 Found 385 hits with Last Name = 'shu' and Initial = 'hh'
Found 385 hits with Last Name = 'shu' and Initial = 'hh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostacyclin receptor
(RAT) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235370
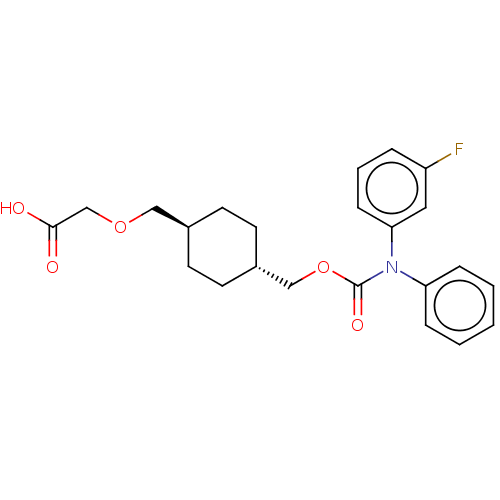
(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(RAT) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(RAT) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant rat IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation count... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235380

(CHEMBL3917503)Show SMILES OS(=O)(=O)CCNC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:15.15,wD:12.11,(32.65,-26.35,;31.1,-26.35,;29.77,-25.58,;31.1,-24.81,;30.34,-27.69,;31.11,-29.02,;30.34,-30.36,;28.8,-30.36,;28.03,-29.02,;28.03,-31.69,;28.8,-33.02,;28.03,-34.36,;26.49,-34.36,;25.72,-33.02,;24.18,-33.02,;23.41,-34.36,;21.87,-34.36,;21.1,-35.69,;19.56,-35.69,;18.79,-34.36,;18.79,-37.02,;19.56,-38.36,;18.79,-39.69,;19.56,-41.02,;21.1,-41.02,;21.87,-39.69,;21.1,-38.36,;17.25,-37.02,;16.48,-35.69,;14.94,-35.69,;14.17,-37.02,;12.63,-37.02,;14.94,-38.36,;16.48,-38.36,;24.18,-35.69,;25.72,-35.69,)| Show InChI InChI=1S/C25H31ClN2O7S/c26-21-10-12-23(13-11-21)28(22-4-2-1-3-5-22)25(30)35-17-20-8-6-19(7-9-20)16-34-18-24(29)27-14-15-36(31,32)33/h1-5,10-13,19-20H,6-9,14-18H2,(H,27,29)(H,31,32,33)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-iloprost from recombinant human IP receptor expressed in CHO-K1 cell membranes incubated for 1 hr by top count scintillation cou... |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP2 receptor expressed in HEK293 cell membranes incubated for 1 hr |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 678 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from recombinant human DP1 receptor |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGD2 from recombinant human DP1 receptor |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to 5-hydroxytryptamine 3 receptor in rat entorhinal cortex using [3H]-BRL 43694 as radioligand |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235375
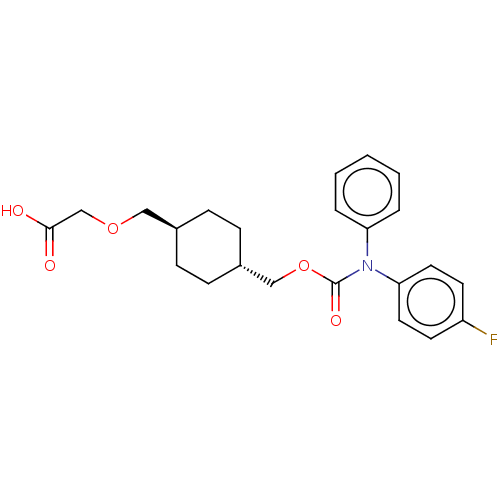
(CHEMBL3975122)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(F)cc2)CC1 |r,wU:6.5,wD:9.9,(31.35,-33.64,;30.02,-34.41,;30.02,-35.95,;28.69,-33.64,;28.69,-32.1,;27.35,-31.33,;26.02,-32.1,;26.02,-33.64,;24.69,-34.41,;23.35,-33.64,;22.02,-34.41,;20.68,-33.64,;19.35,-34.41,;19.35,-35.95,;18.02,-33.64,;18.02,-32.1,;16.68,-31.33,;16.68,-29.79,;18.02,-29.02,;19.35,-29.79,;19.35,-31.33,;16.68,-34.41,;16.68,-35.95,;15.35,-36.72,;14.02,-35.95,;12.68,-36.72,;14.02,-34.41,;15.35,-33.64,;23.35,-32.1,;24.69,-31.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235370

