 Found 224 hits with Last Name = 'sinharoy' and Initial = 'r'
Found 224 hits with Last Name = 'sinharoy' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364407
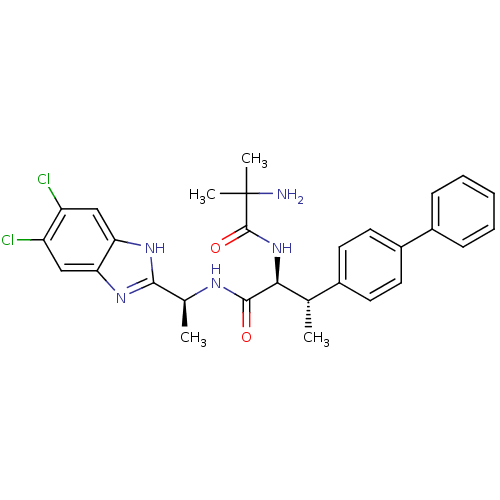
(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364689

(CHEMBL1951478)Show SMILES [O-][n+]1ccc(Cl)cc1C1OC2(CCN(CC2)S(=O)(=O)c2ccc(cn2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C23H19ClF3N3O4S/c24-16-7-10-30(31)19(13-16)21-17-3-1-2-4-18(17)22(34-21)8-11-29(12-9-22)35(32,33)20-6-5-15(14-28-20)23(25,26)27/h1-7,10,13-14,21H,8-9,11-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364386
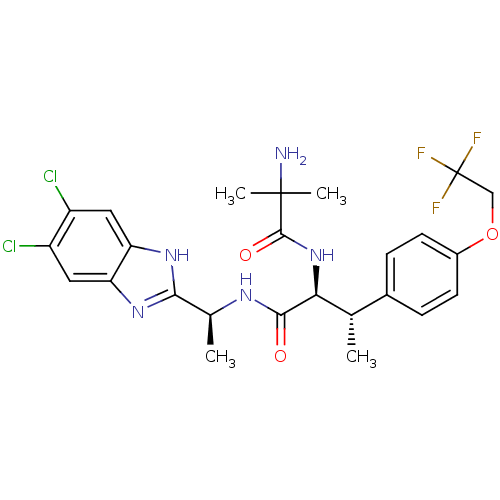
(CHEMBL1950440)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H28Cl2F3N5O3/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(35-23(37)24(3,4)31)22(36)32-13(2)21-33-18-9-16(26)17(27)10-19(18)34-21/h5-10,12-13,20H,11,31H2,1-4H3,(H,32,36)(H,33,34)(H,35,37)/t12-,13-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364676
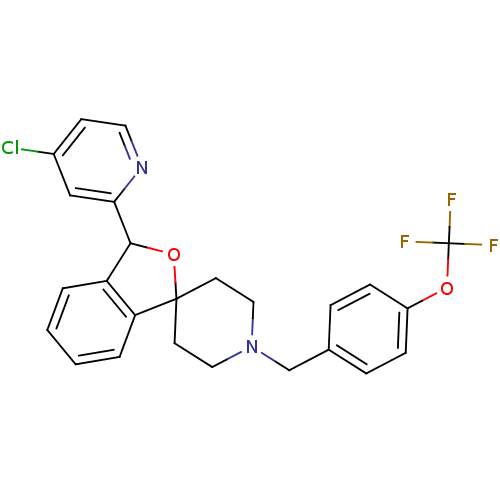
(CHEMBL1951465 | US8785634, 1)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)ccn2)cc1 Show InChI InChI=1S/C25H22ClF3N2O2/c26-18-9-12-30-22(15-18)23-20-3-1-2-4-21(20)24(33-23)10-13-31(14-11-24)16-17-5-7-19(8-6-17)32-25(27,28)29/h1-9,12,15,23H,10-11,13-14,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364685
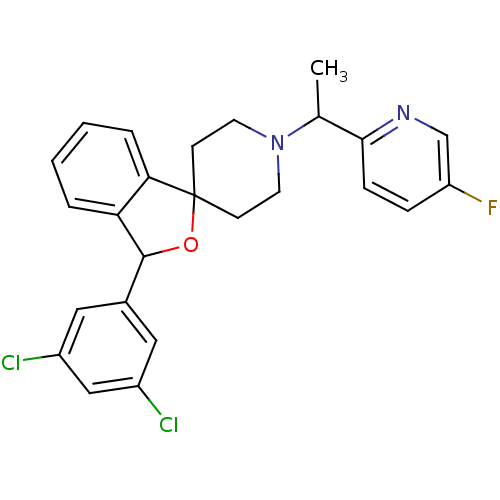
(CHEMBL1951474)Show SMILES CC(N1CCC2(CC1)OC(c1ccccc21)c1cc(Cl)cc(Cl)c1)c1ccc(F)cn1 Show InChI InChI=1S/C25H23Cl2FN2O/c1-16(23-7-6-20(28)15-29-23)30-10-8-25(9-11-30)22-5-3-2-4-21(22)24(31-25)17-12-18(26)14-19(27)13-17/h2-7,12-16,24H,8-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364407

