 Found 1229 hits with Last Name = 'siegl' and Initial = 'pk'
Found 1229 hits with Last Name = 'siegl' and Initial = 'pk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(RAT) | BDBM82431
(L-162,193 | L-162193 | N-[[4-[[[6-[4-(Cyclopropylc...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#8]-[#6](=O)-[#7][S;v6](=O)(=O)c1ccccc1-c1ccc(-[#6]-n2c(-[#6]-[#6]-[#6]-[#6])nc3ccc(cc3c2=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-2-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C38H45N5O6S/c1-3-5-11-35-39-33-19-18-30(41-20-22-42(23-21-41)36(44)29-16-17-29)25-32(33)37(45)43(35)26-27-12-14-28(15-13-27)31-9-7-8-10-34(31)50(47,48)40-38(46)49-24-6-4-2/h7-10,12-15,18-19,25,29H,3-6,11,16-17,20-24,26H2,1-2H3,(H,40,46) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82435
(1-[2,4,6-Trichlorophenyl]-3-butyl-4-[[2'-(1H-t...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(13.29,.94,;11.96,.17,;10.62,.94,;9.29,.17,;7.95,.94,;7.79,2.47,;6.29,2.79,;5.52,1.46,;3.99,1.29,;3.36,-.11,;3.08,2.54,;6.55,.31,;6.23,-1.2,;7.37,-2.23,;7.05,-3.73,;8.2,-4.76,;9.66,-4.29,;9.98,-2.78,;8.84,-1.75,;10.8,-5.32,;12.27,-4.84,;13.41,-5.87,;13.09,-7.38,;11.63,-7.85,;10.48,-6.82,;9.02,-7.3,;7.77,-6.39,;6.53,-7.3,;7,-8.76,;8.54,-8.76,;5.91,4.28,;7.02,5.35,;8.5,4.93,;6.64,6.85,;5.16,7.27,;4.79,8.76,;4.06,6.2,;4.43,4.7,;3.32,3.63,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82259

(CAS_123856 | L-158,809 | NSC_123856)Show SMILES CCc1nc2c(C)cc(C)nc2n1-c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7/c1-4-20-25-21-14(2)13-15(3)24-23(21)30(20)17-11-9-16(10-12-17)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-13H,4H2,1-3H3,(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50030727
(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82436