(CHEMBL3933704)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235378
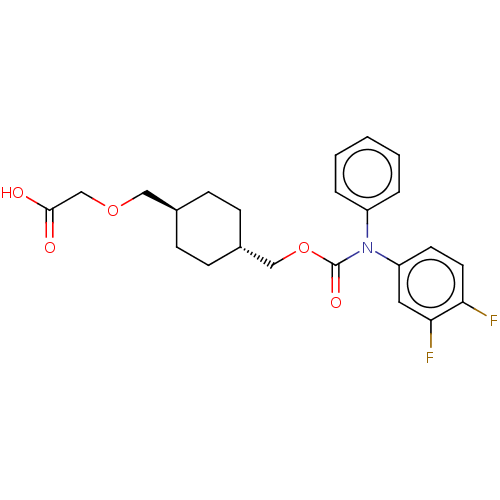
(CHEMBL3981509)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(F)c(F)c2)CC1 |r,wU:6.5,wD:9.9,(31.35,-32.87,;30.02,-33.64,;30.02,-35.18,;28.69,-32.87,;28.69,-31.33,;27.35,-30.56,;26.02,-31.33,;26.02,-32.87,;24.69,-33.64,;23.35,-32.87,;22.02,-33.64,;20.68,-32.87,;19.35,-33.64,;19.35,-35.18,;18.02,-32.87,;18.02,-31.33,;16.68,-30.56,;16.68,-29.02,;18.02,-28.25,;19.35,-29.02,;19.35,-30.56,;16.68,-33.64,;15.35,-32.87,;14.02,-33.64,;14.02,-35.18,;12.68,-35.95,;15.35,-35.95,;15.35,-37.49,;16.68,-35.18,;23.35,-31.33,;24.69,-30.56,)| Show InChI InChI=1S/C23H25F2NO5/c24-20-11-10-19(12-21(20)25)26(18-4-2-1-3-5-18)23(29)31-14-17-8-6-16(7-9-17)13-30-15-22(27)28/h1-5,10-12,16-17H,6-9,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235368
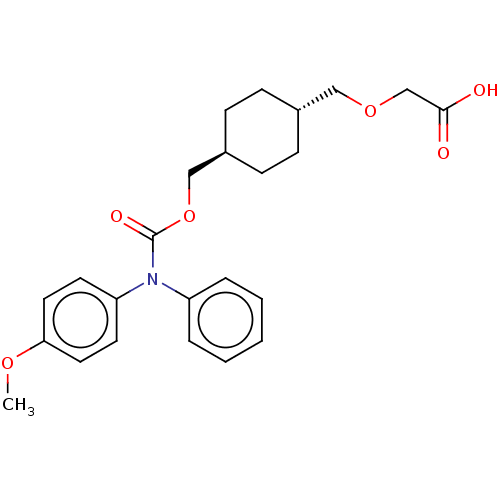
(CHEMBL3893346)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1ccccc1 |r,wU:16.17,wD:13.13,(12.53,-35.95,;13.86,-36.72,;15.19,-35.95,;16.53,-36.72,;17.86,-35.95,;17.86,-34.41,;16.53,-33.64,;15.19,-34.41,;19.19,-33.64,;20.53,-34.41,;20.53,-35.95,;21.86,-33.64,;23.2,-34.41,;24.53,-33.64,;25.86,-34.41,;27.2,-33.64,;27.2,-32.1,;28.53,-31.33,;29.86,-32.1,;29.86,-33.64,;31.2,-34.41,;32.53,-33.64,;31.2,-35.95,;25.86,-31.33,;24.53,-32.1,;19.19,-32.1,;17.86,-31.33,;17.86,-29.79,;19.19,-29.02,;20.53,-29.79,;20.53,-31.33,)| Show InChI InChI=1S/C24H29NO6/c1-29-22-13-11-21(12-14-22)25(20-5-3-2-4-6-20)24(28)31-16-19-9-7-18(8-10-19)15-30-17-23(26)27/h2-6,11-14,18-19H,7-10,15-17H2,1H3,(H,26,27)/t18-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235373
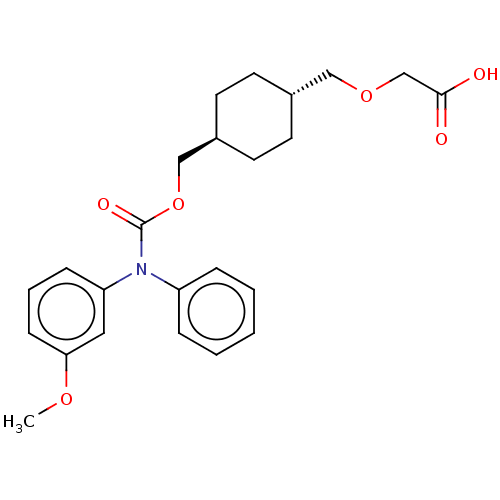
(CHEMBL3928729)Show SMILES COc1cccc(c1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1ccccc1 |r,wU:16.17,wD:13.13,(30.85,-28.01,;29.51,-28.78,;29.51,-30.32,;30.85,-31.09,;30.85,-32.63,;29.51,-33.4,;28.18,-32.63,;28.18,-31.09,;26.85,-33.4,;25.51,-32.63,;25.51,-31.09,;24.18,-33.4,;22.85,-32.63,;21.51,-33.4,;20.18,-32.63,;18.84,-33.4,;18.84,-34.94,;17.51,-35.71,;16.18,-34.94,;16.18,-33.4,;14.84,-32.63,;13.51,-33.4,;14.84,-31.09,;20.18,-35.71,;21.51,-34.94,;26.85,-34.94,;28.18,-35.71,;28.18,-37.25,;26.85,-38.02,;25.51,-37.25,;25.51,-35.71,)| Show InChI InChI=1S/C24H29NO6/c1-29-22-9-5-8-21(14-22)25(20-6-3-2-4-7-20)24(28)31-16-19-12-10-18(11-13-19)15-30-17-23(26)27/h2-9,14,18-19H,10-13,15-17H2,1H3,(H,26,27)/t18-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against cathepsin B |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235376
