

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50560609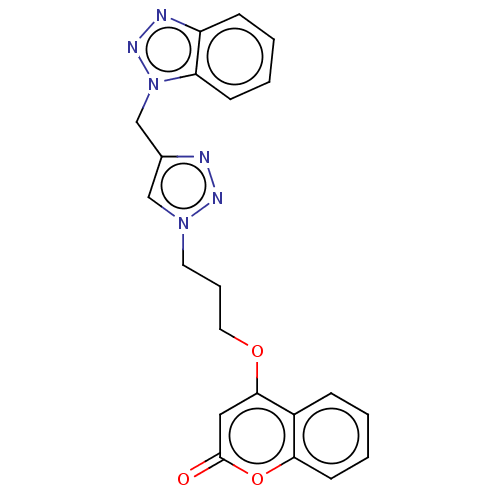 (CHEMBL4749763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of electric eel AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by reciprocal Linew... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127477 BindingDB Entry DOI: 10.7270/Q2W099MW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30369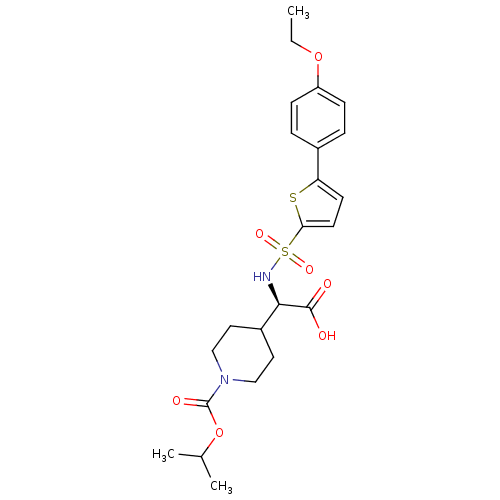 (piperidinyl glycine derivative, 24f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | -54.0 | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30344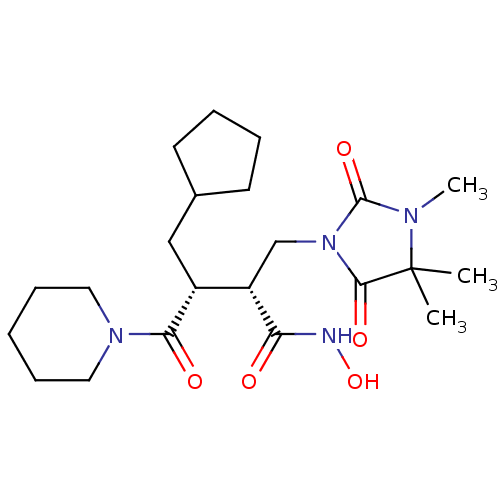 (Cipemastat | Trocade) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | -52.4 | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50602541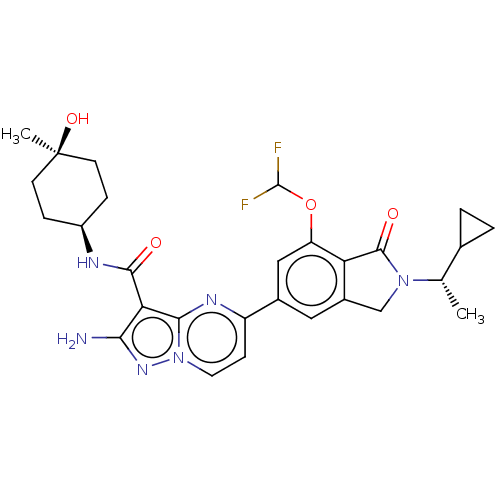 (CHEMBL5209268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01153 BindingDB Entry DOI: 10.7270/Q2KH0SCH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.27 | -50.3 | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303975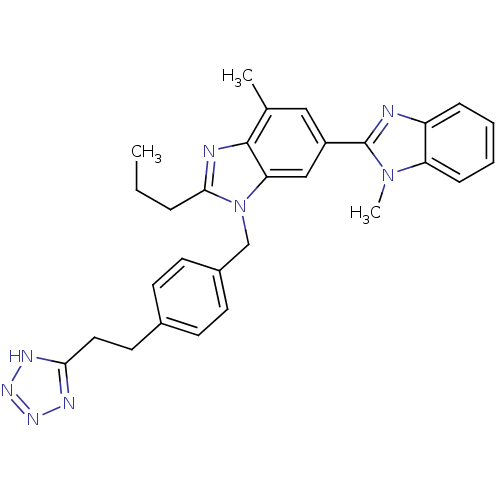 (3'-(4-(2-(1H-Tetrazol-5-yl)ethyl)benzyl)-1,7'-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50515758 (CHEMBL4453575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human dihydrofolate reductase assessed as inhibition constant by UV-Vis spectra based Benesi Hilebrand equation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126631 BindingDB Entry DOI: 10.7270/Q2RB780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50515763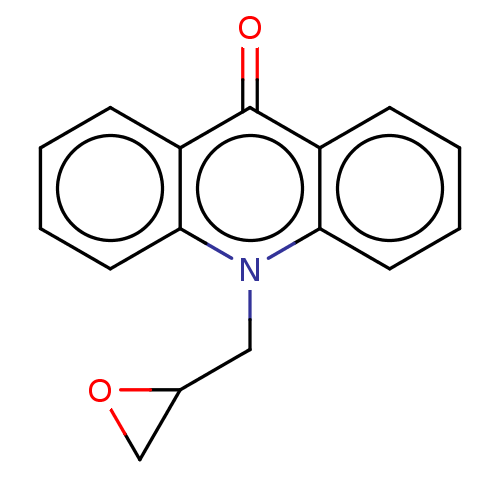 (CHEMBL4458303) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of human dihydrofolate reductase assessed as inhibition constant by UV-Vis spectra based Benesi Hilebrand equation analysis | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126631 BindingDB Entry DOI: 10.7270/Q2RB780V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303976 (3'-(2-(4-(2-(1H-Tetrazol-5-yl)ethyl)phenoxy)ethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303977 (3-(4-(2-(1,7'-Dimethyl-2'-propyl-1H,3'H-2,5'-biben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-322] (Homo sapiens (Human)) | BDBM50166435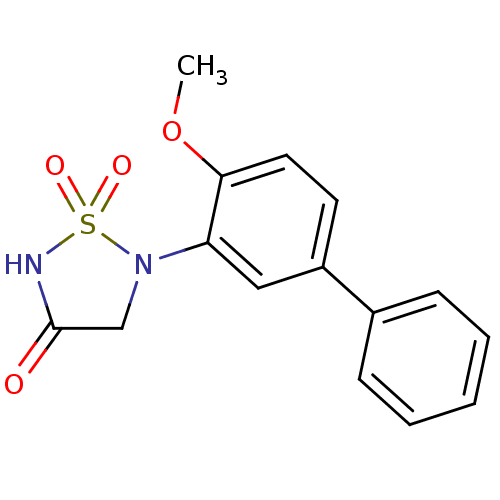 (5-(4-METHOXYBIPHENYL-3-YL)-1,2,5-THIADIAZOLIDIN-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.40E+3 | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Missouri | Assay Description Assays for the reversible inhibition