(1-(2,6-Dichlorophenyl)-4-[[2'-(1H-tetrazol-5-y...)Show SMILES CCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cccc1Cl |(11.96,.91,;10.62,1.68,;9.29,.91,;7.95,1.68,;7.79,3.22,;6.29,3.54,;5.52,2.2,;3.99,2.04,;3.36,.63,;3.08,3.29,;6.55,1.06,;6.23,-.45,;7.37,-1.48,;7.05,-2.98,;8.2,-4.02,;9.66,-3.54,;9.98,-2.03,;8.84,-1,;10.8,-4.57,;12.27,-4.09,;13.41,-5.12,;13.09,-6.63,;11.63,-7.11,;10.48,-6.08,;9.02,-6.55,;7.77,-5.65,;6.53,-6.55,;7,-8.02,;8.54,-8.02,;5.91,5.03,;7.02,6.1,;8.5,5.68,;6.64,7.59,;5.16,8.02,;4.06,6.95,;4.43,5.45,;3.32,4.38,)| Show InChI InChI=1S/C27H22Cl2N6O2/c1-2-6-23-20(24(27(36)37)35(32-23)25-21(28)9-5-10-22(25)29)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)26-30-33-34-31-26/h3-5,7-14H,2,6,15H2,1H3,(H,36,37)(H,30,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50042746
(CHEMBL122212 | L-159,882 | [4-(2-Ethyl-5,7-dimethy...)Show SMILES CCCc1cc(Cn2c(CC)nc3c(C)cc(C)nc23)cc(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C31H37N3O3/c1-6-12-24-17-22(19-34-26(8-3)33-27-20(4)16-21(5)32-30(27)34)18-25(13-7-2)28(24)37-29(31(35)36)23-14-10-9-11-15-23/h9-11,14-18,29H,6-8,12-13,19H2,1-5H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82433
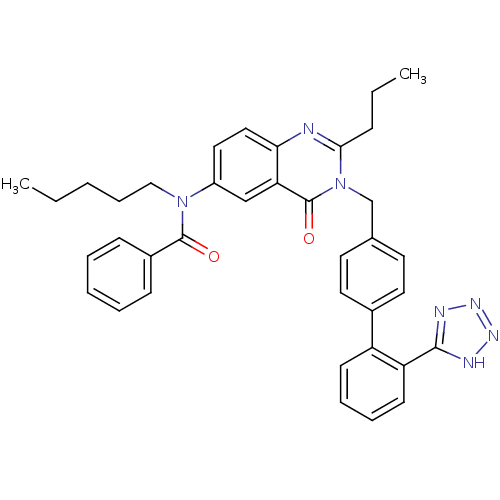
(CHEMBL302102 | L-159,689 | L-159689 | N-Pentyl-N-[...)Show SMILES CCCCCN(C(=O)c1ccccc1)c1ccc2nc(CCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(=O)c2c1 Show InChI InChI=1S/C37H37N7O2/c1-3-5-11-23-43(36(45)28-13-7-6-8-14-28)29-21-22-33-32(24-29)37(46)44(34(38-33)12-4-2)25-26-17-19-27(20-18-26)30-15-9-10-16-31(30)35-39-41-42-40-35/h6-10,13-22,24H,3-5,11-12,23,25H2,1-2H3,(H,39,40,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50006909
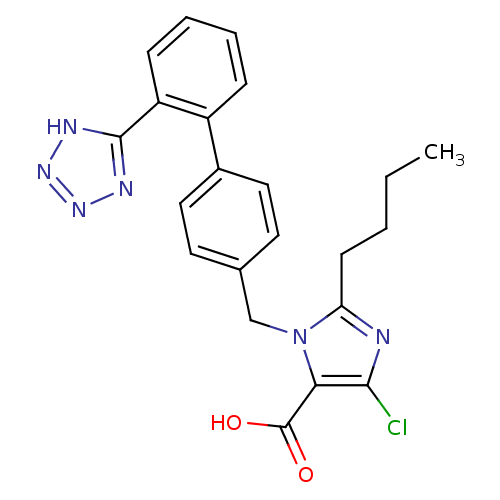
(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82434
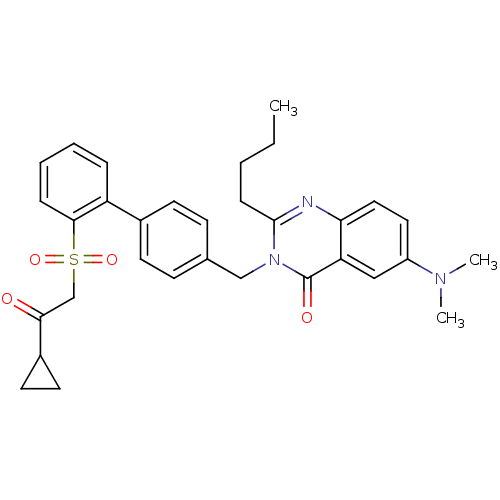
(2-Butyl-6-(dimethylamino)-3-[[2-(cyclopropylcarbon...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1nc2ccc(cc2c(=O)n1-[#6]-c1ccc(cc1)-c1ccccc1[S;v6](=O)(=O)[#6]-[#6](=O)-[#6]-1-[#6]-[#6]-1)-[#7](-[#6])-[#6] Show InChI InChI=1S/C32H35N3O4S/c1-4-5-10-31-33-28-18-17-25(34(2)3)19-27(28)32(37)35(31)20-22-11-13-23(14-12-22)26-8-6-7-9-30(26)40(38,39)21-29(36)24-15-16-24/h6-9,11-14,17-19,24H,4-5,10,15-16,20-21H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82430
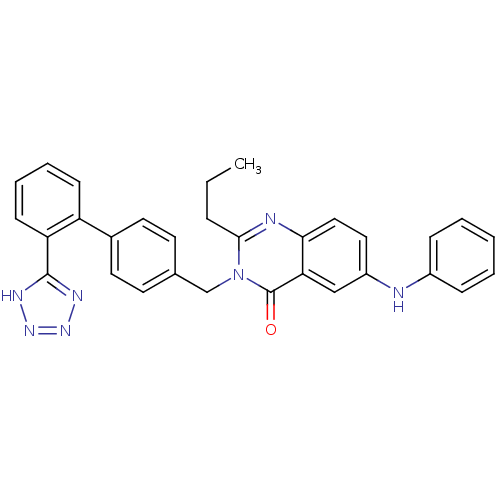
(2-Propyl-3-[[2'-(1H-tetrazol-5-yl)biphenyl-4-y...)Show SMILES CCCc1nc2ccc(Nc3ccccc3)cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H27N7O/c1-2-8-29-33-28-18-17-24(32-23-9-4-3-5-10-23)19-27(28)31(39)38(29)20-21-13-15-22(16-14-21)25-11-6-7-12-26(25)30-34-36-37-35-30/h3-7,9-19,32H,2,8,20H2,1H3,(H,34,35,36,37) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82432
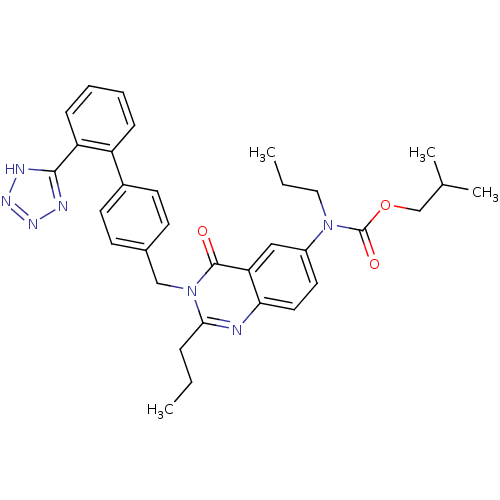
(L-159,530 | L-159530 | N-Propyl-N-[[2-propyl-3-[[2...)Show SMILES CCCN(C(=O)OCC(C)C)c1ccc2nc(CCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(=O)c2c1 Show InChI InChI=1S/C33H37N7O3/c1-5-9-30-34-29-17-16-25(39(18-6-2)33(42)43-21-22(3)4)19-28(29)32(41)40(30)20-23-12-14-24(15-13-23)26-10-7-8-11-27(26)31-35-37-38-36-31/h7-8,10-17,19,22H,5-6,9,18,20-21H2,1-4H3,(H,35,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82429
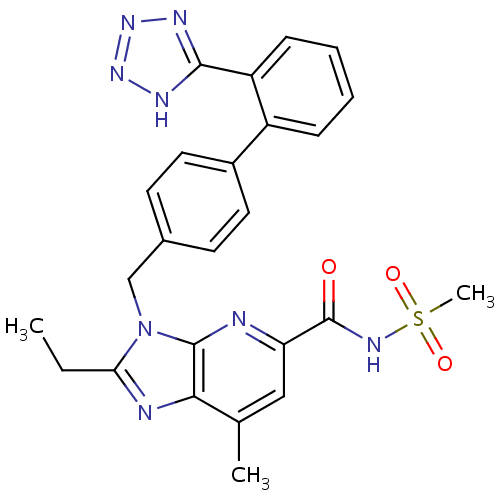
(2-Ethyl-7-methyl-3-[[2'-(1H-tetrazol-5-yl)biph...)Show SMILES CCc1nc2c(C)cc(nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)N[S](C)(=O)=O Show InChI InChI=1S/C25H24N8O3S/c1-4-21-27-22-15(2)13-20(25(34)30-37(3,35)36)26-24(22)33(21)14-16-9-11-17(12-10-16)18-7-5-6-8-19(18)23-28-31-32-29-23/h5-13H,4,14H2,1-3H3,(H,30,34)(H,28,29,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 1.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82259

