

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029499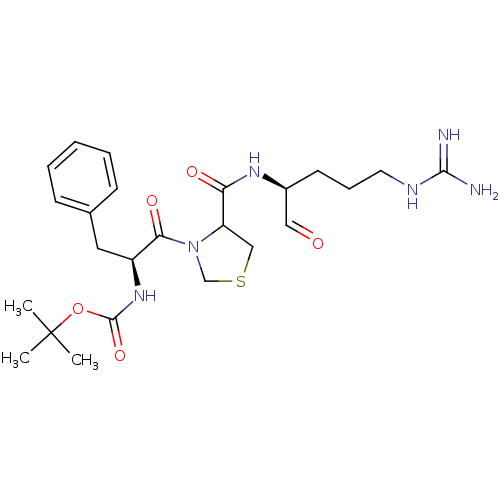 (CHEMBL141834 | tert-butyloxy carbonyl-D-Phe-thiazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046358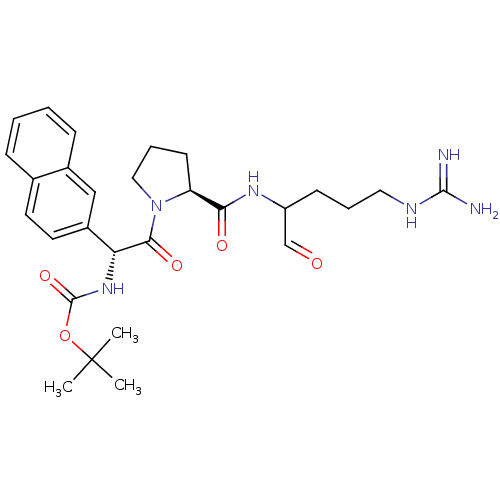 (CHEMBL110780 | {2-[2-(1-Formyl-4-guanidino-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029498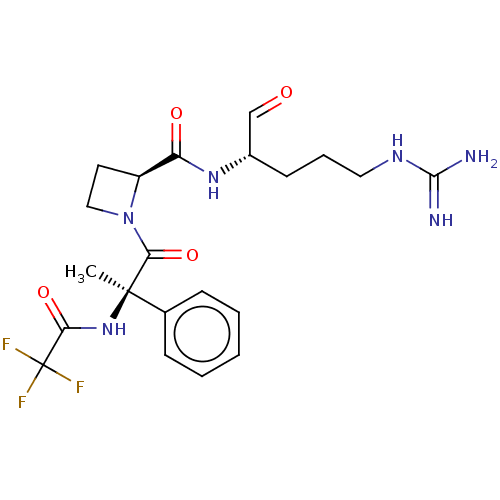 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50029498 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity against t-PA(Tissue plasminogen activator). | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50029498 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Coagulation factor X | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50029498 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Plasmin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029498 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029515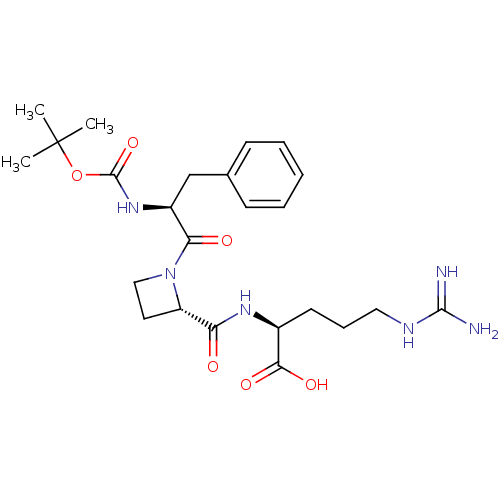 (CHEMBL141584 | tert-butyloxy carbonyl-D-Phe-azetid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029506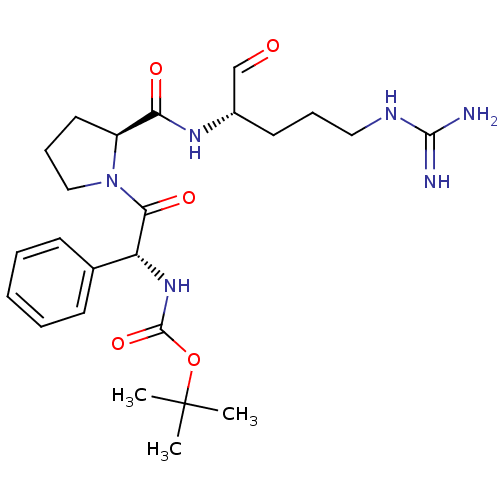 (CHEMBL318998 | tert-butyloxy carbonyl-D-ethylpheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029514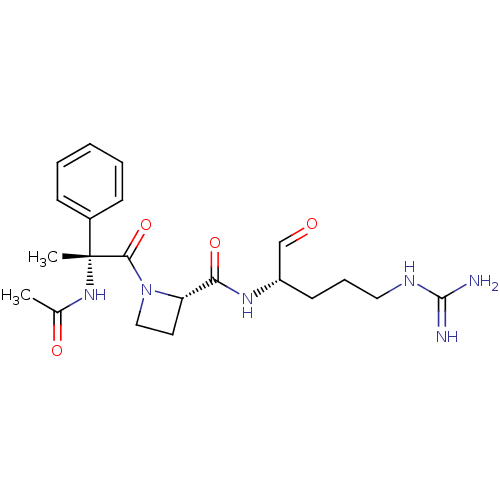 ((S)-1-((R)-2-Acetylamino-2-phenyl-propionyl)-azeti...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029510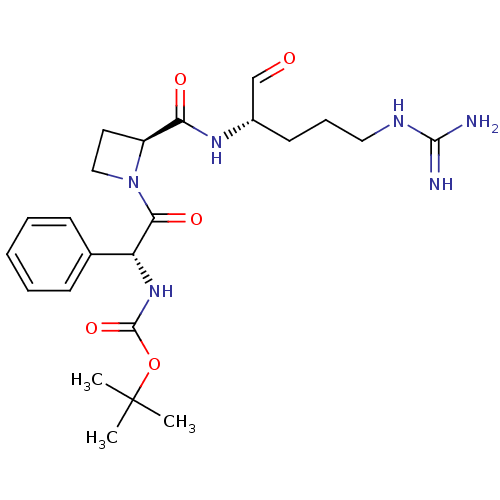 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50228863 ((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029510 (1N-[2-{2-[4-amino(imino)methylamino-1-formyl-(1S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046355 (CHEMBL110898 | {2-[2-(1-Formyl-4-guanidino-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029511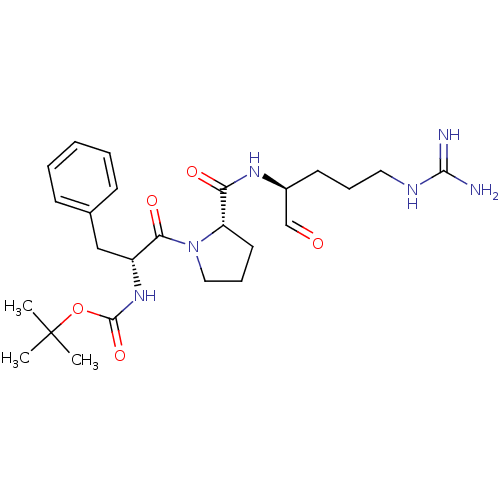 (CHEMBL104472 | tert-butyloxy carbonyl-D-Phe-pro-Ar...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046373 (CHEMBL108670 | {1-(3,4-Dichloro-phenyl)-2-[2-(1-fo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029506 (CHEMBL318998 | tert-butyloxy carbonyl-D-ethylpheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029515 (CHEMBL141584 | tert-butyloxy