

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50434373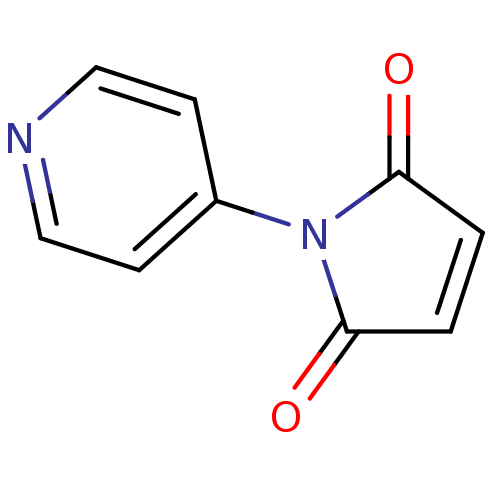 (CHEMBL2386992) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylcholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem Lett 23: 2984-9 (2013) Article DOI: 10.1016/j.bmcl.2013.03.026 BindingDB Entry DOI: 10.7270/Q289177M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438537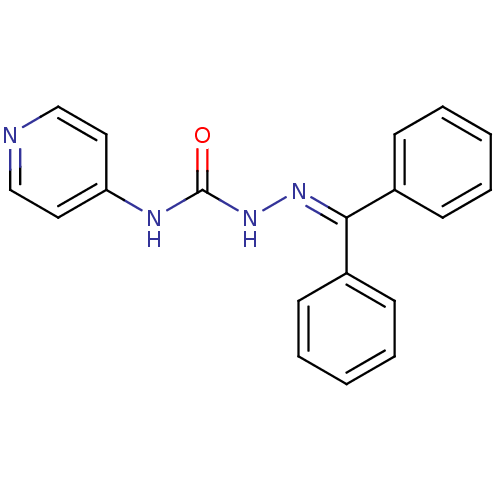 (CHEMBL2414861) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438536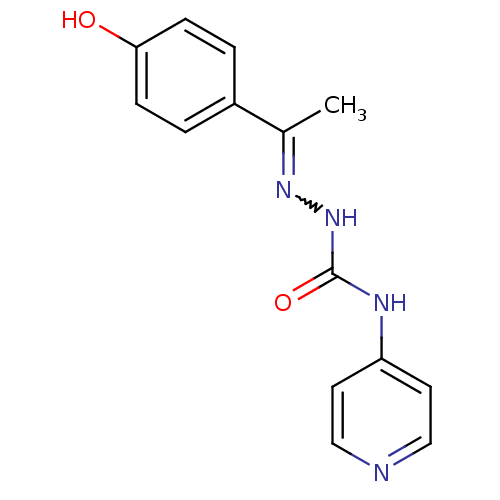 (CHEMBL2414862) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438536 (CHEMBL2414862) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 628 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50212069 (CHEMBL3912967) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Uncompetitive inhibition of electric eel AChE using varying levels of acetylthiocholine iodide substrate preincubated for 20 mins followed by substra... | Bioorg Med Chem 25: 1471-1480 (2017) Article DOI: 10.1016/j.bmc.2017.01.010 BindingDB Entry DOI: 10.7270/Q27M0B3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438541 (CHEMBL2414858) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50397031 (CHEMBL2171190) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki intercept using Histone H4... | J Med Chem 55: 7978-87 (2012) Article DOI: 10.1021/jm300521m BindingDB Entry DOI: 10.7270/Q2PN96R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438539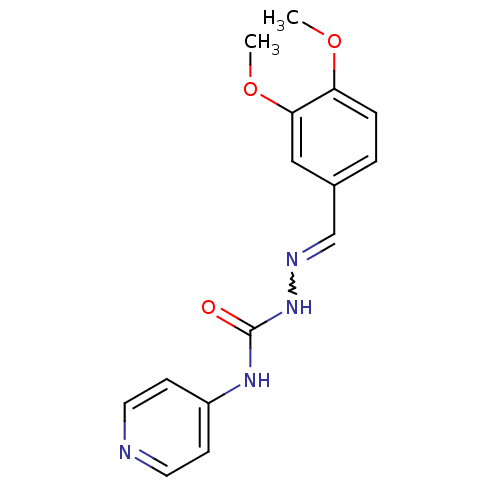 (CHEMBL2414860) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50397030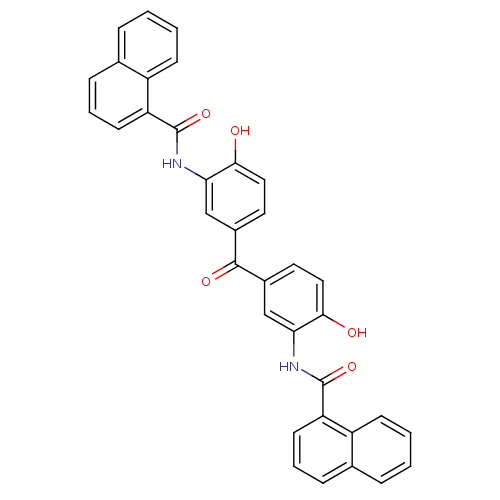 (CHEMBL2171189) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki intercept using Histone H4... | J Med Chem 55: 7978-87 (2012) Article DOI: 10.1021/jm300521m BindingDB Entry DOI: 10.7270/Q2PN96R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438537 (CHEMBL2414861) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438538 (CHEMBL2413097) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438542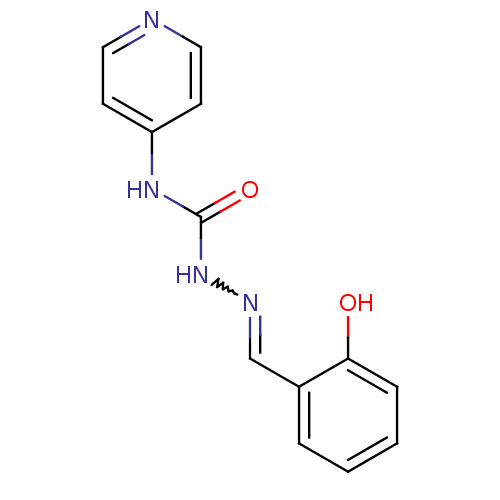 (CHEMBL2414857) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50397030 (CHEMBL2171189) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Non competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki slope using SAM preinc... | J Med Chem 55: 7978-87 (2012) Article DOI: 10.1021/jm300521m BindingDB Entry DOI: 10.7270/Q2PN96R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438540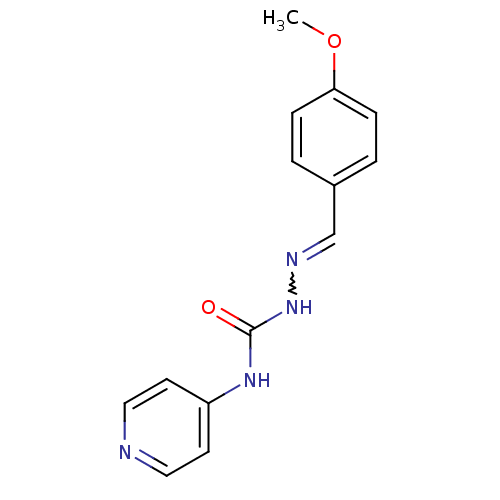 (CHEMBL2414859) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438541 (CHEMBL2414858) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50438543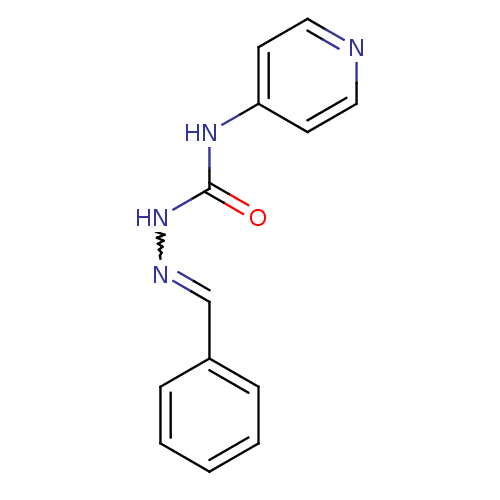 (CHEMBL2414856) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438542 (CHEMBL2414857) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50397031 (CHEMBL2171190) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.57E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Non competitive inhibition of histidine tagged recombinant PRMT1 expressed in Escherichia coli BL21 (DE3) cells assessed as Ki slope using SAM preinc... | J Med Chem 55: 7978-87 (2012) Article DOI: 10.1021/jm300521m BindingDB Entry DOI: 10.7270/Q2PN96R4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438539 (CHEMBL2414860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438538 (CHEMBL2413097) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Non competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438540 (CHEMBL2414859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50438543 (CHEMBL2414856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Banaras Hindu University Curated by ChEMBL | Assay Description Competitive inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Bioorg