(CAS_123856 | L-158,809 | NSC_123856)Show SMILES CCc1nc2c(C)cc(C)nc2n1-c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C23H21N7/c1-4-20-25-21-14(2)13-15(3)24-23(21)30(20)17-11-9-16(10-12-17)18-7-5-6-8-19(18)22-26-28-29-27-22/h5-13H,4H2,1-3H3,(H,26,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50030727

(4''-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,...)Show SMILES CCCCc1nn(-c2ccccc2C(F)(F)F)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C33H29F3N4O4S/c1-2-3-17-30-37-40(28-15-9-8-14-27(28)33(34,35)36)32(42)39(30)22-23-18-20-24(21-19-23)26-13-7-10-16-29(26)45(43,44)38-31(41)25-11-5-4-6-12-25/h4-16,18-21H,2-3,17,22H2,1H3,(H,38,41) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50035429

(4'-[2-Butyl-6-(3-cyclohexyl-ureido)-benzoimidazol-...)Show SMILES CCCCc1nc2ccc(NC(=O)NC3CCCCC3)cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O Show InChI InChI=1S/C32H36N4O3/c1-2-3-13-30-35-28-19-18-25(34-32(39)33-24-9-5-4-6-10-24)20-29(28)36(30)21-22-14-16-23(17-15-22)26-11-7-8-12-27(26)31(37)38/h7-8,11-12,14-20,24H,2-6,9-10,13,21H2,1H3,(H,37,38)(H2,33,34,39) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... |
J Med Chem 37: 4464-78 (1995)
BindingDB Entry DOI: 10.7270/Q2WD3ZK5 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82431

(L-162,193 | L-162193 | N-[[4-[[[6-[4-(Cyclopropylc...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#8]-[#6](=O)-[#7][S;v6](=O)(=O)c1ccccc1-c1ccc(-[#6]-n2c(-[#6]-[#6]-[#6]-[#6])nc3ccc(cc3c2=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#6]-2-[#6]-[#6]-2)cc1 Show InChI InChI=1S/C38H45N5O6S/c1-3-5-11-35-39-33-19-18-30(41-20-22-42(23-21-41)36(44)29-16-17-29)25-32(33)37(45)43(35)26-27-12-14-28(15-13-27)31-9-7-8-10-34(31)50(47,48)40-38(46)49-24-6-4-2/h7-10,12-15,18-19,25,29H,3-6,11,16-17,20-24,26H2,1-2H3,(H,40,46) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 51.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50042746

(CHEMBL122212 | L-159,882 | [4-(2-Ethyl-5,7-dimethy...)Show SMILES CCCc1cc(Cn2c(CC)nc3c(C)cc(C)nc23)cc(CCC)c1OC(C(O)=O)c1ccccc1 Show InChI InChI=1S/C31H37N3O3/c1-6-12-24-17-22(19-34-26(8-3)33-27-20(4)16-21(5)32-30(27)34)18-25(13-7-2)28(24)37-29(31(35)36)23-14-10-9-11-15-23/h9-11,14-18,29H,6-8,12-13,19H2,1-5H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50006909

(2-Butyl-5-chloro-3-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCCCc1nc(Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H21ClN6O2/c1-2-3-8-18-24-20(23)19(22(30)31)29(18)13-14-9-11-15(12-10-14)16-6-4-5-7-17(16)21-25-27-28-26-21/h4-7,9-12H,2-3,8,13H2,1H3,(H,30,31)(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82429

(2-Ethyl-7-methyl-3-[[2'-(1H-tetrazol-5-yl)biph...)Show SMILES CCc1nc2c(C)cc(nc2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(=O)N[S](C)(=O)=O Show InChI InChI=1S/C25H24N8O3S/c1-4-21-27-22-15(2)13-20(25(34)30-37(3,35)36)26-24(22)33(21)14-16-9-11-17(12-10-16)18-7-5-6-8-19(18)23-28-31-32-29-23/h5-13H,4,14H2,1-3H3,(H,30,34)(H,28,29,31,32) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82433

(CHEMBL302102 | L-159,689 | L-159689 | N-Pentyl-N-[...)Show SMILES CCCCCN(C(=O)c1ccccc1)c1ccc2nc(CCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(=O)c2c1 Show InChI InChI=1S/C37H37N7O2/c1-3-5-11-23-43(36(45)28-13-7-6-8-14-28)29-21-22-33-32(24-29)37(46)44(34(38-33)12-4-2)25-26-17-19-27(20-18-26)30-15-9-10-16-31(30)35-39-41-42-40-35/h6-10,13-22,24H,3-5,11-12,23,25H2,1-2H3,(H,39,40,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82434