carbonyl-D-Phe-azetid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046358 (CHEMBL110780 | {2-[2-(1-Formyl-4-guanidino-butylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046356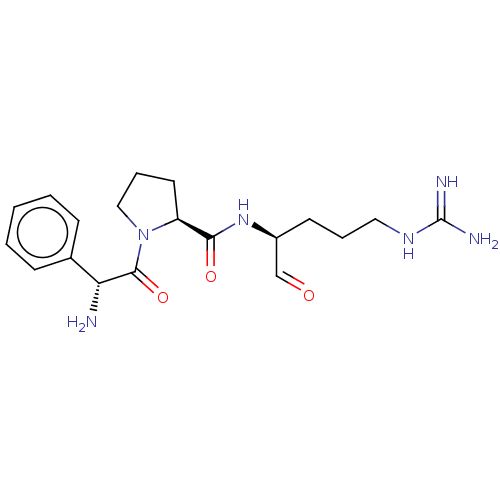 (1-(2-Amino-2-phenyl-acetyl)-pyrrolidine-2-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50060000 ((S)-1-((R)-2-Methylamino-2-phenyl-acetyl)-pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060000 ((S)-1-((R)-2-Methylamino-2-phenyl-acetyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046352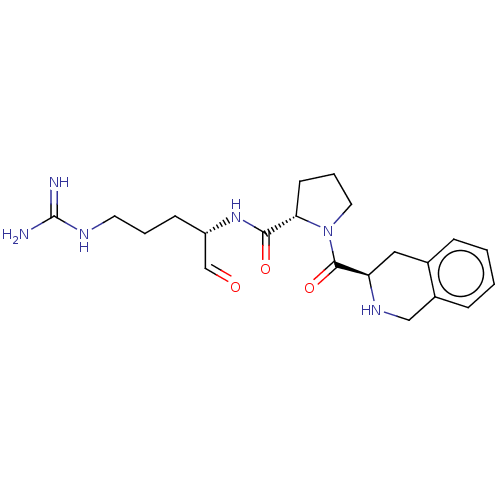 (1-(1,2,3,4-Tetrahydro-isoquinoline-3-carbonyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029501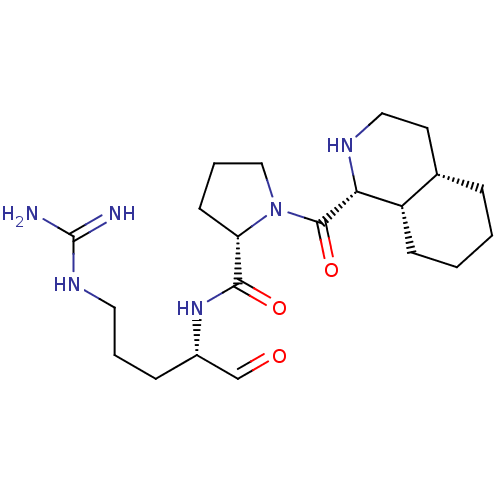 (5N-[4-amino(imino)methylamino-1-formyl-(1S)-butyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029514 ((S)-1-((R)-2-Acetylamino-2-phenyl-propionyl)-azeti...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50046356 (1-(2-Amino-2-phenyl-acetyl)-pyrrolidine-2-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046368 (CHEMBL320988 | [2-[2-(1-Formyl-4-guanidino-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50046356 (1-(2-Amino-2-phenyl-acetyl)-pyrrolidine-2-carboxyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046352 (1-(1,2,3,4-Tetrahydro-isoquinoline-3-carbonyl)-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50046352 (1-(1,2,3,4-Tetrahydro-isoquinoline-3-carbonyl)-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046356 (1-(2-Amino-2-phenyl-acetyl)-pyrrolidine-2-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50046352 (1-(1,2,3,4-Tetrahydro-isoquinoline-3-carbonyl)-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029500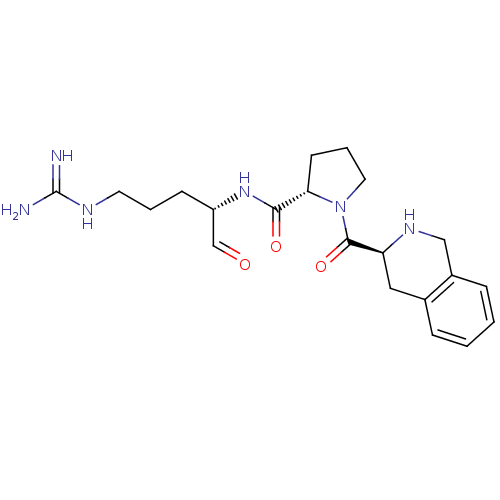 ((S)-1-((S)-1,2,3,4-Tetrahydro-isoquinoline-3-carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029507 (CHEMBL2370860 | {(R)-2-[2-((S)-1-Formyl-4-guanidin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029507 (CHEMBL2370860 | {(R)-2-[2-((S)-1-Formyl-4-guanidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50029507 (CHEMBL2370860 | {(R)-2-[2-((S)-1-Formyl-4-guanidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Plasmin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50029507 (CHEMBL2370860 | {(R)-2-[2-((S)-1-Formyl-4-guanidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity against t-PA(Tissue plasminogen activator). | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Bos taurus) | BDBM50029507 (CHEMBL2370860 | {(R)-2-[2-((S)-1-Formyl-4-guanidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Coagulation factor X | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50029500 ((S)-1-((S)-1,2,3,4-Tetrahydro-isoquinoline-3-carbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Trypsin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046355 (CHEMBL110898 | {2-[2-(1-Formyl-4-guanidino-butylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046368 (CHEMBL320988 | [2-[2-(1-Formyl-4-guanidino-butylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046373 (CHEMBL108670 | {1-(3,4-Dichloro-phenyl)-2-[2-(1-fo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046359 (CHEMBL109109 | {2-[2-(1-Formyl-4-guanidino-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50046360 (CHEMBL104472 | {1-Benzyl-2-[2-(1-formyl-4-guanidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Bos taurus (Bovine)) | BDBM50029511 (CHEMBL104472 | tert-butyloxy carbonyl-D-Phe-pro-Ar...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro Enzyme Inhibitory activity measured against Thrombin | J Med Chem 38: 4446-53 (1995) BindingDB Entry DOI: 10.7270/Q2RB73MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50046360 (CHEMBL104472 | {1-Benzyl-2-[2-(1-formyl-4-guanidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against t-PA (tissue plasminogen activator) | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50046360 (CHEMBL104472 | {1-Benzyl-2-[2-(1-formyl-4-guanidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against trypsin | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50046360 (CHEMBL104472 | {1-Benzyl-2-[2-(1-formyl-4-guanidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50046358 (CHEMBL110780 | {2-[2-(1-Formyl-4-guanidino-butylca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against factor Xa | J Med Chem 36: 314-9 (1993) BindingDB Entry DOI: 10.7270/Q2930S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 201 total ) | Next | Last >> |