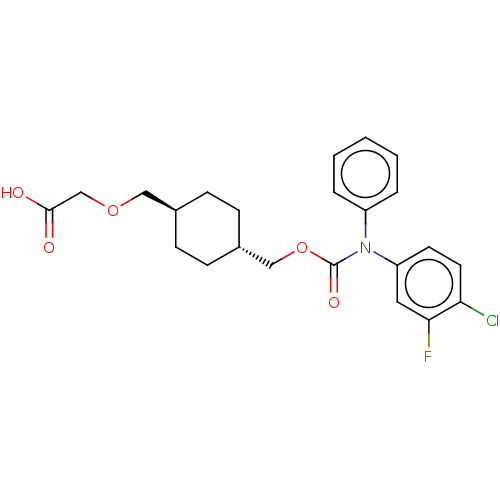
(CHEMBL3926078)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)c(F)c2)CC1 |r,wU:6.5,wD:9.9,(31.59,-32.87,;30.26,-33.64,;30.26,-35.18,;28.93,-32.87,;28.93,-31.33,;27.59,-30.56,;26.26,-31.33,;26.26,-32.87,;24.92,-33.64,;23.59,-32.87,;22.26,-33.64,;20.92,-32.87,;19.59,-33.64,;19.59,-35.18,;18.26,-32.87,;18.26,-31.33,;16.92,-30.56,;16.92,-29.02,;18.26,-28.25,;19.59,-29.02,;19.59,-30.56,;16.92,-33.64,;15.59,-32.87,;14.26,-33.64,;14.26,-35.18,;12.92,-35.95,;15.59,-35.95,;15.59,-37.49,;16.92,-35.18,;23.59,-31.33,;24.92,-30.56,)| Show InChI InChI=1S/C23H25ClFNO5/c24-20-11-10-19(12-21(20)25)26(18-4-2-1-3-5-18)23(29)31-14-17-8-6-16(7-9-17)13-30-15-22(27)28/h1-5,10-12,16-17H,6-9,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235367
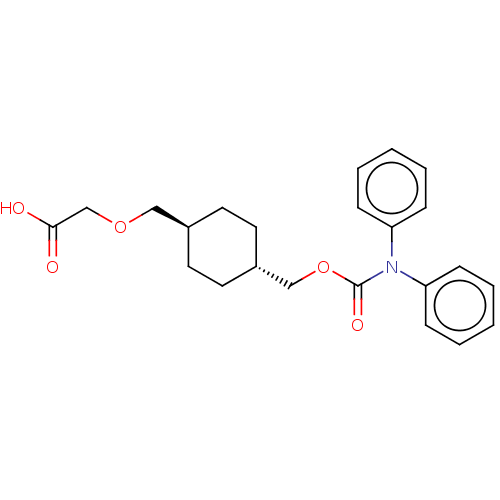
(CHEMBL3952237)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccccc2)CC1 |r,wU:6.5,wD:9.9,(30.68,-33.76,;29.35,-34.53,;29.35,-36.07,;28.02,-33.76,;28.02,-32.22,;26.68,-31.45,;25.35,-32.22,;25.35,-33.76,;24.02,-34.53,;22.68,-33.76,;21.35,-34.53,;20.01,-33.76,;18.68,-34.53,;18.68,-36.07,;17.35,-33.76,;16.01,-34.53,;16.01,-36.07,;14.68,-36.84,;13.35,-36.07,;13.35,-34.53,;14.68,-33.76,;17.35,-32.22,;16.01,-31.45,;16.01,-29.91,;17.35,-29.14,;18.68,-29.91,;18.68,-31.45,;22.68,-32.22,;24.02,-31.45,)| Show InChI InChI=1S/C23H27NO5/c25-22(26)17-28-15-18-11-13-19(14-12-18)16-29-23(27)24(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-10,18-19H,11-17H2,(H,25,26)/t18-,19- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235389

(CHEMBL3983767)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccc(F)cc2)c2cccc(F)c2)CC1 |r,wU:6.5,wD:9.9,(30.69,-33.67,;29.35,-34.44,;29.35,-35.98,;28.02,-33.67,;28.02,-32.13,;26.69,-31.36,;25.35,-32.13,;25.35,-33.67,;24.02,-34.44,;22.69,-33.67,;21.35,-34.44,;20.02,-33.67,;18.68,-34.44,;18.68,-35.98,;17.35,-33.67,;17.35,-32.13,;16.02,-31.36,;16.02,-29.82,;17.35,-29.05,;17.35,-27.51,;18.68,-29.82,;18.68,-31.36,;16.02,-34.44,;14.68,-33.67,;13.35,-34.44,;13.35,-35.98,;14.68,-36.75,;14.68,-38.29,;16.02,-35.98,;22.69,-32.13,;24.02,-31.36,)| Show InChI InChI=1S/C23H25F2NO5/c24-18-8-10-20(11-9-18)26(21-3-1-2-19(25)12-21)23(29)31-14-17-6-4-16(5-7-17)13-30-15-22(27)28/h1-3,8-12,16-17H,4-7,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235385