(2-Butyl-6-(dimethylamino)-3-[[2-(cyclopropylcarbon...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1nc2ccc(cc2c(=O)n1-[#6]-c1ccc(cc1)-c1ccccc1[S;v6](=O)(=O)[#6]-[#6](=O)-[#6]-1-[#6]-[#6]-1)-[#7](-[#6])-[#6] Show InChI InChI=1S/C32H35N3O4S/c1-4-5-10-31-33-28-18-17-25(34(2)3)19-27(28)32(37)35(31)20-22-11-13-23(14-12-22)26-8-6-7-9-30(26)40(38,39)21-29(36)24-15-16-24/h6-9,11-14,17-19,24H,4-5,10,15-16,20-21H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82436

(1-(2,6-Dichlorophenyl)-4-[[2'-(1H-tetrazol-5-y...)Show SMILES CCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cccc1Cl |(11.96,.91,;10.62,1.68,;9.29,.91,;7.95,1.68,;7.79,3.22,;6.29,3.54,;5.52,2.2,;3.99,2.04,;3.36,.63,;3.08,3.29,;6.55,1.06,;6.23,-.45,;7.37,-1.48,;7.05,-2.98,;8.2,-4.02,;9.66,-3.54,;9.98,-2.03,;8.84,-1,;10.8,-4.57,;12.27,-4.09,;13.41,-5.12,;13.09,-6.63,;11.63,-7.11,;10.48,-6.08,;9.02,-6.55,;7.77,-5.65,;6.53,-6.55,;7,-8.02,;8.54,-8.02,;5.91,5.03,;7.02,6.1,;8.5,5.68,;6.64,7.59,;5.16,8.02,;4.06,6.95,;4.43,5.45,;3.32,4.38,)| Show InChI InChI=1S/C27H22Cl2N6O2/c1-2-6-23-20(24(27(36)37)35(32-23)25-21(28)9-5-10-22(25)29)15-16-11-13-17(14-12-16)18-7-3-4-8-19(18)26-30-33-34-31-26/h3-5,7-14H,2,6,15H2,1H3,(H,36,37)(H,30,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 526 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82435

(1-[2,4,6-Trichlorophenyl]-3-butyl-4-[[2'-(1H-t...)Show SMILES CCCCc1nn(c(C(O)=O)c1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)-c1c(Cl)cc(Cl)cc1Cl |(13.29,.94,;11.96,.17,;10.62,.94,;9.29,.17,;7.95,.94,;7.79,2.47,;6.29,2.79,;5.52,1.46,;3.99,1.29,;3.36,-.11,;3.08,2.54,;6.55,.31,;6.23,-1.2,;7.37,-2.23,;7.05,-3.73,;8.2,-4.76,;9.66,-4.29,;9.98,-2.78,;8.84,-1.75,;10.8,-5.32,;12.27,-4.84,;13.41,-5.87,;13.09,-7.38,;11.63,-7.85,;10.48,-6.82,;9.02,-7.3,;7.77,-6.39,;6.53,-7.3,;7,-8.76,;8.54,-8.76,;5.91,4.28,;7.02,5.35,;8.5,4.93,;6.64,6.85,;5.16,7.27,;4.79,8.76,;4.06,6.2,;4.43,4.7,;3.32,3.63,)| Show InChI InChI=1S/C28H23Cl3N6O2/c1-2-3-8-24-21(25(28(38)39)37(34-24)26-22(30)14-18(29)15-23(26)31)13-16-9-11-17(12-10-16)19-6-4-5-7-20(19)27-32-35-36-33-27/h4-7,9-12,14-15H,2-3,8,13H2,1H3,(H,38,39)(H,32,33,35,36) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82432

(L-159,530 | L-159530 | N-Propyl-N-[[2-propyl-3-[[2...)Show SMILES CCCN(C(=O)OCC(C)C)c1ccc2nc(CCC)n(Cc3ccc(cc3)-c3ccccc3-c3nnn[nH]3)c(=O)c2c1 Show InChI InChI=1S/C33H37N7O3/c1-5-9-30-34-29-17-16-25(39(18-6-2)33(42)43-21-22(3)4)19-28(29)32(41)40(30)20-23-12-14-24(15-13-23)26-10-7-8-11-27(26)31-35-37-38-36-31/h7-8,10-17,19,22H,5-6,9,18,20-21H2,1-4H3,(H,35,36,37,38) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82430