(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364679
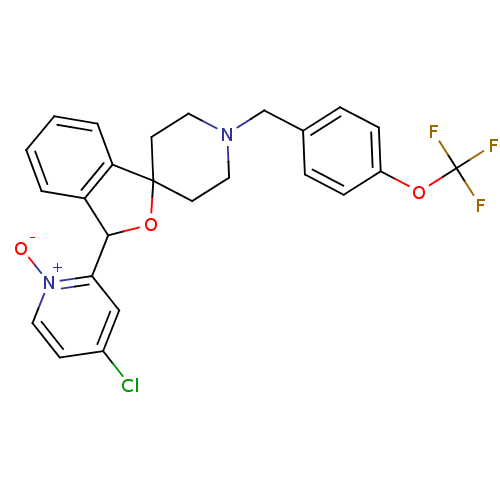
(CHEMBL1951468 | US8785634, 2)Show SMILES [O-][n+]1ccc(Cl)cc1C1OC2(CCN(Cc3ccc(OC(F)(F)F)cc3)CC2)c2ccccc12 Show InChI InChI=1S/C25H22ClF3N2O3/c26-18-9-12-31(32)22(15-18)23-20-3-1-2-4-21(20)24(34-23)10-13-30(14-11-24)16-17-5-7-19(8-6-17)33-25(27,28)29/h1-9,12,15,23H,10-11,13-14,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364687
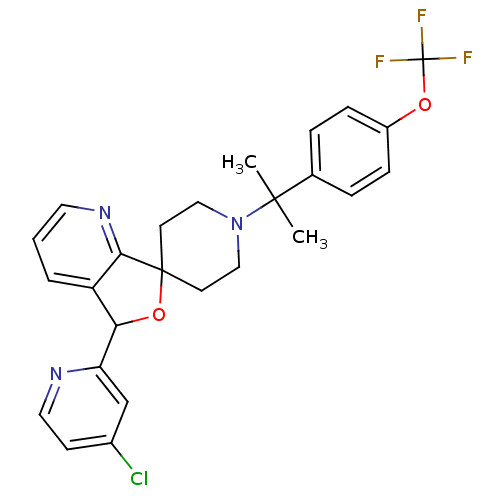
(CHEMBL1951476 | US8785634, 8)Show SMILES CC(C)(N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)ccn1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H25ClF3N3O2/c1-24(2,17-5-7-19(8-6-17)34-26(28,29)30)33-14-10-25(11-15-33)23-20(4-3-12-32-23)22(35-25)21-16-18(27)9-13-31-21/h3-9,12-13,16,22H,10-11,14-15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338033
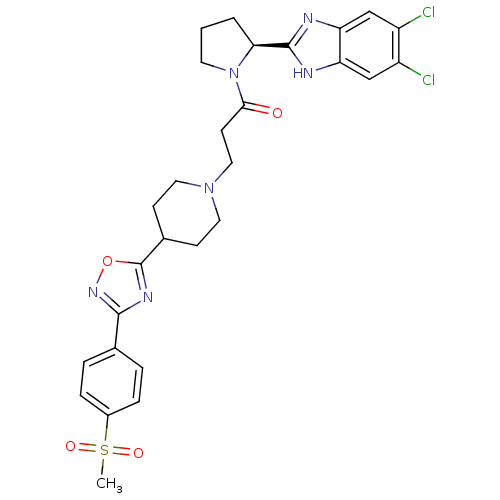
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H30Cl2N6O4S/c1-41(38,39)19-6-4-17(5-7-19)26-33-28(40-34-26)18-8-12-35(13-9-18)14-10-25(37)36-11-2-3-24(36)27-31-22-15-20(29)21(30)16-23(22)32-27/h4-7,15-16,18,24H,2-3,8-14H2,1H3,(H,31,32)/t24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364676