(APD-811 | Ralinepag)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:6.5,wD:9.9,(-11.29,-9.03,;-9.96,-9.8,;-9.96,-11.34,;-8.62,-9.03,;-7.29,-9.8,;-5.96,-9.03,;-4.62,-9.8,;-3.29,-9.03,;-1.96,-9.8,;-1.96,-11.34,;-.62,-12.11,;.71,-11.34,;2.04,-12.11,;2.04,-13.65,;3.38,-11.34,;3.38,-9.8,;2.04,-9.03,;2.04,-7.49,;3.38,-6.72,;4.71,-7.49,;4.71,-9.03,;4.71,-12.11,;6.05,-11.34,;7.38,-12.11,;7.38,-13.65,;8.71,-14.42,;6.05,-14.42,;4.71,-13.65,;-3.29,-12.11,;-4.62,-11.34,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235374
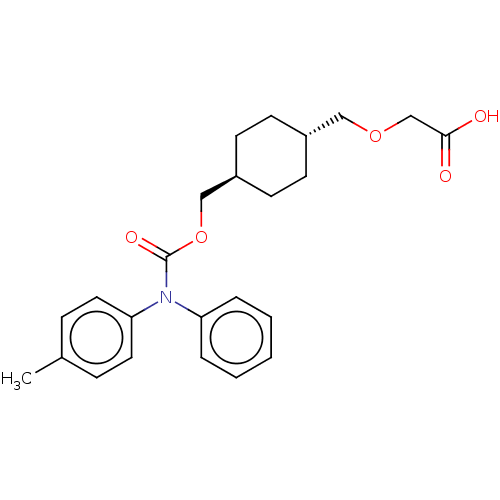
(CHEMBL3935924)Show SMILES Cc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1ccccc1 |r,wU:15.16,wD:12.12,(13.2,-36.56,;14.53,-35.79,;15.86,-36.56,;17.2,-35.79,;17.2,-34.25,;15.86,-33.48,;14.53,-34.25,;18.53,-33.48,;19.87,-34.25,;19.87,-35.79,;21.2,-33.48,;22.53,-34.25,;23.87,-33.48,;25.2,-34.25,;26.53,-33.48,;26.53,-31.94,;27.87,-31.17,;29.2,-31.94,;29.2,-33.48,;30.53,-34.25,;31.87,-33.48,;30.53,-35.79,;25.2,-31.17,;23.87,-31.94,;18.53,-31.94,;17.2,-31.17,;17.2,-29.63,;18.53,-28.86,;19.87,-29.63,;19.87,-31.17,)| Show InChI InChI=1S/C24H29NO5/c1-18-7-13-22(14-8-18)25(21-5-3-2-4-6-21)24(28)30-16-20-11-9-19(10-12-20)15-29-17-23(26)27/h2-8,13-14,19-20H,9-12,15-17H2,1H3,(H,26,27)/t19-,20- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235379
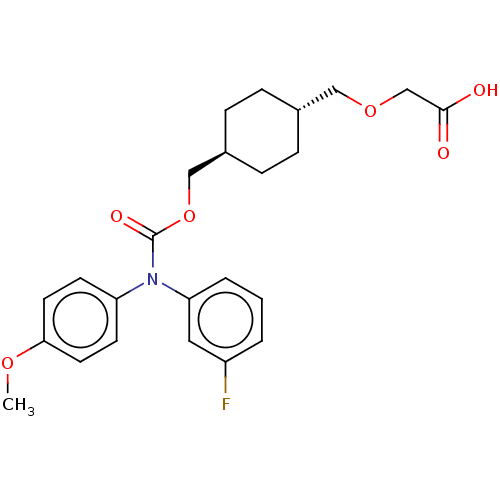
(CHEMBL3932106 | US10668033, Compound 55)Show SMILES COc1ccc(cc1)N(C(=O)OC[C@H]1CC[C@H](COCC(O)=O)CC1)c1cccc(F)c1 |r,wU:16.17,wD:13.13,(31.51,-37.36,;31.51,-35.82,;30.18,-35.05,;30.18,-33.51,;28.85,-32.74,;27.51,-33.51,;27.51,-35.05,;28.85,-35.82,;26.18,-32.74,;24.85,-33.51,;23.51,-32.74,;24.85,-35.05,;23.51,-35.82,;22.18,-35.05,;22.18,-33.51,;20.84,-32.74,;19.51,-33.51,;18.18,-32.74,;16.84,-33.51,;15.51,-32.74,;14.18,-33.51,;12.84,-32.74,;14.18,-35.05,;19.51,-35.05,;20.84,-35.82,;26.18,-31.2,;27.51,-30.43,;27.51,-28.89,;26.18,-28.12,;24.85,-28.89,;23.51,-28.12,;24.85,-30.43,)| Show InChI InChI=1S/C24H28FNO6/c1-30-22-11-9-20(10-12-22)26(21-4-2-3-19(25)13-21)24(29)32-15-18-7-5-17(6-8-18)14-31-16-23(27)28/h2-4,9-13,17-18H,5-8,14-16H2,1H3,(H,27,28)/t17-,18- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235384
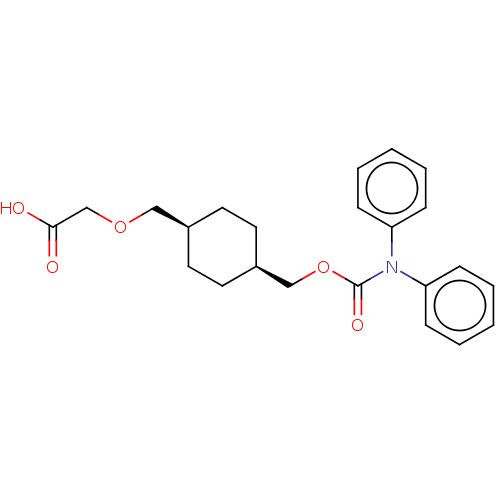
(CHEMBL3900038)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2ccccc2)CC1 |r,wU:9.9,6.5,(30.68,-33.76,;29.35,-34.53,;29.35,-36.07,;28.02,-33.76,;28.02,-32.22,;26.68,-31.45,;25.35,-32.22,;25.35,-33.76,;24.02,-34.53,;22.68,-33.76,;21.35,-34.53,;20.01,-33.76,;18.68,-34.53,;18.68,-36.07,;17.35,-33.76,;16.01,-34.53,;16.01,-36.07,;14.68,-36.84,;13.35,-36.07,;13.35,-34.53,;14.68,-33.76,;17.35,-32.22,;16.01,-31.45,;16.01,-29.91,;17.35,-29.14,;18.68,-29.91,;18.68,-31.45,;22.68,-32.22,;24.02,-31.45,)| Show InChI InChI=1S/C23H27NO5/c25-22(26)17-28-15-18-11-13-19(14-12-18)16-29-23(27)24(20-7-3-1-4-8-20)21-9-5-2-6-10-21/h1-10,18-19H,11-17H2,(H,25,26)/t18-,19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235372