of PTP1B (72 nM) contained the test compound(2a, 2b, 5a, or 5b) in Bis-Tris (50 mM), NaCl (100 mM), EDTA (2 mM),... | Biochemistry 56: 2051-2060 (2017) Article DOI: 10.1021/acs.biochem.7b00151 BindingDB Entry DOI: 10.7270/Q2HX1BJ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303979 (CHEMBL571763 | Ethyl 3-(4-(2-(1,7'-Dimethyl-2'-pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303978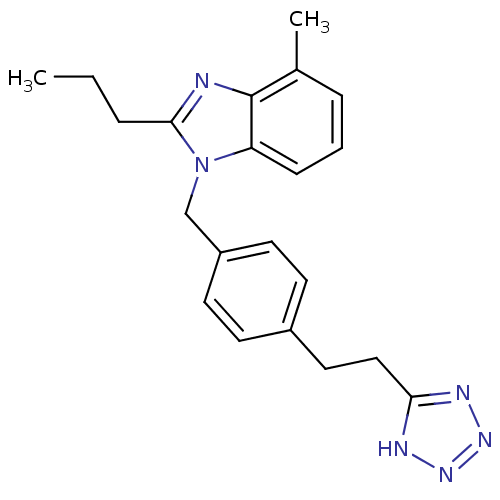 (1-(4-(2-(1H-tetrazol-5-yl)ethyl)benzyl)-4-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303980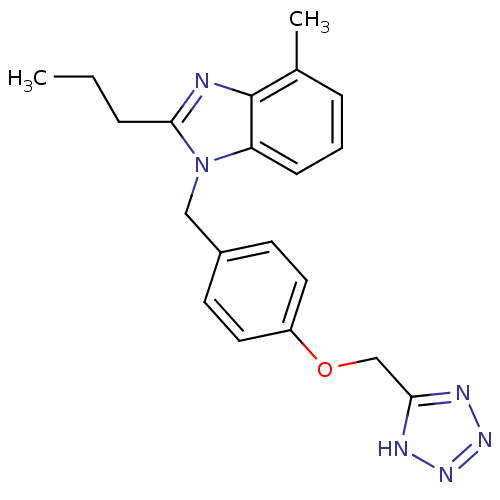 (1-(4-((1H-Tetrazol-5-yl)methoxy)benzyl)-4-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303985 (CHEMBL567269 | Ethyl 2,2-Dimethyl-3-(4-(2-(4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303986 (CHEMBL584704 | Ethyl 2-Methyl-3-(4-(2-(4-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303984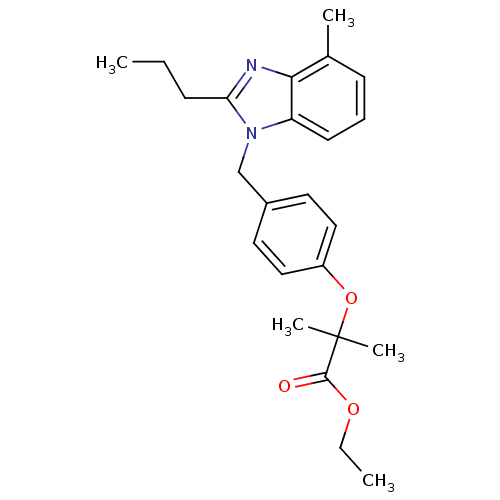 (CHEMBL565379 | Ethyl 2-Methyl-2-(4-((4-methyl-2-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303982 (CHEMBL567689 | Methyl 3-(4-((4-Methyl-2-propyl-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303981 (CHEMBL567063 | Ethyl 3-(4-((4-Methyl-2-propyl-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50303983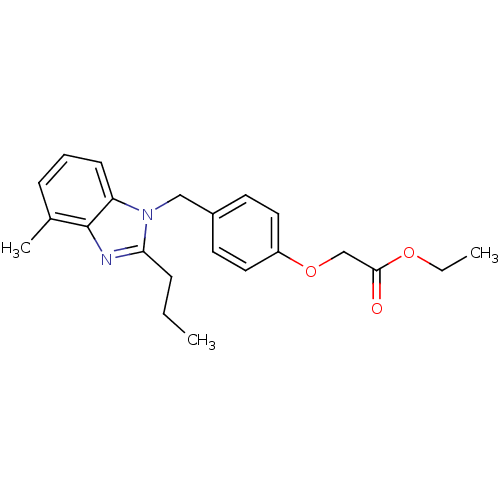 (CHEMBL565793 | ethyl 2-(4-((4-methyl-2-propyl-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Mississippi Curated by ChEMBL | Assay Description Displacement of [125I]SI-Ang2 from AT1 receptor in Sprague-Dawley rat liver membrane | J Med Chem 53: 1076-85 (2010) Article DOI: 10.1021/jm901272d BindingDB Entry DOI: 10.7270/Q22V2H2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-322] (Homo sapiens (Human)) | BDBM223197 (2-Bromo-N-[3'-(1,1-dioxido-4-oxo-1,2,5-thiadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70E+4 | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Missouri | Assay Description Assays for the reversible inhibition of PTP1B (72 nM) contained the test compound(2a, 2b, 5a, or 5b) in Bis-Tris (50 mM), NaCl (100 mM), EDTA (2 mM),... | Biochemistry 56: 2051-2060 (2017) Article DOI: 10.1021/acs.biochem.7b00151 BindingDB Entry DOI: 10.7270/Q2HX1BJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-322] (Homo sapiens (Human)) | BDBM223198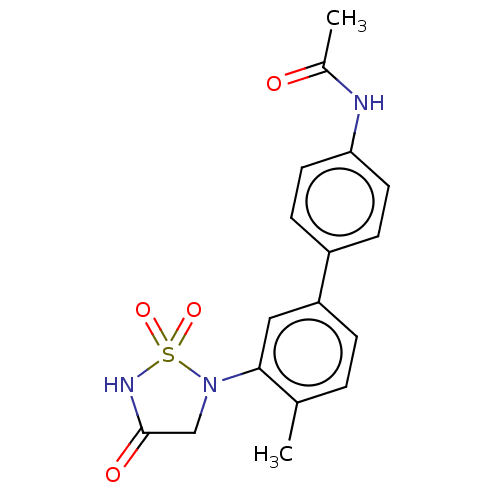 (N-[3'-(1,1-Dioxido-4-oxo-1,2,5-thiadiazolidin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+4 | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Missouri | Assay Description Assays for the reversible inhibition of PTP1B (72 nM) contained the test compound(2a, 2b, 5a, or 5b) in Bis-Tris (50 mM), NaCl (100 mM), EDTA (2 mM),... | Biochemistry 56: 2051-2060 (2017) Article DOI: 10.1021/acs.biochem.7b00151 BindingDB Entry DOI: 10.7270/Q2HX1BJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-322] (Homo sapiens (Human)) | BDBM223199 (5-(4-Methyl[1,1'-biphenyl]-3-yl)-1,2,5-thiadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.85E+4 | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
University of Missouri | Assay Description Assays for the reversible inhibition of PTP1B (72 nM) contained the test compound(2a, 2b, 5a, or 5b) in Bis-Tris (50 mM), NaCl (100 mM), EDTA (2 mM),... | Biochemistry 56: 2051-2060 (2017) Article DOI: 10.1021/acs.biochem.7b00151 BindingDB Entry DOI: 10.7270/Q2HX1BJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM50056999 (CHEMBL56367 | nimesulide) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate preinc... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM25753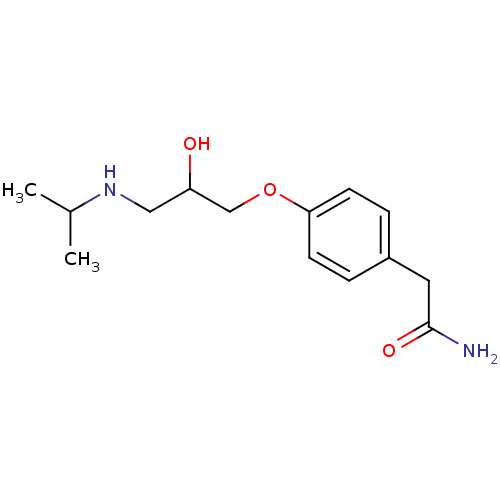 (2-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM25756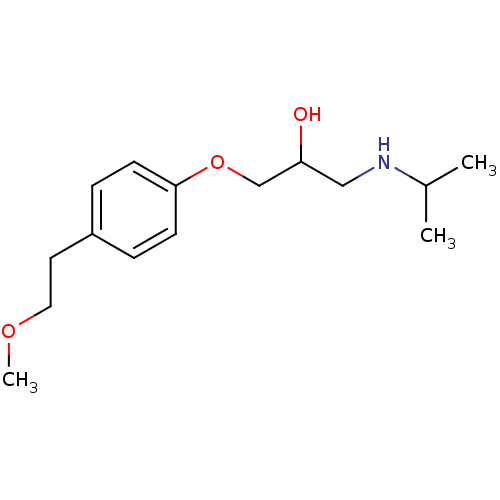 ((2R,3R)-2,3-dihydroxysuccinic acid;1-(isopropylami...