(2-Propyl-3-[[2'-(1H-tetrazol-5-yl)biphenyl-4-y...)Show SMILES CCCc1nc2ccc(Nc3ccccc3)cc2c(=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H27N7O/c1-2-8-29-33-28-18-17-24(32-23-9-4-3-5-10-23)19-27(28)31(39)38(29)20-21-13-15-22(16-14-21)25-11-6-7-12-26(25)30-34-36-37-35-30/h3-7,9-19,32H,2,8,20H2,1H3,(H,34,35,36,37) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128419
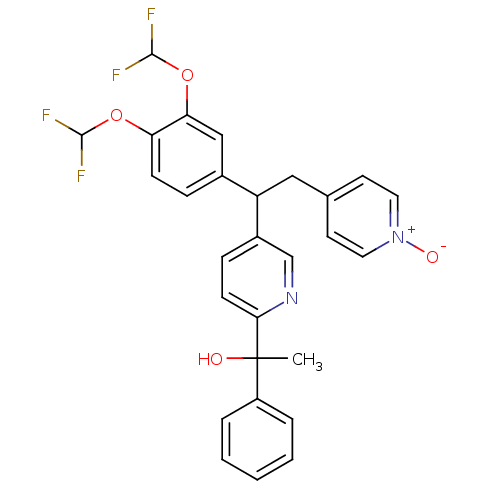
(1-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(O)(c1ccccc1)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C28H24F4N2O4/c1-28(35,21-5-3-2-4-6-21)25-10-8-20(17-33-25)22(15-18-11-13-34(36)14-12-18)19-7-9-23(37-26(29)30)24(16-19)38-27(31)32/h2-14,16-17,22,26-27,35H,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM82428
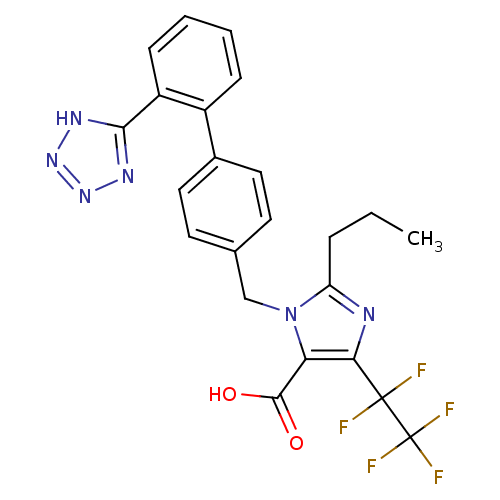
(CAS_124750-95-4 | CB91356279 | CHEMBL443269 | DuP ...)Show SMILES CCCc1nc(c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)C(F)(F)C(F)(F)F Show InChI InChI=1S/C23H19F5N6O2/c1-2-5-17-29-19(22(24,25)23(26,27)28)18(21(35)36)34(17)12-13-8-10-14(11-9-13)15-6-3-4-7-16(15)20-30-32-33-31-20/h3-4,6-11H,2,5,12H2,1H3,(H,35,36)(H,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 272: 612-8 (1995)
BindingDB Entry DOI: 10.7270/Q2FB51GP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409702
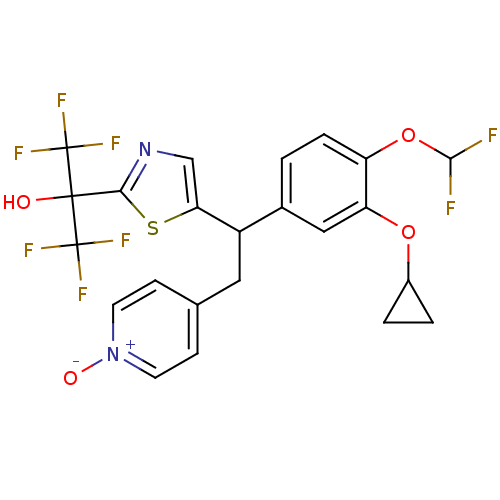
(CHEMBL2112296)Show SMILES OC(c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-4-1-13(10-17(16)36-14-2-3-14)15(9-12-5-7-33(35)8-6-12)18-11-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1,4-8,10-11,14-15,20,34H,2-3,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409699
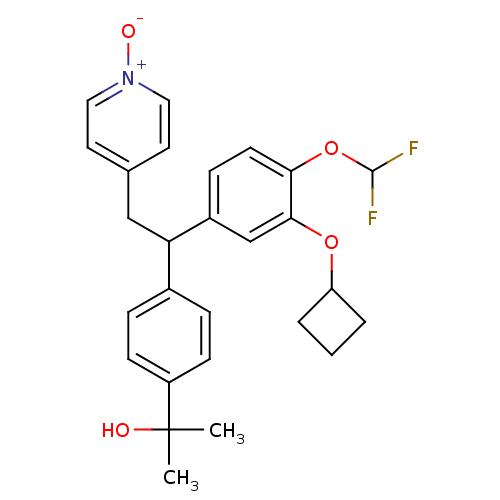
(CHEMBL2112294)Show SMILES CC(C)(O)c1ccc(cc1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C27H29F2NO4/c1-27(2,31)21-9-6-19(7-10-21)23(16-18-12-14-30(32)15-13-18)20-8-11-24(34-26(28)29)25(17-20)33-22-4-3-5-22/h6-15,17,22-23,26,31H,3-5,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128424

(2-{5-[1-(3,4-Bis-difluoromethoxy-phenyl)-2-(1-oxy-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1 Show InChI InChI=1S/C23H22F4N2O4/c1-23(2,30)20-6-4-16(13-28-20)17(11-14-7-9-29(31)10-8-14)15-3-5-18(32-21(24)25)19(12-15)33-22(26)27/h3-10,12-13,17,21-22,30H,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128686

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C26H28F2N2O4/c1-26(2,31)24-9-7-19(16-29-24)21(14-17-10-12-30(32)13-11-17)18-6-8-22(34-25(27)28)23(15-18)33-20-4-3-5-20/h6-13,15-16,20-21,25,31H,3-5,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128689
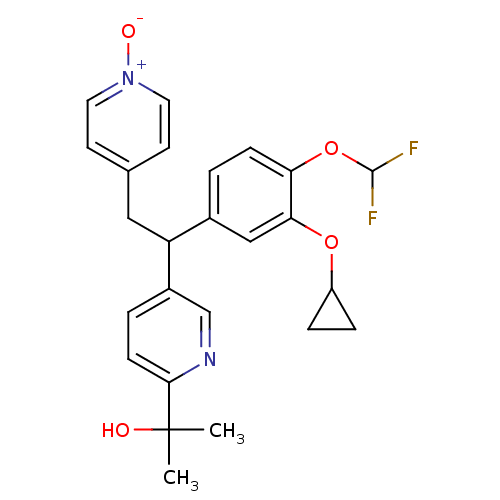
(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128689