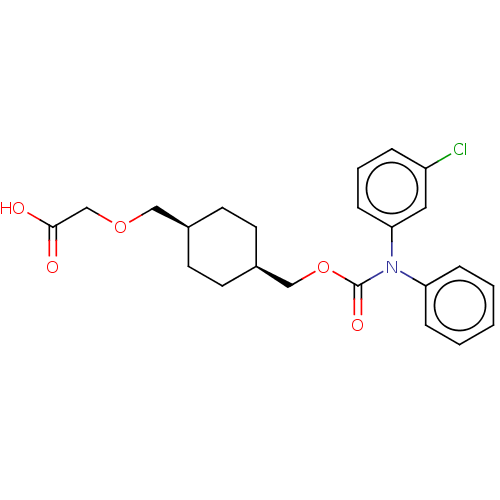
(CHEMBL3966307)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2cccc(Cl)c2)CC1 |r,wU:9.9,6.5,(14.02,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.02,-35.33,;19.36,-34.56,;19.36,-33.02,;20.69,-32.25,;22.03,-33.02,;23.36,-32.25,;24.69,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.69,-35.33,;28.69,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.69,-32.25,;30.03,-33.02,;31.36,-32.25,;31.36,-30.71,;30.03,-29.94,;30.03,-28.4,;28.69,-30.71,;22.03,-34.56,;20.69,-35.33,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103396
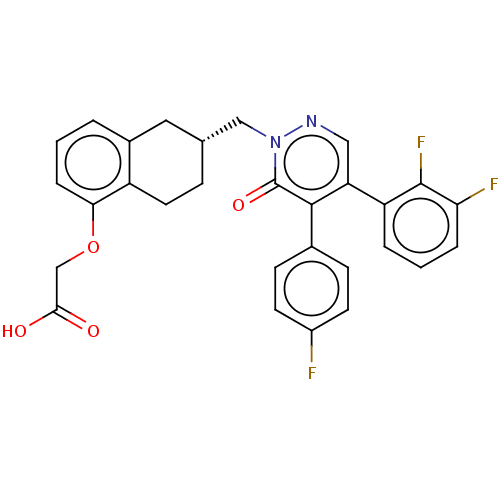
(CHEMBL3398236)Show SMILES OC(=O)COc1cccc2C[C@H](Cn3ncc(-c4cccc(F)c4F)c(-c4ccc(F)cc4)c3=O)CCc12 |r| Show InChI InChI=1S/C29H23F3N2O4/c30-20-10-8-18(9-11-20)27-23(22-4-2-5-24(31)28(22)32)14-33-34(29(27)37)15-17-7-12-21-19(13-17)3-1-6-25(21)38-16-26(35)36/h1-6,8-11,14,17H,7,12-13,15-16H2,(H,35,36)/t17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103395
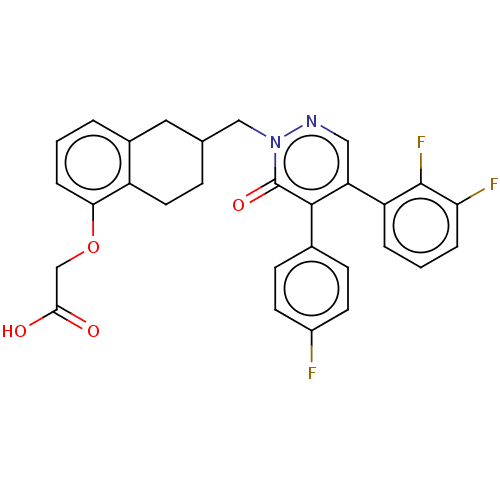
(CHEMBL3398235)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4cccc(F)c4F)c(-c4ccc(F)cc4)c3=O)CCc12 Show InChI InChI=1S/C29H23F3N2O4/c30-20-10-8-18(9-11-20)27-23(22-4-2-5-24(31)28(22)32)14-33-34(29(27)37)15-17-7-12-21-19(13-17)3-1-6-25(21)38-16-26(35)36/h1-6,8-11,14,17H,7,12-13,15-16H2,(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235383
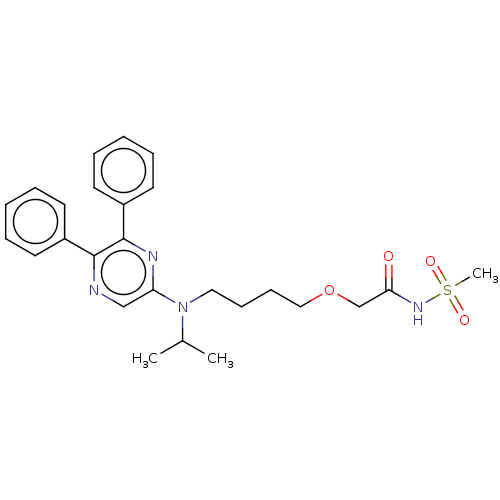
(ACT-293987 | NS-304 | Selexipag | Uptravi)Show SMILES CC(C)N(CCCCOCC(=O)NS(C)(=O)=O)c1cnc(-c2ccccc2)c(n1)-c1ccccc1 Show InChI InChI=1S/C26H32N4O4S/c1-20(2)30(16-10-11-17-34-19-24(31)29-35(3,32)33)23-18-27-25(21-12-6-4-7-13-21)26(28-23)22-14-8-5-9-15-22/h4-9,12-15,18,20H,10-11,16-17,19H2,1-3H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103394
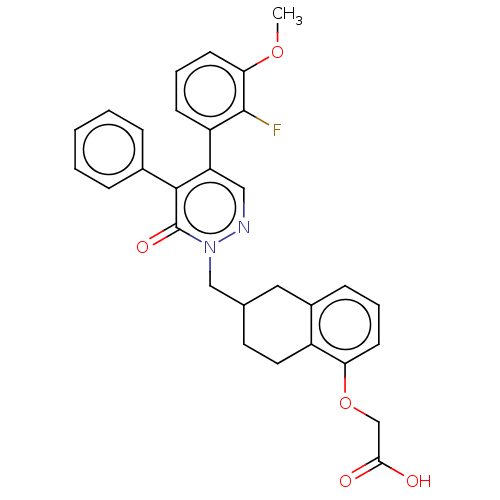
(CHEMBL3398229)Show SMILES COc1cccc(c1F)-c1cnn(CC2CCc3c(C2)cccc3OCC(O)=O)c(=O)c1-c1ccccc1 Show InChI InChI=1S/C30H27FN2O5/c1-37-26-12-6-10-23(29(26)31)24-16-32-33(30(36)28(24)20-7-3-2-4-8-20)17-19-13-14-22-21(15-19)9-5-11-25(22)38-18-27(34)35/h2-12,16,19H,13-15,17-18H2,1H3,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103404
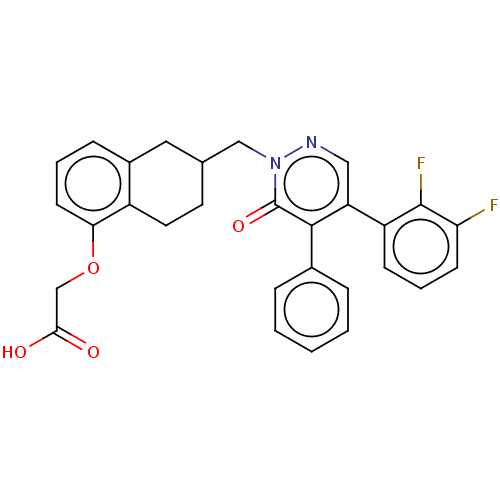