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM26197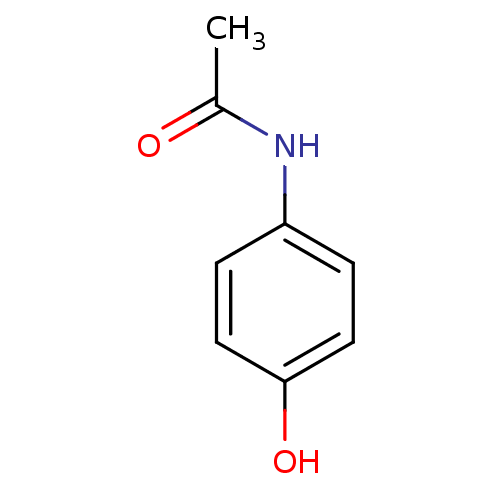 (CHEMBL112 | N-(4-hydroxyphenyl)acetamide | Norco |...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM25758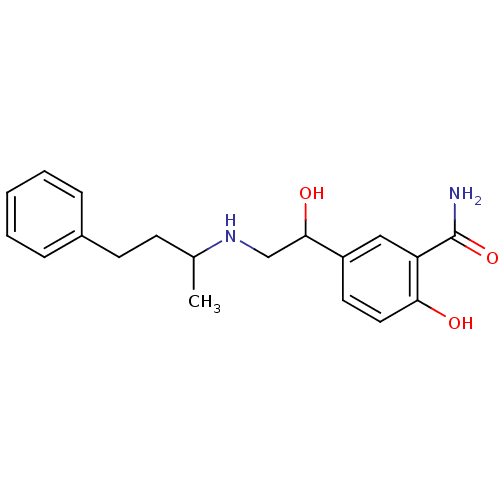 (2-hydroxy-5-{1-hydroxy-2-[(4-phenylbutan-2-yl)amin...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article | 2.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 3 (Bos mutus) | BDBM50174201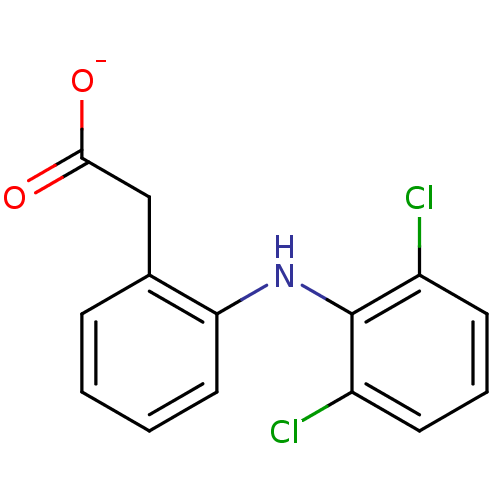 (ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein... | Citation and Details Article DOI: 10.1007/s00044-010-9454-7 BindingDB Entry DOI: 10.7270/Q2Z60RZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10512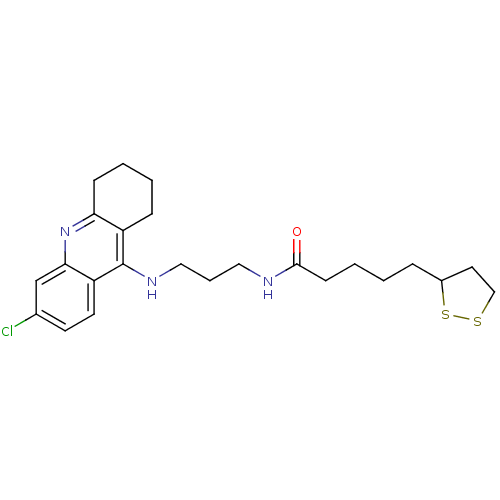 (CHEMBL194823 | N-{3-[(6-chloro-1,2,3,4-tetrahydroa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 151: 62-97 (2018) Article DOI: 10.1016/j.ejmech.2018.03.057 BindingDB Entry DOI: 10.7270/Q2NC63V1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30345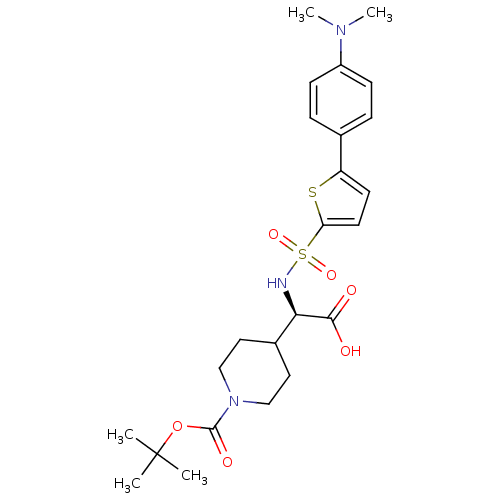 (BOC-piperidinyl glycine derivative, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30356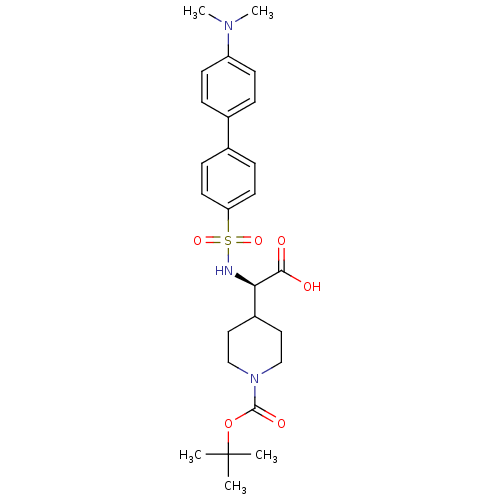 (BOC-piperidinyl