(CHEMBL1951465 | US8785634, 1)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)ccn2)cc1 Show InChI InChI=1S/C25H22ClF3N2O2/c26-18-9-12-30-22(15-18)23-20-3-1-2-4-21(20)24(33-23)10-13-31(14-11-24)16-17-5-7-19(8-6-17)32-25(27,28)29/h1-9,12,15,23H,10-11,13-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364687

(CHEMBL1951476 | US8785634, 8)Show SMILES CC(C)(N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)ccn1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H25ClF3N3O2/c1-24(2,17-5-7-19(8-6-17)34-26(28,29)30)33-14-10-25(11-15-33)23-20(4-3-12-32-23)22(35-25)21-16-18(27)9-13-31-21/h3-9,12-13,16,22H,10-11,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338029
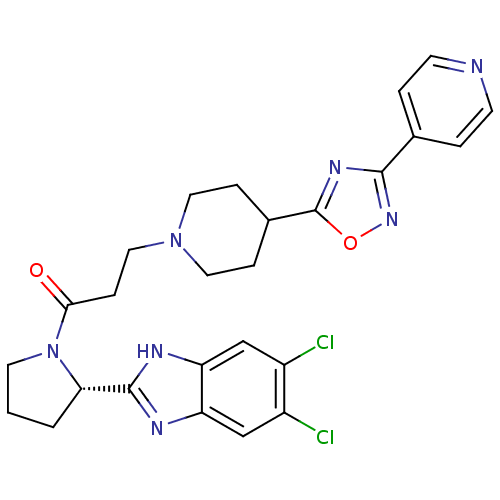
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccncc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-14-20-21(15-19(18)28)31-25(30-20)22-2-1-10-35(22)23(36)7-13-34-11-5-17(6-12-34)26-32-24(33-37-26)16-3-8-29-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364669
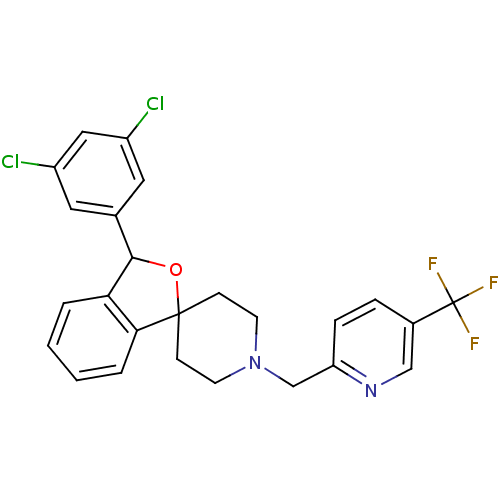
(CHEMBL1951458)Show SMILES FC(F)(F)c1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)cc(Cl)c2)nc1 Show InChI InChI=1S/C25H21Cl2F3N2O/c26-18-11-16(12-19(27)13-18)23-21-3-1-2-4-22(21)24(33-23)7-9-32(10-8-24)15-20-6-5-17(14-31-20)25(28,29)30/h1-6,11-14,23H,7-10,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364685