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-8-4-18(15-28-23)20(13-16-9-11-29(31)12-10-16)17-3-7-21(33-24(26)27)22(14-17)32-19-5-6-19/h3-4,7-12,14-15,19-20,24,30H,5-6,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409700
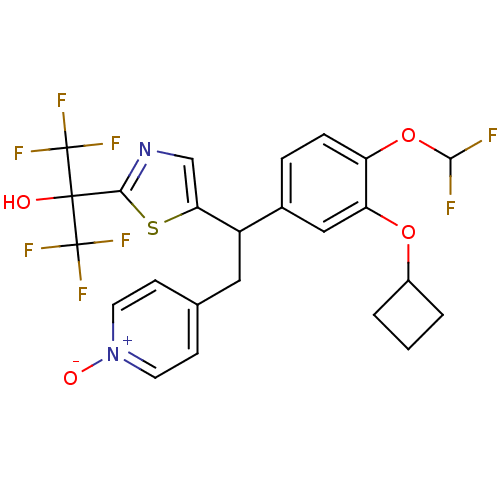
(CHEMBL2112295)Show SMILES OC(c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H20F8N2O4S/c25-21(26)38-17-5-4-14(11-18(17)37-15-2-1-3-15)16(10-13-6-8-34(36)9-7-13)19-12-33-20(39-19)22(35,23(27,28)29)24(30,31)32/h4-9,11-12,15-16,21,35H,1-3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50409701

(CHEMBL383762)Show SMILES OC(c1ncc(s1)[C@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128683
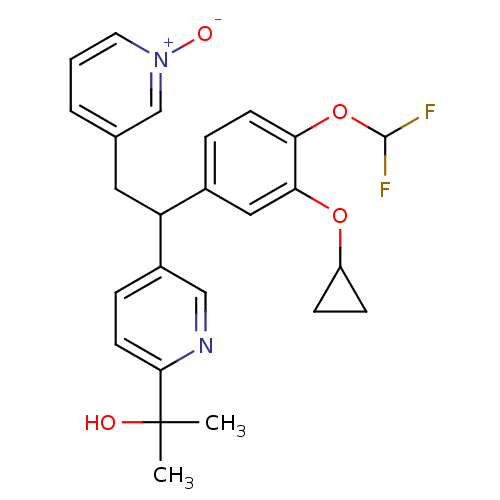
(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128683

(2-{5-[1-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,30)23-10-6-18(14-28-23)20(12-16-4-3-11-29(31)15-16)17-5-9-21(33-24(26)27)22(13-17)32-19-7-8-19/h3-6,9-11,13-15,19-20,24,30H,7-8,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128692
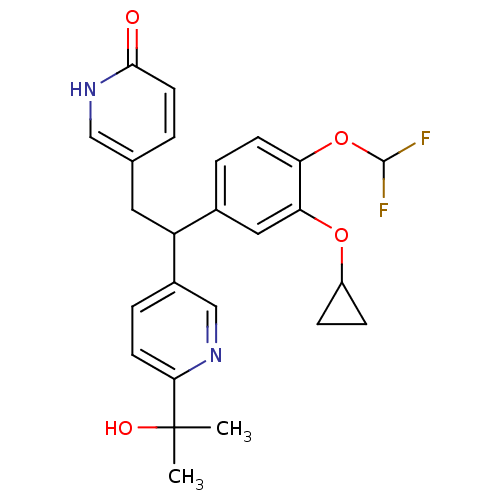
(5-{2-(3-Cyclopropoxy-4-difluoromethoxy-phenyl)-2-[...)Show SMILES CC(C)(O)c1ccc(cn1)C(Cc1ccc(=O)[nH]c1)c1ccc(OC(F)F)c(OC2CC2)c1 Show InChI InChI=1S/C25H26F2N2O4/c1-25(2,31)22-9-5-17(14-28-22)19(11-15-3-10-23(30)29-13-15)16-4-8-20(33-24(26)27)21(12-16)32-18-6-7-18/h3-5,8-10,12-14,18-19,24,31H,6-7,11H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50128685