glycine derivative, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30384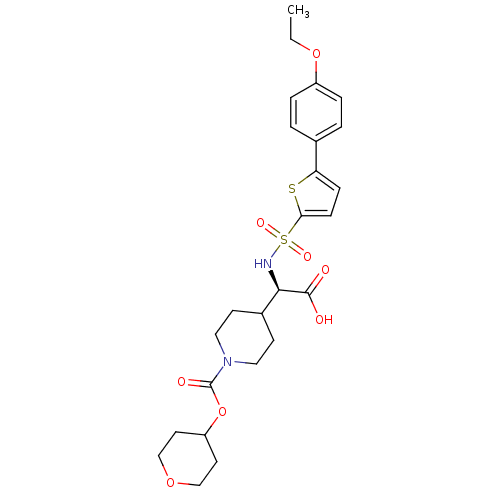 (piperidinyl glycine derivative, 24u) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30380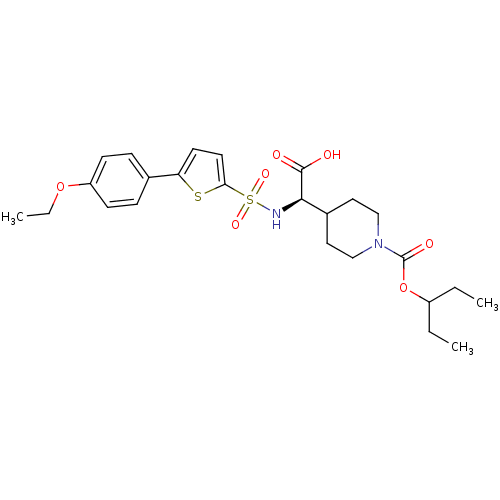 (piperidinyl glycine derivative, 24q) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30383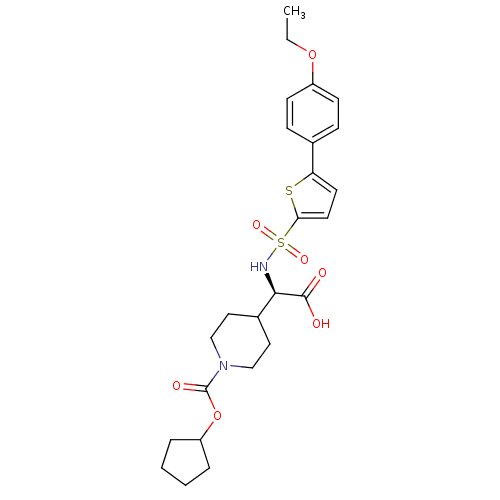 (piperidinyl glycine derivative, 24t) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30360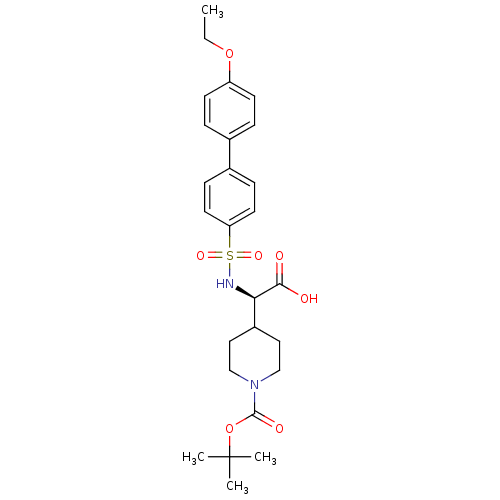 (BOC-piperidinyl glycine derivative, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30359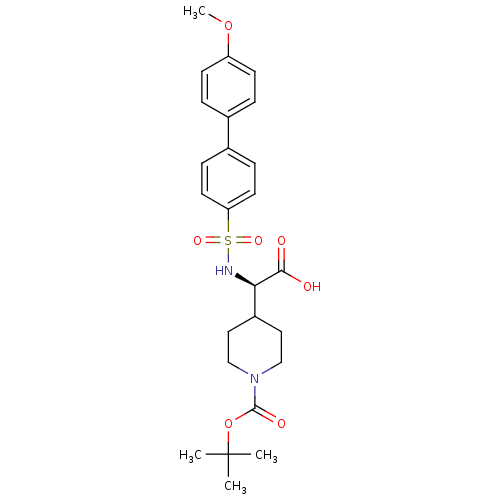 (BOC-piperidinyl glycine derivative, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30379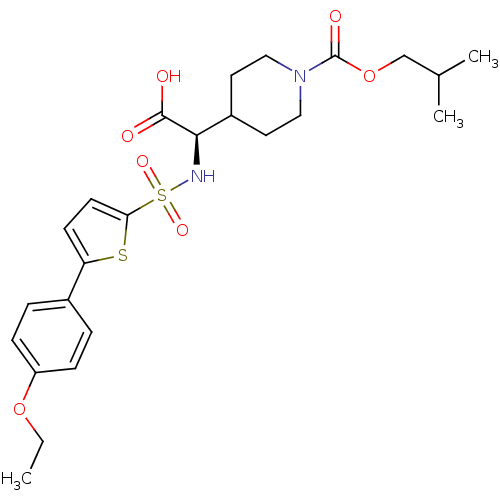 (piperidinyl glycine derivative, 24p) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30382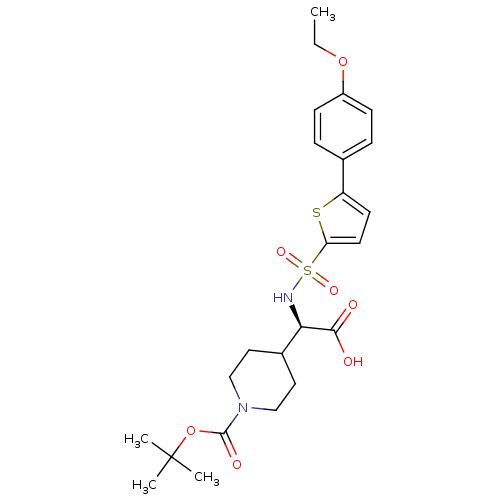 (piperidinyl glycine derivative, 24s) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30367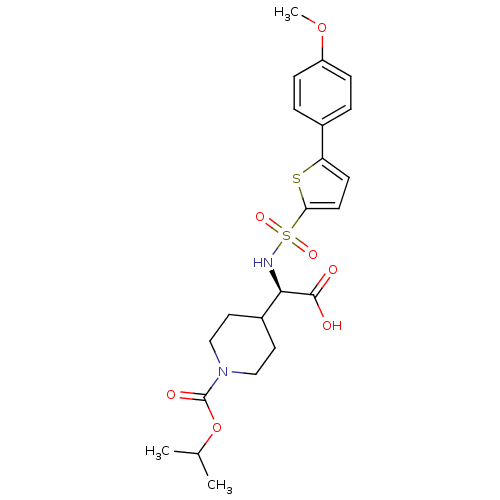 (piperidinyl glycine derivative, 24d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30396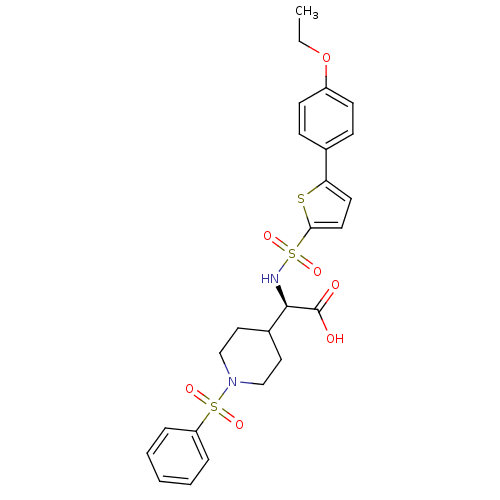 (piperidinyl glycine derivative, 