(CHEMBL1951474)Show SMILES CC(N1CCC2(CC1)OC(c1ccccc21)c1cc(Cl)cc(Cl)c1)c1ccc(F)cn1 Show InChI InChI=1S/C25H23Cl2FN2O/c1-16(23-7-6-20(28)15-29-23)30-10-8-25(9-11-30)22-5-3-2-4-21(22)24(31-25)17-12-18(26)14-19(27)13-17/h2-7,12-16,24H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364387
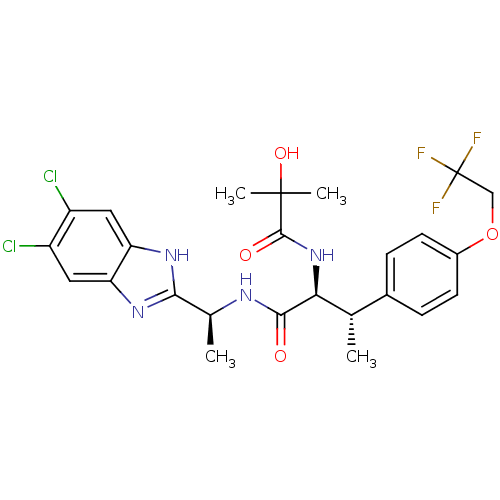
(CHEMBL1950439)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)O)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H27Cl2F3N4O4/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(34-23(36)24(3,4)37)22(35)31-13(2)21-32-18-9-16(26)17(27)10-19(18)33-21/h5-10,12-13,20,37H,11H2,1-4H3,(H,31,35)(H,32,33)(H,34,36)/t12-,13-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364689

(CHEMBL1951478)Show SMILES [O-][n+]1ccc(Cl)cc1C1OC2(CCN(CC2)S(=O)(=O)c2ccc(cn2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C23H19ClF3N3O4S/c24-16-7-10-30(31)19(13-16)21-17-3-1-2-4-18(17)22(34-21)8-11-29(12-9-22)35(32,33)20-6-5-15(14-28-20)23(25,26)27/h1-7,10,13-14,21H,8-9,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364681
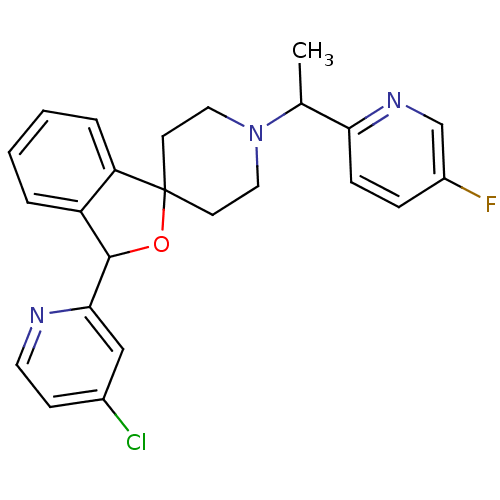
(CHEMBL1951470)Show SMILES CC(N1CCC2(CC1)OC(c1ccccc21)c1cc(Cl)ccn1)c1ccc(F)cn1 Show InChI InChI=1S/C24H23ClFN3O/c1-16(21-7-6-18(26)15-28-21)29-12-9-24(10-13-29)20-5-3-2-4-19(20)23(30-24)22-14-17(25)8-11-27-22/h2-8,11,14-16,23H,9-10,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364408

(CHEMBL1950443)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NCC(C)(C)N)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C28H31Cl2N5O/c1-17(26-34-23-14-21(29)22(30)15-24(23)35-26)33-27(36)25(32-16-28(2,3)31)13-18-9-11-20(12-10-18)19-7-5-4-6-8-19/h4-12,14-15,17,25,32H,13,16,31H2,1-3H3,(H,33,36)(H,34,35)/t17-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364681