Med Chem 21: 5451-60 (2013) Article DOI: 10.1016/j.bmc.2013.06.003 BindingDB Entry DOI: 10.7270/Q27W6DM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16250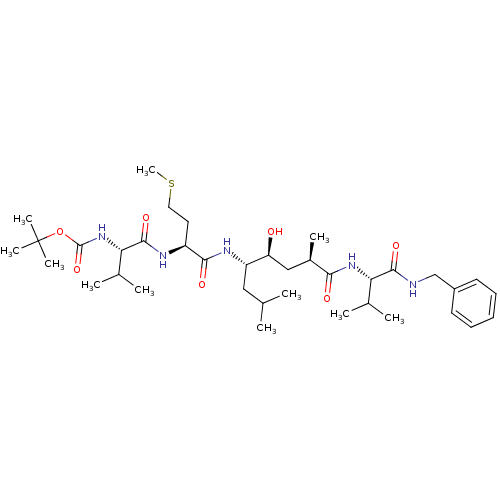 (CHEMBL290001 | N-(tert-butoxycarbonyl)-L-valyl-N-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against human beta-Secretase (BACE) | J Med Chem 46: 4625-30 (2003) Article DOI: 10.1021/jm030247h BindingDB Entry DOI: 10.7270/Q2PR7VCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM16047 ((4S)-4-[(2S)-2-[(2R,4S,5S)-5-[(2S)-2-[(2S)-2-[(4S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against human beta-Secretase (BACE) | J Med Chem 46: 4625-30 (2003) Article DOI: 10.1021/jm030247h BindingDB Entry DOI: 10.7270/Q2PR7VCB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50302846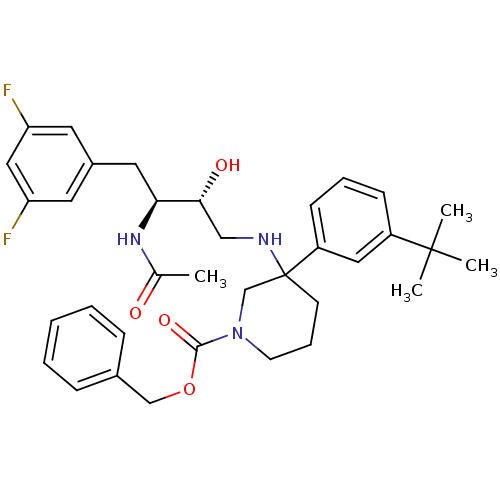 (CHEMBL570165 | benzyl 3-((2R,3S)-3-acetamido-4-(3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin D assessed as reduction in polarization after 110 mins by oregon green based fluorescence polarization assay | Bioorg Med Chem Lett 19: 6386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.09.061 BindingDB Entry DOI: 10.7270/Q23779NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523559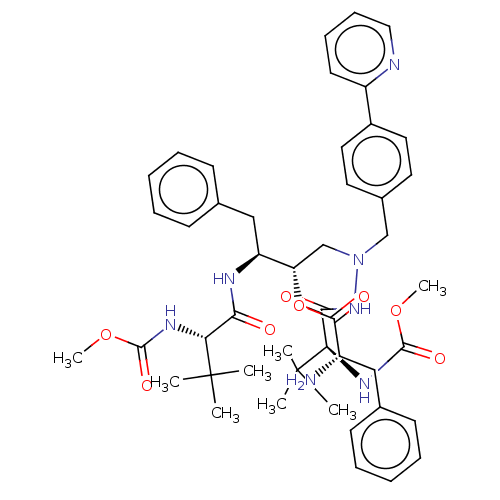 (CHEMBL4573907) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523558 (CHEMBL4460401) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523567 (CHEMBL4522729) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523566 (CHEMBL4574382) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523569 (CHEMBL4463796) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523568 (CHEMBL4447493) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523560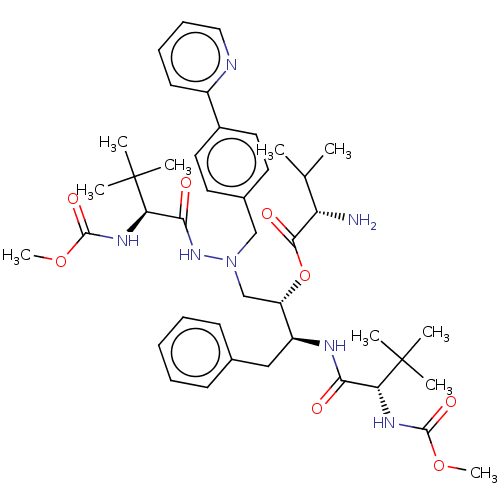 (CHEMBL4300203) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523561 (CHEMBL4437104) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523562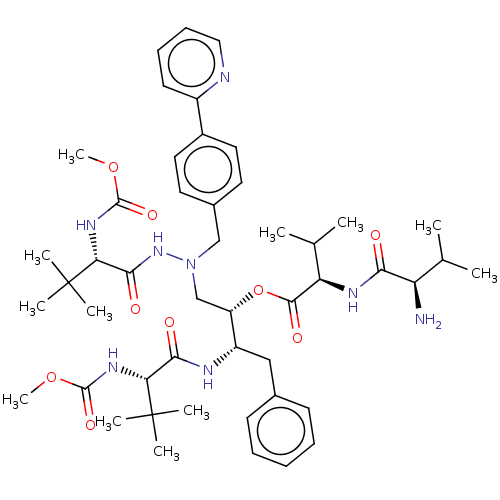 (CHEMBL4474072) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523563 (CHEMBL4554438) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523564 (CHEMBL4525849) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50523565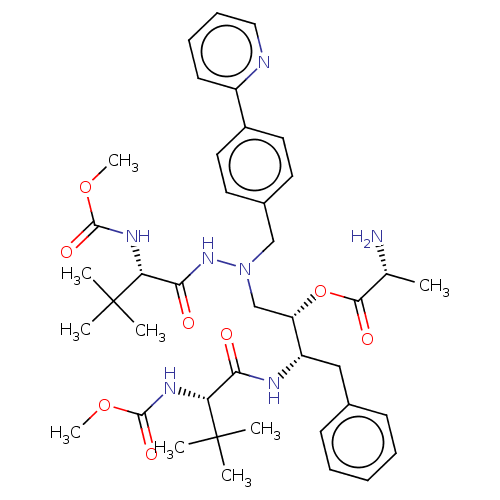 (CHEMBL4542773) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | J Med Chem 62: 3553-3574 (2019) Article DOI: 10.1021/acs.jmedchem.9b00002 BindingDB Entry DOI: 10.7270/Q2BP066S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15797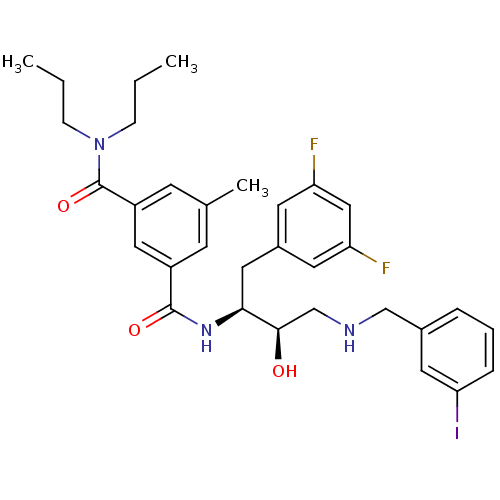 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM531207 ((R)-3-((3-chloro-2-hydroxyphenyl)amino)-4-((5,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK-Gqi5 cells stably expressing human CCR6 were cultured in DMEM high glucose, 10% FBS, 1% PSA, 400 ug/ml geneticin and 50 ug/ml hygromycin. Appropr... | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM531086 ((R)-6-chloro-3-((2-((2,2-dimethyl-1-phenylpropyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK-Gqi5 cells stably expressing human CCR6 were cultured in DMEM high glucose, 10% FBS, 1% PSA, 400 ug/ml geneticin and 50 ug/ml hygromycin. Appropr... | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50328048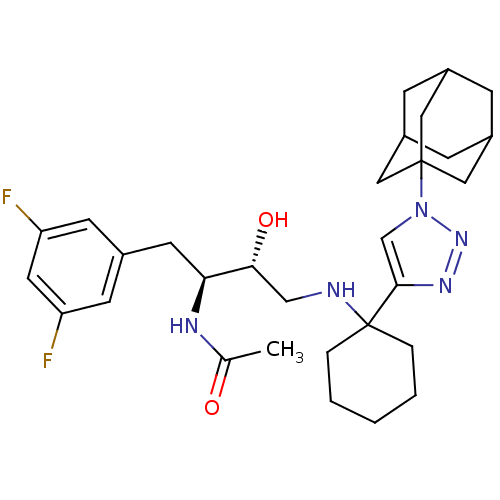 (CHEMBL1257185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 20: 6034-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.070 BindingDB Entry DOI: 10.7270/Q2XK8FSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM531125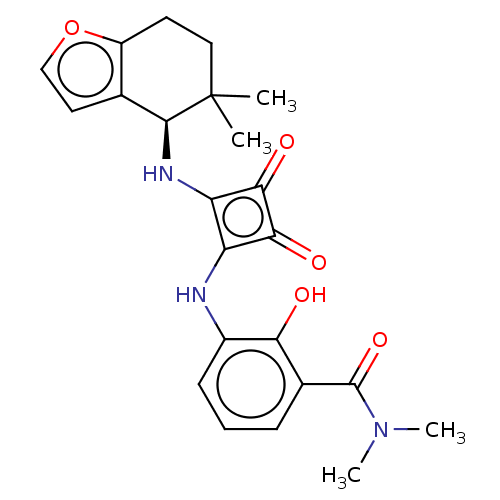 ((R)-3-((2-((5,5-dimethyl-4,5,6,7-tetrahydrobenzofu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK-Gqi5 cells stably expressing human CCR6 were cultured in DMEM high glucose, 10% FBS, 1% PSA, 400 ug/ml geneticin and 50 ug/ml