

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as inhibition constant using acetylthiocholine iodide as substrate by Cornish-Bowden plot an... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10439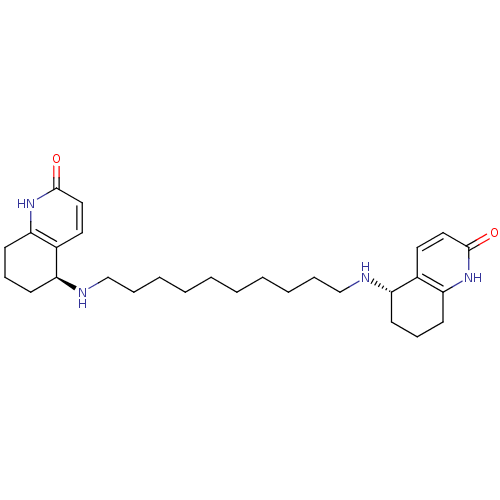 ((5S)-5-[(10-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.800 | -51.4 | 2.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed-type inhibition of recombinant human AChE assessed as dissociation constant for protein-substrate-compound complex using acetylthiocholine iodi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507212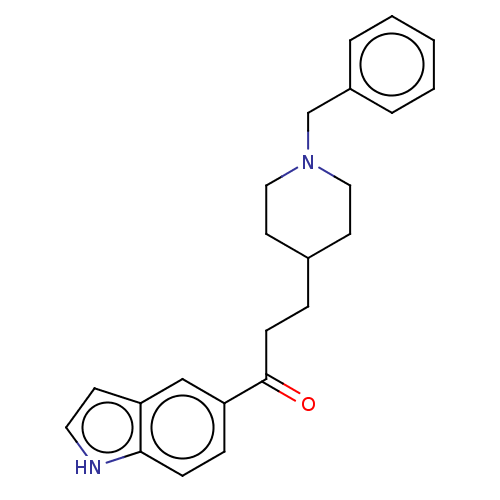 (CHEMBL4585990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10440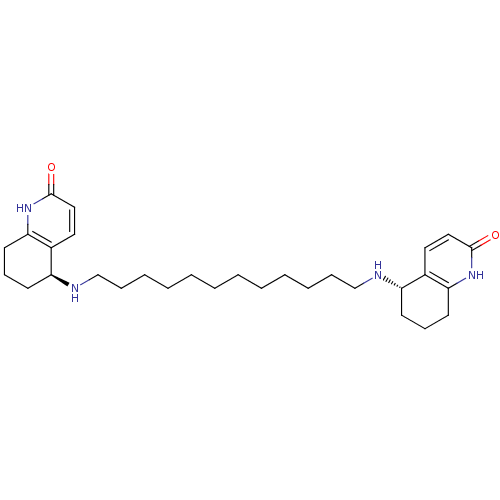 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.5 | -47.2 | 16 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507211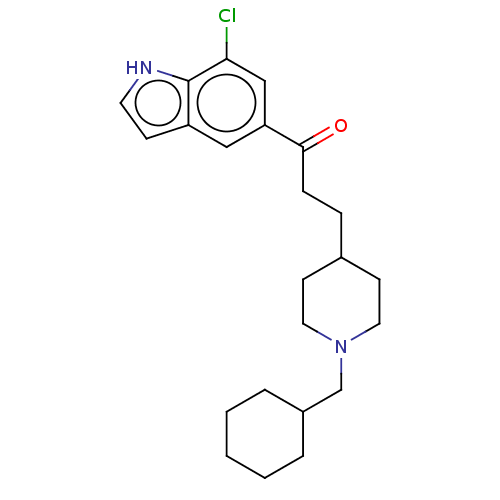 (CHEMBL4444843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507209 (CHEMBL4537101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507208 (CHEMBL4526050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Agonist activity at 5-HT4R (unknown origin) | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50540764 (CHEMBL4648322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10440 ((5S)-5-[(12-{[(5S)-2-oxo-1,2,5,6,7,8-hexahydroquin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 19.6 | -43.6 | 52 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507215 (CHEMBL4562793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507211 (CHEMBL4444843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507213 (CHEMBL4529710) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507209 (CHEMBL4537101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 47.1 | -41.4 | 114 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507214 (CHEMBL4567394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507210 (CHEMBL4454706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507208 (CHEMBL4526050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507212 (CHEMBL4585990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -38.2 | 414 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 125: 363-73 (2003) Article DOI: 10.1021/ja021111w BindingDB Entry DOI: 10.7270/Q26D5R69 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10632 ((-)-Huperzine B | (1R,10R)-16-methyl-6,14-diazatet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 334 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10630 (3-[1-(dimethylamino)ethyl]phenol | NAP) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 700 | -35.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Biochemistry 41: 3555-64 (2002) Article DOI: 10.1021/bi020016x BindingDB Entry DOI: 10.7270/Q2NG4NVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10630 (3-[1-(dimethylamino)ethyl]phenol | NAP) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 3.30E+4 | -25.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at... | Biochemistry 41: 3555-64 (2002) Article DOI: 10.1021/bi020016x BindingDB Entry DOI: 10.7270/Q2NG4NVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556952 (CHEMBL4754487) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523281 (CHEMBL4583650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... | Eur J Med Chem 168: 58-77 (2019) Article DOI: 10.1016/j.ejmech.2018.12.063 BindingDB Entry DOI: 10.7270/Q2J969S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556958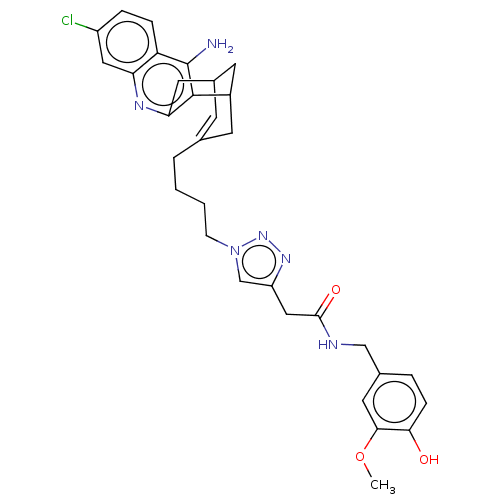 (CHEMBL4744528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556953 (CHEMBL4748114) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10487 (1-chloro-6H,7H,8H,9H,10H-cyclohepta[b]quinolin-11-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556954 (CHEMBL4782921) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10503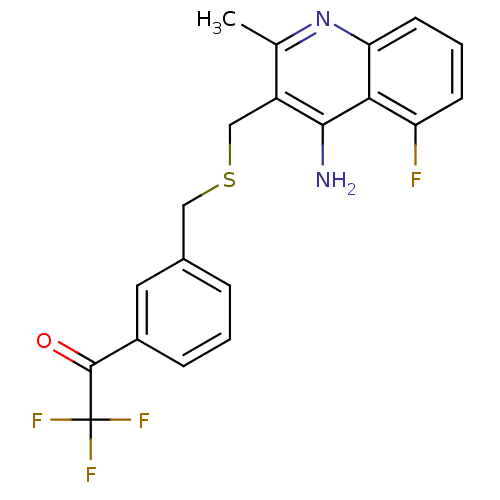 (1-[3-({[(4-amino-5-fluoro-2-methylquinolin-3-yl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10501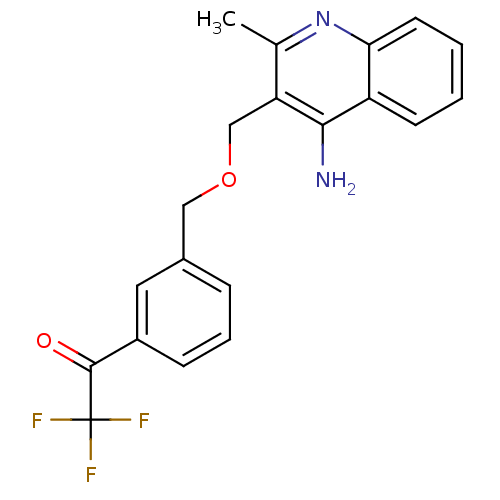 (1-(3-{[(4-amino-2-methylquinolin-3-yl)methoxy]meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10499 (1-(3-{[(4-amino-5-fluoro-2-methylquinolin-3-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM9047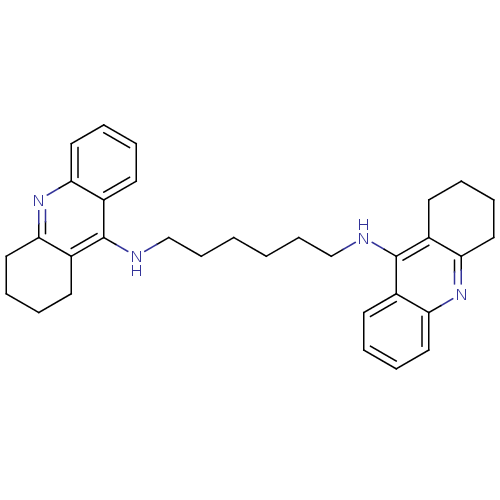 (Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science Curated by ChEMBL | Assay Description Inhibition of rat brain AChE | J Med Chem 49: 5491-500 (2006) Article DOI: 10.1021/jm060164b BindingDB Entry DOI: 10.7270/Q2154GPX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10406 ((1S,12S,14R)-4-[8-(1,3-dioxo-2,3-dihydro-1H-isoind...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Am Chem Soc 126: 15405-11 (2004) Article DOI: 10.1021/ja0466154 BindingDB Entry DOI: 10.7270/Q2FX77N1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556956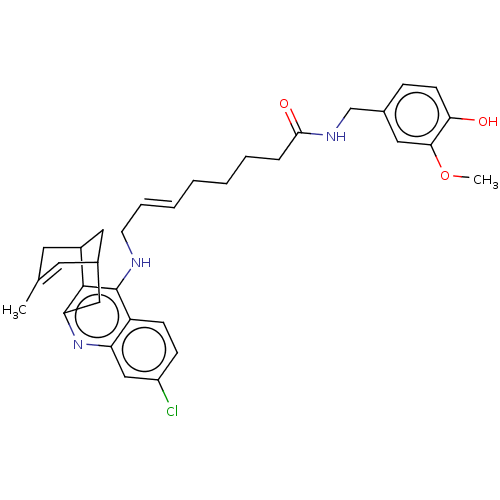 (CHEMBL4741162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50556951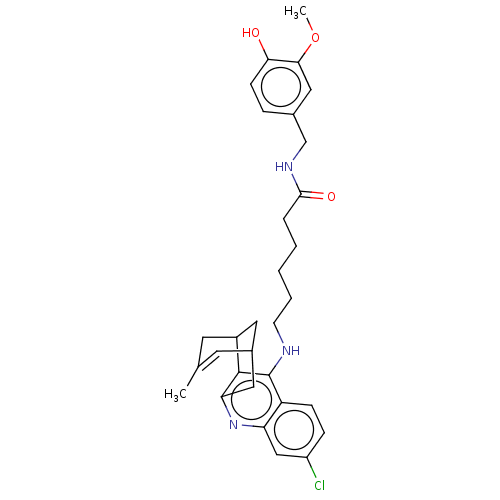 (CHEMBL4788434) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholineiodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01775 BindingDB Entry DOI: 10.7270/Q2K077XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10500 (1-(3-{[(4-amino-5-chloro-2-methylquinolin-3-yl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Syngenta | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | J Med Chem 44: 3203-15 (2001) Article DOI: 10.1021/jm010826r BindingDB Entry DOI: 10.7270/Q2PC30MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 219 total ) | Next | Last >> |