27c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30397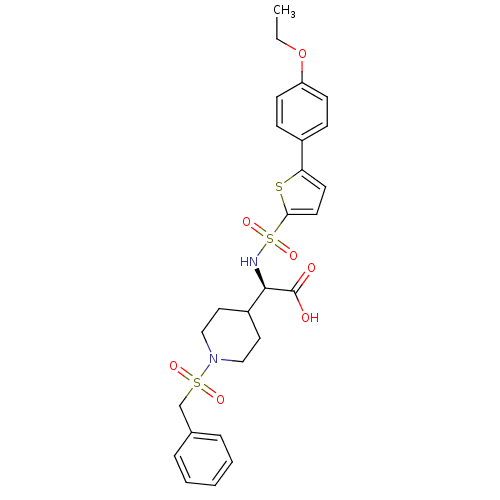 (piperidinyl glycine derivative, 27d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30357 (BOC-piperidinyl glycine derivative, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30347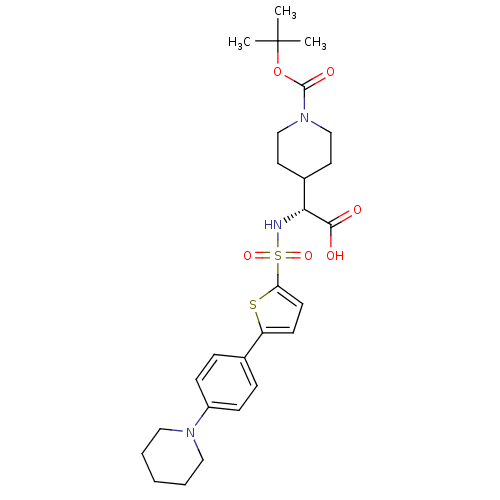 (BOC-piperidinyl glycine derivative, 22a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Sus scrofa) | BDBM50235214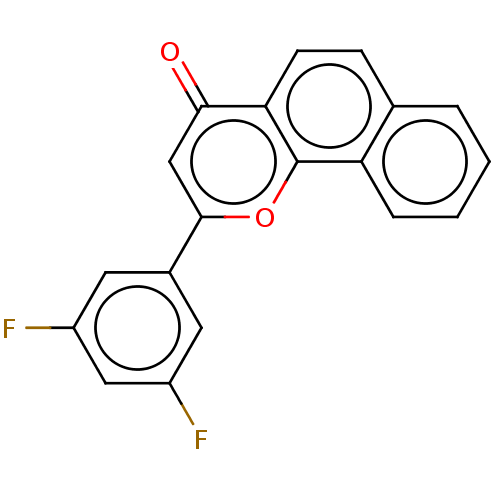 (CHEMBL4084903) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University Curated by ChEMBL | Assay Description Inhibition of porcine cholesterol esterase using para-nitrophenyl butyrate as substrate after 5 mins in presence of sodium taurocholate by spectropho... | Bioorg Med Chem Lett 27: 850-854 (2017) Article DOI: 10.1016/j.bmcl.2017.01.020 BindingDB Entry DOI: 10.7270/Q2PC34PC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30387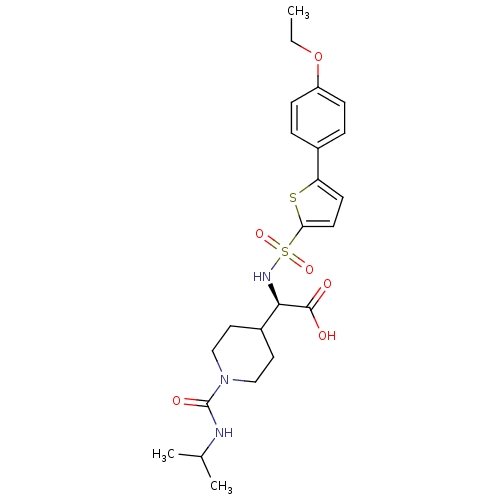 (piperidinyl glycine derivative, 25c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30378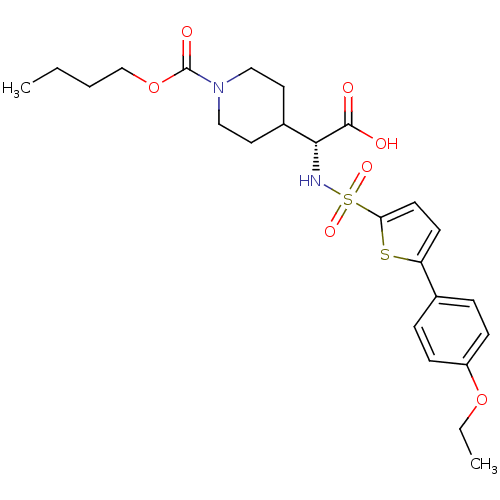 (piperidinyl glycine derivative, 24o) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 586 total ) | Next | Last >> |