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C24H26F2N2O4S/c1-24(2,29)22-27-14-21(33-22)18(12-15-8-10-28(30)11-9-15)16-6-7-19(32-23(25)26)20(13-16)31-17-4-3-5-17/h6-11,13-14,17-18,23,29H,3-5,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50174020
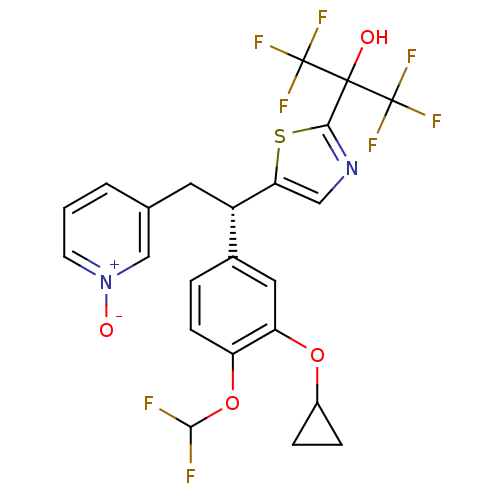
((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro displacement of [35S]-MK- 499 from HEK 293 cells stably transfected with hERG voltage-gated potassium channel subunit Kv11.1 |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50035458
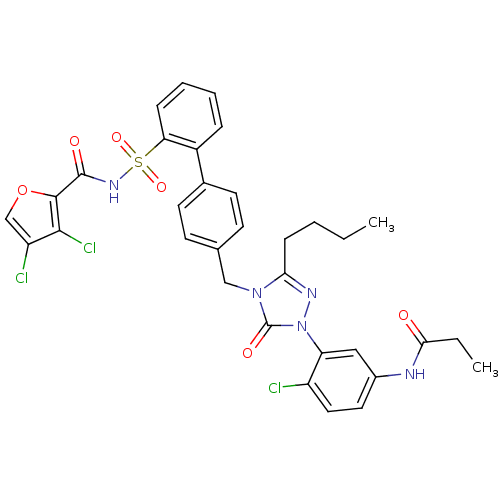
(CHEMBL138690 | N-[3-(3-Butyl-4-{2'-[(3,4-dichloro-...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2Cl)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1occ(Cl)c1Cl Show InChI InChI=1S/C33H30Cl3N5O6S/c1-3-5-10-28-38-41(26-17-22(15-16-24(26)34)37-29(42)4-2)33(44)40(28)18-20-11-13-21(14-12-20)23-8-6-7-9-27(23)48(45,46)39-32(43)31-30(36)25(35)19-47-31/h6-9,11-17,19H,3-5,10,18H2,1-2H3,(H,37,42)(H,39,43) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... |
J Med Chem 37: 4464-78 (1995)
BindingDB Entry DOI: 10.7270/Q2WD3ZK5 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50035434
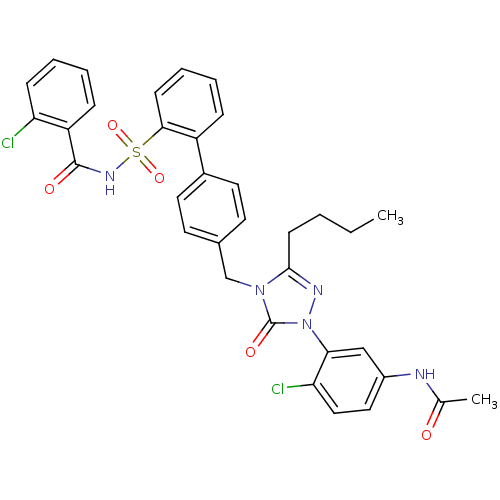
(CHEMBL265797 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(C)=O)ccc2Cl)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C34H31Cl2N5O5S/c1-3-4-13-32-38-41(30-20-25(37-22(2)42)18-19-29(30)36)34(44)40(32)21-23-14-16-24(17-15-23)26-9-6-8-12-31(26)47(45,46)39-33(43)27-10-5-7-11-28(27)35/h5-12,14-20H,3-4,13,21H2,1-2H3,(H,37,42)(H,39,43) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... |
J Med Chem 37: 4464-78 (1995)
BindingDB Entry DOI: 10.7270/Q2WD3ZK5 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50035434

(CHEMBL265797 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(C)=O)ccc2Cl)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C34H31Cl2N5O5S/c1-3-4-13-32-38-41(30-20-25(37-22(2)42)18-19-29(30)36)34(44)40(32)21-23-14-16-24(17-15-23)26-9-6-8-12-31(26)47(45,46)39-33(43)27-10-5-7-11-28(27)35/h5-12,14-20H,3-4,13,21H2,1-2H3,(H,37,42)(H,39,43) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro ability of the compound to inhibit the binding of radioligand 125I[Sar1,IIe8]AII to AT1 receptor from rabbit aorta |
Bioorg Med Chem Lett 4: 2787-2792 (1994)
Article DOI: 10.1016/S0960-894X(01)80595-3
BindingDB Entry DOI: 10.7270/Q2WS8T68 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50030726