hygromycin. Appropr... | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50302862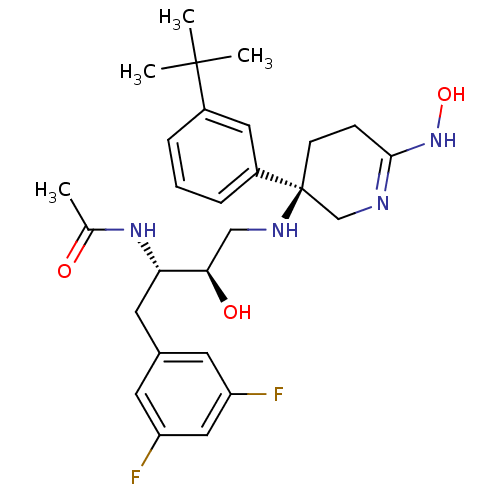 (CHEMBL566603 | N-((2S,3R)-4-((R)-3-(3-tert-butylph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli after 3 hrs by oregon green based fluorescence polarization assay | Bioorg Med Chem Lett 19: 6386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.09.061 BindingDB Entry DOI: 10.7270/Q23779NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50328038 (CHEMBL1258467 | N-((2S,3R)-1-(3,5-difluorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 20: 6034-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.070 BindingDB Entry DOI: 10.7270/Q2XK8FSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM531139 (6-chloro-3-((2-((2,2-dimethyl-1-phenylbutyl)amino)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK-Gqi5 cells stably expressing human CCR6 were cultured in DMEM high glucose, 10% FBS, 1% PSA, 400 ug/ml geneticin and 50 ug/ml hygromycin. Appropr... | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 6 (Homo sapiens (Human)) | BDBM531128 ((R)-6-chloro-3-((2-((5,5-dimethyl-4,5,6,7-tetrahyd...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description HEK-Gqi5 cells stably expressing human CCR6 were cultured in DMEM high glucose, 10% FBS, 1% PSA, 400 ug/ml geneticin and 50 ug/ml hygromycin. Appropr... | Citation and Details BindingDB Entry DOI: 10.7270/Q22F7RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50378120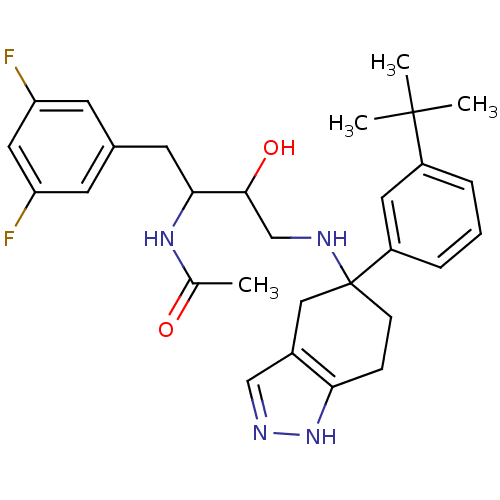 (CHEMBL609987) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Elan Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant BACE1 expressed in Escherichia coli after 3 hrs by oregon green based fluorescence polarization assay | Bioorg Med Chem Lett 19: 6386-91 (2009) Article DOI: 10.1016/j.bmcl.2009.09.061 BindingDB Entry DOI: 10.7270/Q23779NV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 674 total ) | Next | Last >> |