(CHEMBL3398228)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4cccc(F)c4F)c(-c4ccccc4)c3=O)CCc12 Show InChI InChI=1S/C29H24F2N2O4/c30-24-10-5-9-22(28(24)31)23-15-32-33(29(36)27(23)19-6-2-1-3-7-19)16-18-12-13-21-20(14-18)8-4-11-25(21)37-17-26(34)35/h1-11,15,18H,12-14,16-17H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235371
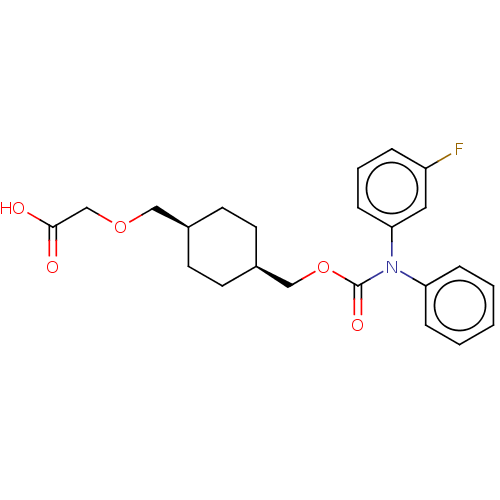
(CHEMBL3922000)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2cccc(F)c2)CC1 |r,wU:9.9,6.5,(14.03,-33.02,;15.36,-32.25,;15.36,-30.71,;16.69,-33.02,;16.69,-34.56,;18.03,-35.33,;19.36,-34.56,;19.36,-33.02,;20.7,-32.25,;22.03,-33.02,;23.36,-32.25,;24.7,-33.02,;26.03,-32.25,;26.03,-30.71,;27.36,-33.02,;27.36,-34.56,;28.7,-35.33,;28.7,-36.87,;27.36,-37.64,;26.03,-36.87,;26.03,-35.33,;28.7,-32.25,;30.03,-33.02,;31.37,-32.25,;31.37,-30.71,;30.03,-29.94,;30.03,-28.4,;28.7,-30.71,;22.03,-34.56,;20.7,-35.33,)| Show InChI InChI=1S/C23H26FNO5/c24-19-5-4-8-21(13-19)25(20-6-2-1-3-7-20)23(28)30-15-18-11-9-17(10-12-18)14-29-16-22(26)27/h1-8,13,17-18H,9-12,14-16H2,(H,26,27)/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235377
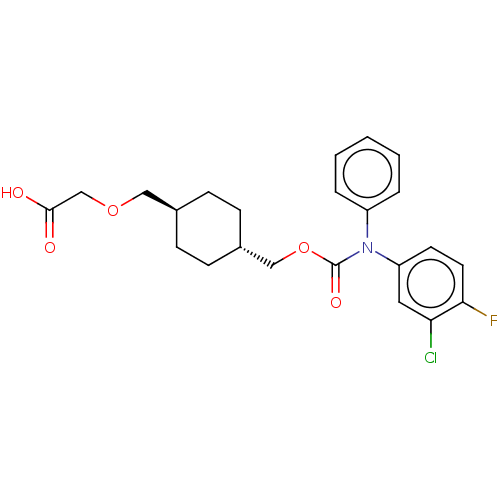
(CHEMBL3890685)Show SMILES OC(=O)COC[C@H]1CC[C@H](COC(=O)N(c2ccccc2)c2ccc(F)c(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(31.35,-32.86,;30.02,-33.63,;30.02,-35.17,;28.69,-32.86,;28.69,-31.32,;27.35,-30.55,;26.02,-31.32,;26.02,-32.86,;24.69,-33.63,;23.35,-32.86,;22.02,-33.63,;20.68,-32.86,;19.35,-33.63,;19.35,-35.17,;18.02,-32.86,;18.02,-31.32,;16.68,-30.55,;16.68,-29.01,;18.02,-28.24,;19.35,-29.01,;19.35,-30.55,;16.68,-33.63,;15.35,-32.86,;14.02,-33.63,;14.02,-35.17,;12.68,-35.94,;15.35,-35.94,;15.35,-37.48,;16.68,-35.17,;23.35,-31.32,;24.69,-30.55,)| Show InChI InChI=1S/C23H25ClFNO5/c24-20-12-19(10-11-21(20)25)26(18-4-2-1-3-5-18)23(29)31-14-17-8-6-16(7-9-17)13-30-15-22(27)28/h1-5,10-12,16-17H,6-9,13-15H2,(H,27,28)/t16-,17- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235386
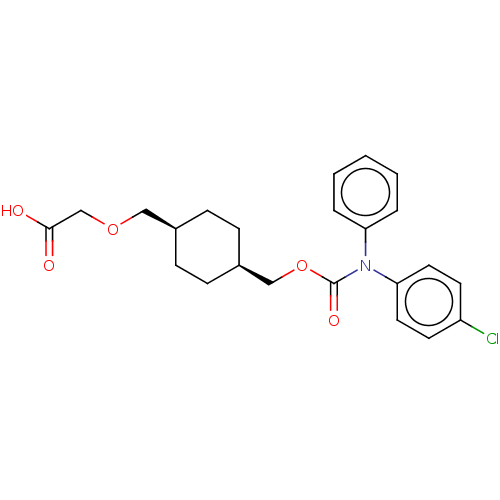
(CHEMBL3919269)Show SMILES OC(=O)COC[C@H]1CC[C@@H](COC(=O)N(c2ccccc2)c2ccc(Cl)cc2)CC1 |r,wU:9.9,6.5,(31.59,-33.64,;30.26,-34.41,;30.26,-35.95,;28.93,-33.64,;28.93,-32.1,;27.59,-31.33,;26.26,-32.1,;26.26,-33.64,;24.92,-34.41,;23.59,-33.64,;22.26,-34.41,;20.92,-33.64,;19.59,-34.41,;19.59,-35.95,;18.26,-33.64,;18.26,-32.1,;16.92,-31.33,;16.92,-29.79,;18.26,-29.02,;19.59,-29.79,;19.59,-31.33,;16.92,-34.41,;16.92,-35.95,;15.59,-36.72,;14.26,-35.95,;12.92,-36.72,;14.26,-34.41,;15.59,-33.64,;23.59,-32.1,;24.92,-31.33,)| Show InChI InChI=1S/C23H26ClNO5/c24-19-10-12-21(13-11-19)25(20-4-2-1-3-5-20)23(28)30-15-18-8-6-17(7-9-18)14-29-16-22(26)27/h1-5,10-13,17-18H,6-9,14-16H2,(H,26,27)/t17-,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103412
