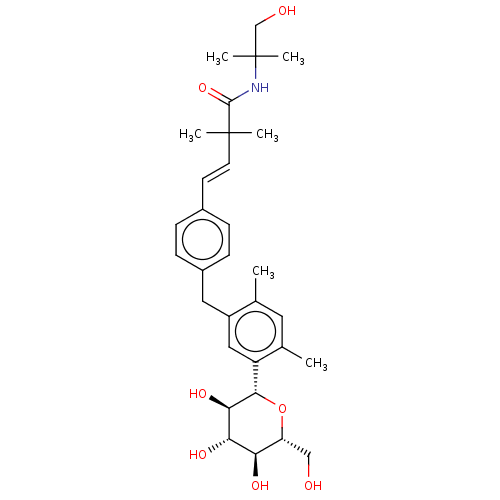 (CHEMBL4450704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242568 (US9422240, 1-378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242567 (US9422240, 1-377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242554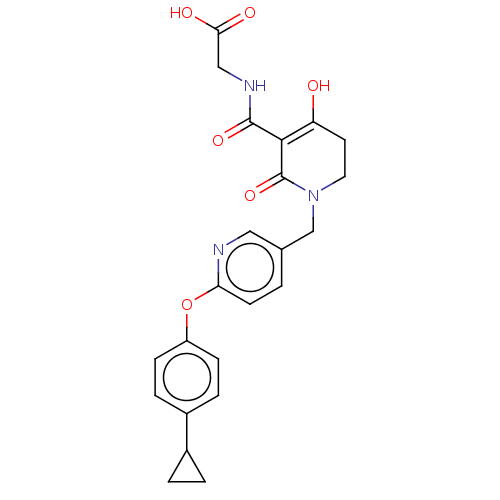 (US9422240, 1-296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503834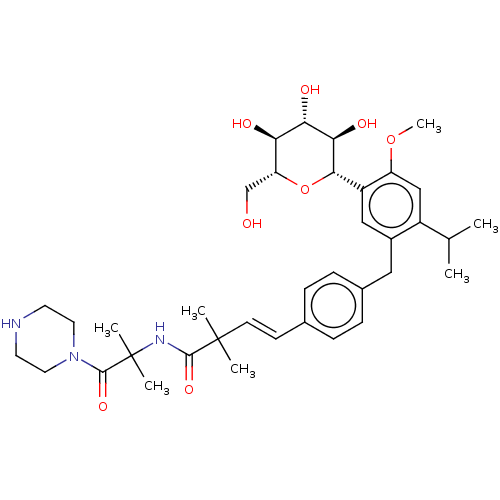 (CHEMBL4591009) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503827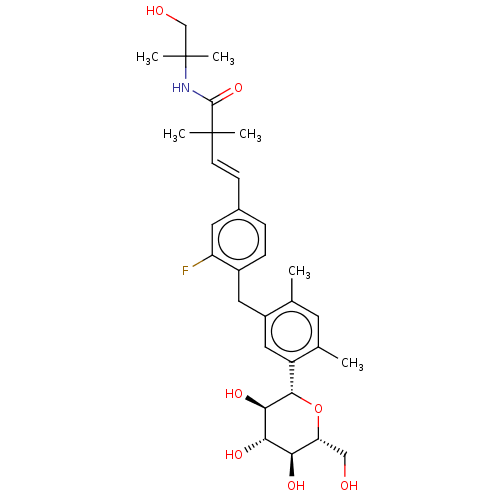 (CHEMBL4439652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503822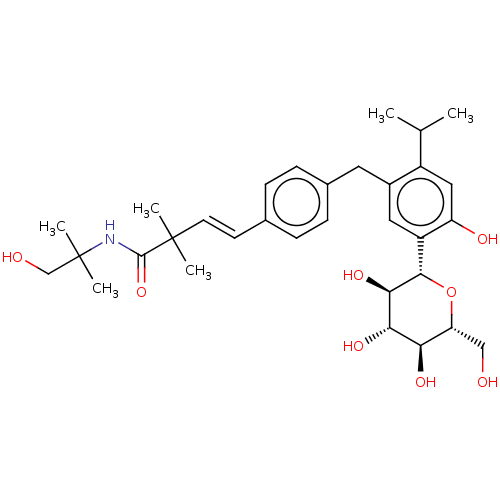 (CHEMBL4460788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242526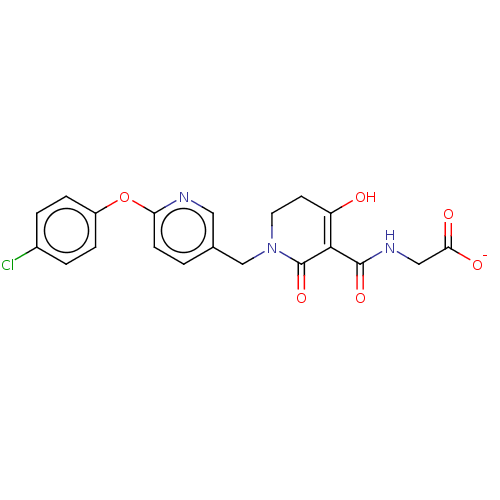 (US9422240, 1-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242563 (US9422240, 1-366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 2 (Homo sapiens (Human)) | BDBM50503841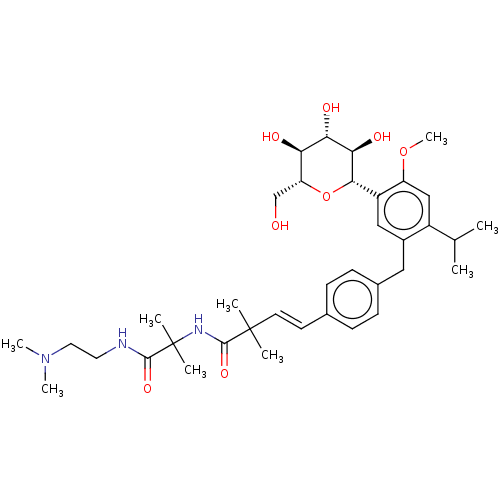 (CHEMBL4554040) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT2 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 1 hr in presence of [14C]-methyl-al... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242577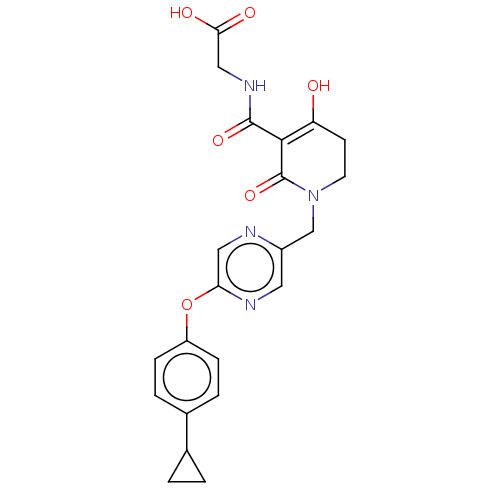 (US9422240, 1-443) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503835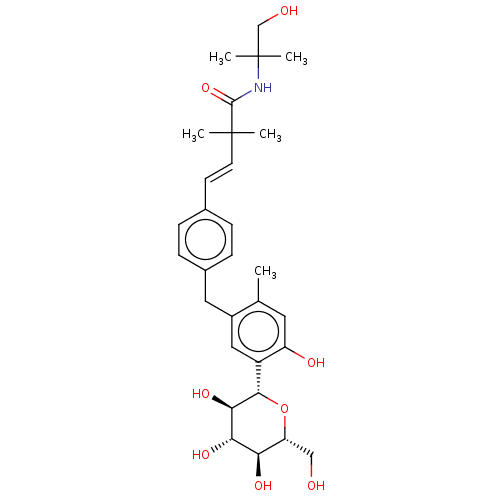 (CHEMBL4524341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242566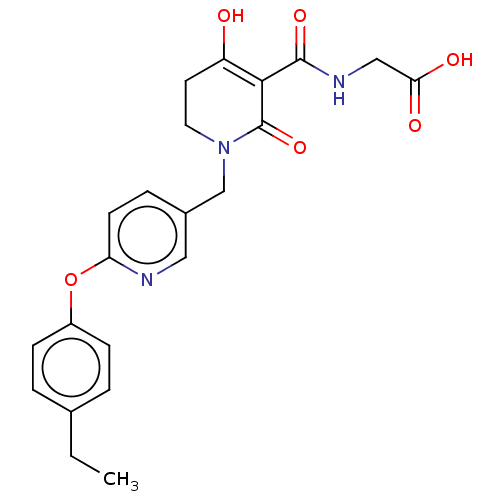 (US9422240, 1-376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503838 (CHEMBL4450704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM50503822 (CHEMBL4460788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SGLT1 expressed in CHOK1 cells assessed as reduction in sodium-dependent glucose uptake after 30 mins in presence of [14C]-methyl... | Bioorg Med Chem 27: 394-409 (2019) Article DOI: 10.1016/j.bmc.2018.12.015 BindingDB Entry DOI: 10.7270/Q2X63R7F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50305146 (4-amino-5-(2,6-difluorobenzoyl)-2-(4-phenoxyphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of Src | Bioorg Med Chem Lett 20: 108-11 (2010) Article DOI: 10.1016/j.bmcl.2009.11.014 BindingDB Entry DOI: 10.7270/Q2Z60P5W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM242542 (US9422240, 1-265 | US9422240, 1-355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
TAISHO PHARMACEUTICAL CO., LTD US Patent | Assay Description The enzyme and the substrate were each diluted with a 50 mM tris-hydrochloric acid buffer (pH 7.5) containing 12.5 mM KCl, 3.75 mM MgCl2, 25 μM ... | US Patent US9422240 (2016) BindingDB Entry DOI: 10.7270/Q24B307K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 281 total ) | Next | Last >> |