(3-[4-(2'-Benzoylsulfamoyl-3-fluoro-biphenyl-4-ylme...)Show SMILES CCCNC(=O)c1ccc(c(c1)-n1nc(CCC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)c2ccccc2)c1=O)C(F)(F)F Show InChI InChI=1S/C36H33F4N5O5S/c1-3-10-32-42-45(30-21-25(33(46)41-19-4-2)17-18-28(30)36(38,39)40)35(48)44(32)22-26-16-15-24(20-29(26)37)27-13-8-9-14-31(27)51(49,50)43-34(47)23-11-6-5-7-12-23/h5-9,11-18,20-21H,3-4,10,19,22H2,1-2H3,(H,41,46)(H,43,47) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50030685
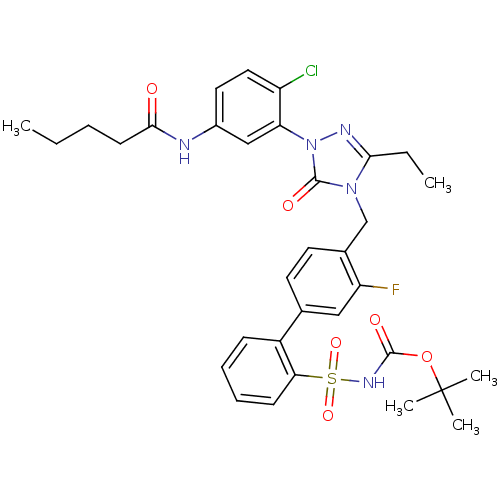
(CHEMBL338888 | Pentanoic acid {4-chloro-3-[3-ethyl...)Show SMILES CCCCC(=O)Nc1ccc(Cl)c(c1)-n1nc(CC)n(Cc2ccc(cc2F)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C33H37ClFN5O6S/c1-6-8-13-30(41)36-23-16-17-25(34)27(19-23)40-32(43)39(29(7-2)37-40)20-22-15-14-21(18-26(22)35)24-11-9-10-12-28(24)47(44,45)38-31(42)46-33(3,4)5/h9-12,14-19H,6-8,13,20H2,1-5H3,(H,36,41)(H,38,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against angiotensin II type 2 receptor in rat adrenal membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50035444
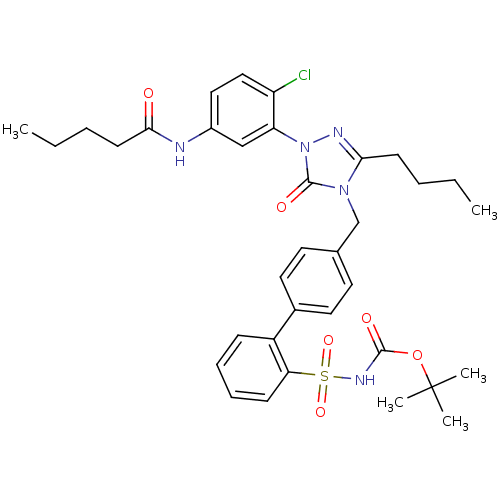
(4-[[2'-[N-(tert-Butoxycarbonyl)sulfamoyl]biphenyl-...)Show SMILES CCCCC(=O)Nc1ccc(Cl)c(c1)-n1nc(CCCC)n(Cc2ccc(cc2)-c2ccccc2S(=O)(=O)NC(=O)OC(C)(C)C)c1=O Show InChI InChI=1S/C35H42ClN5O6S/c1-6-8-14-31-38-41(29-22-26(20-21-28(29)36)37-32(42)15-9-7-2)34(44)40(31)23-24-16-18-25(19-17-24)27-12-10-11-13-30(27)48(45,46)39-33(43)47-35(3,4)5/h10-13,16-22H,6-9,14-15,23H2,1-5H3,(H,37,42)(H,39,43) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... |
J Med Chem 37: 4464-78 (1995)
BindingDB Entry DOI: 10.7270/Q2WD3ZK5 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50030692
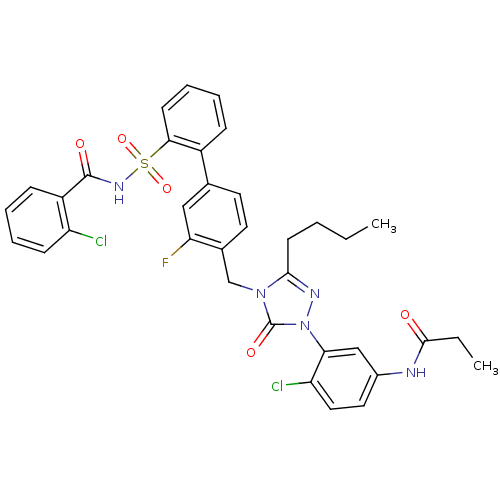
(CHEMBL339672 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C35H32Cl2FN5O5S/c1-3-5-14-32-40-43(30-20-24(17-18-28(30)37)39-33(44)4-2)35(46)42(32)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)49(47,48)41-34(45)26-11-6-8-12-27(26)36/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,39,44)(H,41,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50030692

(CHEMBL339672 | N-(3-{3-Butyl-4-[2'-(2-chloro-benzo...)Show SMILES CCCCc1nn(-c2cc(NC(=O)CC)ccc2Cl)c(=O)n1Cc1ccc(cc1F)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C35H32Cl2FN5O5S/c1-3-5-14-32-40-43(30-20-24(17-18-28(30)37)39-33(44)4-2)35(46)42(32)21-23-16-15-22(19-29(23)38)25-10-7-9-13-31(25)49(47,48)41-34(45)26-11-6-8-12-27(26)36/h6-13,15-20H,3-5,14,21H2,1-2H3,(H,39,44)(H,41,45) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Angiotensin II receptor, type 1 in rabbit aorta membrane preparations. |
J Med Chem 38: 3741-58 (1995)
BindingDB Entry DOI: 10.7270/Q2Q81C35 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50035454
(CHEMBL343309 | Cyclopropanecarboxylic acid (3-{3-b...)Show SMILES CCCCc1nn(-c2cc(NC(=O)C3CC3)ccc2Cl)c(=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1Cl Show InChI InChI=1S/C36H33Cl2N5O5S/c1-2-3-12-33-40-43(31-21-26(19-20-30(31)38)39-34(44)25-17-18-25)36(46)42(33)22-23-13-15-24(16-14-23)27-8-5-7-11-32(27)49(47,48)41-35(45)28-9-4-6-10-29(28)37/h4-11,13-16,19-21,25H,2-3,12,17-18,22H2,1H3,(H,39,44)(H,41,45) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin I (AT1) receptor to competitively block the specific binding of [125I]- [Sar1,Ile8] AII to a... |
J Med Chem 37: 4464-78 (1995)
BindingDB Entry DOI: 10.7270/Q2WD3ZK5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data