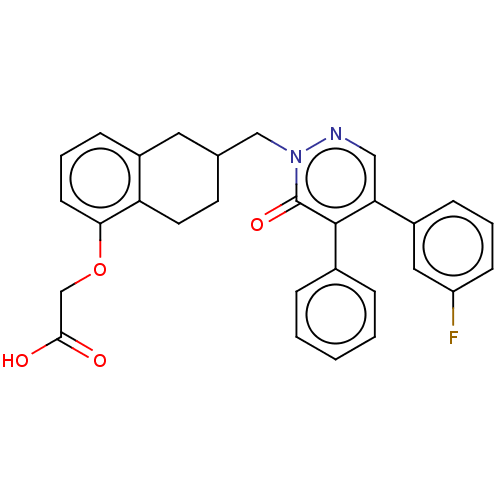
(CHEMBL3398224)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4cccc(F)c4)c(-c4ccccc4)c3=O)CCc12 Show InChI InChI=1S/C29H25FN2O4/c30-23-10-4-8-22(15-23)25-16-31-32(29(35)28(25)20-6-2-1-3-7-20)17-19-12-13-24-21(14-19)9-5-11-26(24)36-18-27(33)34/h1-11,15-16,19H,12-14,17-18H2,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103413
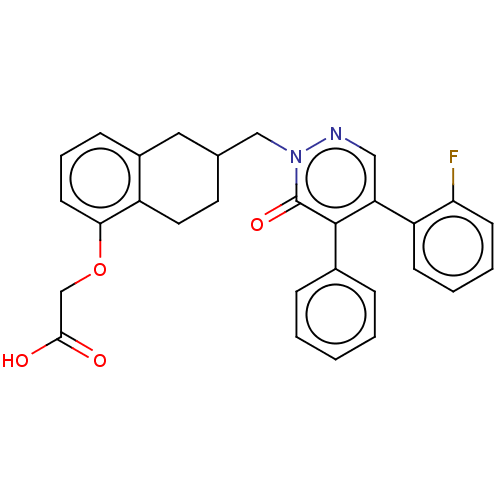
(CHEMBL3398227)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4ccccc4F)c(-c4ccccc4)c3=O)CCc12 Show InChI InChI=1S/C29H25FN2O4/c30-25-11-5-4-10-23(25)24-16-31-32(29(35)28(24)20-7-2-1-3-8-20)17-19-13-14-22-21(15-19)9-6-12-26(22)36-18-27(33)34/h1-12,16,19H,13-15,17-18H2,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103392

(CHEMBL3398232)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4ccccc4)c(-c4ccc(F)cc4)c3=O)CCc12 Show InChI InChI=1S/C29H25FN2O4/c30-23-12-10-21(11-13-23)28-25(20-5-2-1-3-6-20)16-31-32(29(28)35)17-19-9-14-24-22(15-19)7-4-8-26(24)36-18-27(33)34/h1-8,10-13,16,19H,9,14-15,17-18H2,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103397
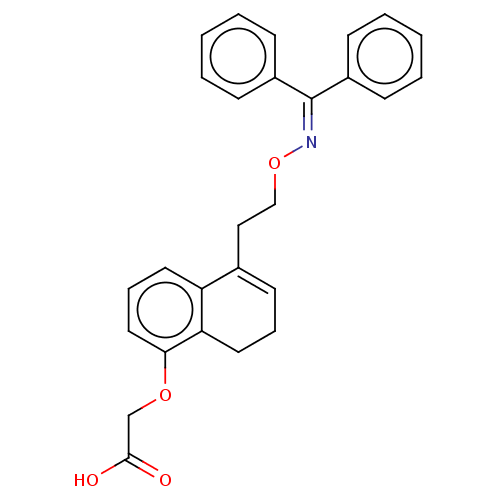
(CHEMBL3398237)Show SMILES [#8]-[#6](=O)-[#6]-[#8]-c1cccc2-[#6](-[#6]-[#6]-[#8]\[#7]=[#6](\c3ccccc3)-c3ccccc3)=[#6]-[#6]-[#6]-c12 |c:29| Show InChI InChI=1S/C27H25NO4/c29-26(30)19-31-25-16-8-14-23-20(13-7-15-24(23)25)17-18-32-28-27(21-9-3-1-4-10-21)22-11-5-2-6-12-22/h1-6,8-14,16H,7,15,17-19H2,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50235387
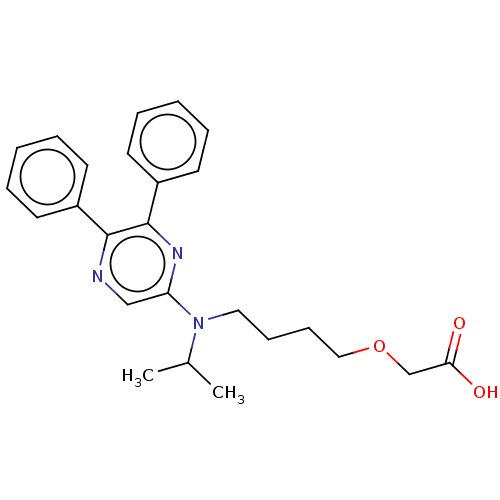
(CHEMBL239226)Show SMILES CC(C)N(CCCCOCC(O)=O)c1cnc(-c2ccccc2)c(n1)-c1ccccc1 Show InChI InChI=1S/C25H29N3O3/c1-19(2)28(15-9-10-16-31-18-23(29)30)22-17-26-24(20-11-5-3-6-12-20)25(27-22)21-13-7-4-8-14-21/h3-8,11-14,17,19H,9-10,15-16,18H2,1-2H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at IP receptor in human primary platelets assessed as inhibition of ADP-induced platelet aggregation |
J Med Chem 60: 913-927 (2017)
Article DOI: 10.1021/acs.jmedchem.6b00871
BindingDB Entry DOI: 10.7270/Q2VX0JSM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103410

(CHEMBL3398222)Show SMILES OC(=O)COc1cccc2CC(Cn3ncc(-c4ccccc4)c(-c4ccccc4)c3=O)CCc12 Show InChI InChI=1S/C29H26N2O4/c32-27(33)19-35-26-13-7-12-23-16-20(14-15-24(23)26)18-31-29(34)28(22-10-5-2-6-11-22)25(17-30-31)21-8-3-1-4-9-21/h1-13,17,20H,14-16,18-19H2,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(Homo sapiens (Human)) | BDBM50103391

(CHEMBL3398233)Show SMILES COc1ccc(cc1)-c1c(cnn(CC2CCc3c(C2)cccc3OCC(O)=O)c1=O)-c1ccccc1 Show InChI InChI=1S/C30H28N2O5/c1-36-24-13-11-22(12-14-24)29-26(21-6-3-2-4-7-21)17-31-32(30(29)35)18-20-10-15-25-23(16-20)8-5-9-27(25)37-19-28(33)34/h2-9,11-14,17,20H,10,15-16,18-19H2,1H3,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human IP receptor in human platelets assessed as inhibition of ADP-induced platelet aggregation |
Bioorg Med Chem Lett 25: 1030-5 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.024
BindingDB Entry DOI: 10.7270/Q2057HQM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50055992
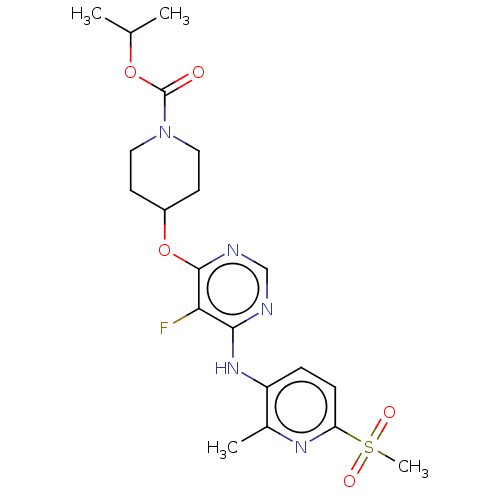
(CHEMBL3325842)Show SMILES CC(C)OC(=O)N1CCC(CC1)Oc1ncnc(Nc2ccc(nc2C)S(C)(=O)=O)c1F Show InChI InChI=1S/C20H26FN5O5S/c1-12(2)30-20(27)26-9-7-14(8-10-26)31-19-17(21)18(22-11-23-19)25-15-5-6-16(24-13(15)3)32(4,28)29/h5-6,11-12,14H,7-10H2,1-4H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 4332-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.071
BindingDB Entry DOI: 10.7270/Q2C82BZ7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50364559

(CHEMBL1951032)Show SMILES COc1c(Nc2ccc(nc2C)S(C)(=O)=O)ncnc1OC1CCN(CC1)C(=O)OC(C)C Show InChI InChI=1S/C21H29N5O6S/c1-13(2)31-21(27)26-10-8-15(9-11-26)32-20-18(30-4)19(22-12-23-20)25-16-6-7-17(24-14(16)3)33(5,28)29/h6-7,12-13,15H,8-11H2,1-5H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 4332-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.071
BindingDB Entry DOI: 10.7270/Q2C82BZ7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50056004
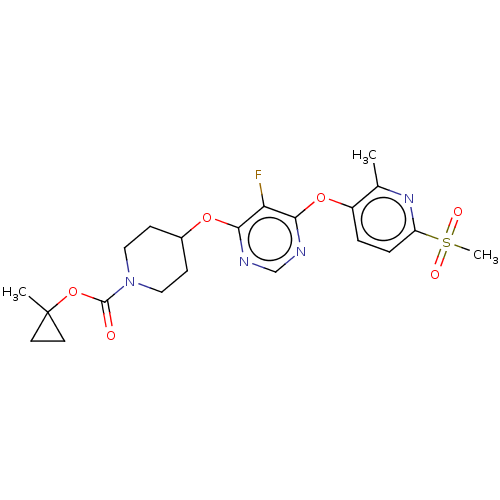
(CHEMBL3326667)Show SMILES Cc1nc(ccc1Oc1ncnc(OC2CCN(CC2)C(=O)OC2(C)CC2)c1F)S(C)(=O)=O Show InChI InChI=1S/C21H25FN4O6S/c1-13-15(4-5-16(25-13)33(3,28)29)31-19-17(22)18(23-12-24-19)30-14-6-10-26(11-7-14)20(27)32-21(2)8-9-21/h4-5,12,14H,6-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
Bioorg Med Chem Lett 24: 4332-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.071
BindingDB Entry DOI: 10.7270/Q2C82BZ7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50056004

(CHEMBL3326667)Show SMILES Cc1nc(ccc1Oc1ncnc(OC2CCN(CC2)C(=O)OC2(C)CC2)c1F)S(C)(=O)=O Show InChI InChI=1S/C21H25FN4O6S/c1-13-15(4-5-16(25-13)33(3,28)29)31-19-17(22)18(23-12-24-19)30-14-6-10-26(11-7-14)20(27)32-21(2)8-9-21/h4-5,12,14H,6-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 4332-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.071
BindingDB Entry DOI: 10.7270/Q2C82BZ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data