(CHEMBL1951470)Show SMILES CC(N1CCC2(CC1)OC(c1ccccc21)c1cc(Cl)ccn1)c1ccc(F)cn1 Show InChI InChI=1S/C24H23ClFN3O/c1-16(21-7-6-18(26)15-28-21)29-12-9-24(10-13-29)20-5-3-2-4-19(20)23(30-24)22-14-17(25)8-11-27-22/h2-8,11,14-16,23H,9-10,12-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364666
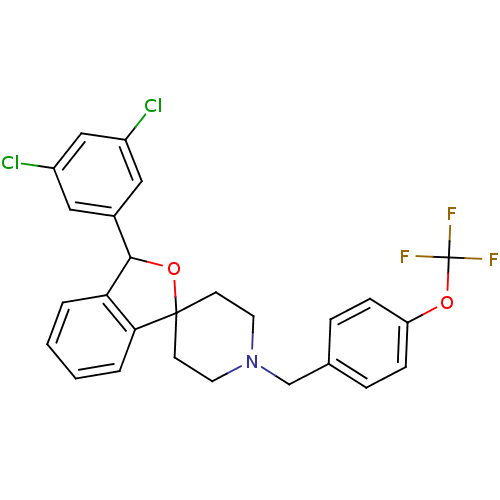
(CHEMBL1951456)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C26H22Cl2F3NO2/c27-19-13-18(14-20(28)15-19)24-22-3-1-2-4-23(22)25(34-24)9-11-32(12-10-25)16-17-5-7-21(8-6-17)33-26(29,30)31/h1-8,13-15,24H,9-12,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate after 30 mins by fluorescence analysis |
Bioorg Med Chem Lett 22: 1727-30 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.098
BindingDB Entry DOI: 10.7270/Q2GT5NNH |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364684

(CHEMBL1951473)Show SMILES CC(N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)cc(Cl)c1)c1ccc(F)cn1 Show InChI InChI=1S/C24H22Cl2FN3O/c1-15(21-5-4-19(27)14-29-21)30-9-6-24(7-10-30)23-20(3-2-8-28-23)22(31-24)16-11-17(25)13-18(26)12-16/h2-5,8,11-15,22H,6-7,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364679

(CHEMBL1951468 | US8785634, 2)Show SMILES [O-][n+]1ccc(Cl)cc1C1OC2(CCN(Cc3ccc(OC(F)(F)F)cc3)CC2)c2ccccc12 Show InChI InChI=1S/C25H22ClF3N2O3/c26-18-9-12-31(32)22(15-18)23-20-3-1-2-4-21(20)24(34-23)10-13-30(14-11-24)16-17-5-7-19(8-6-17)33-25(27,28)29/h1-9,12,15,23H,10-11,13-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364673
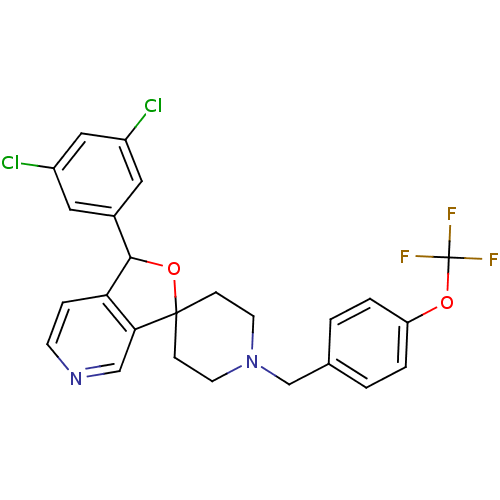
(CHEMBL1951462)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccncc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C25H21Cl2F3N2O2/c26-18-11-17(12-19(27)13-18)23-21-5-8-31-14-22(21)24(34-23)6-9-32(10-7-24)15-16-1-3-20(4-2-16)33-25(28,29)30/h1-5,8,11-14,23H,6-7,9-10,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338007

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Cn1nc(cc1-c1ccncc1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H31Cl2N7O/c1-35-26(19-4-9-31-10-5-19)17-22(34-35)18-6-12-36(13-7-18)14-8-27(38)37-11-2-3-25(37)28-32-23-15-20(29)21(30)16-24(23)33-28/h4-5,9-10,15-18,25H,2-3,6-8,11-14H2,1H3,(H,32,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364672
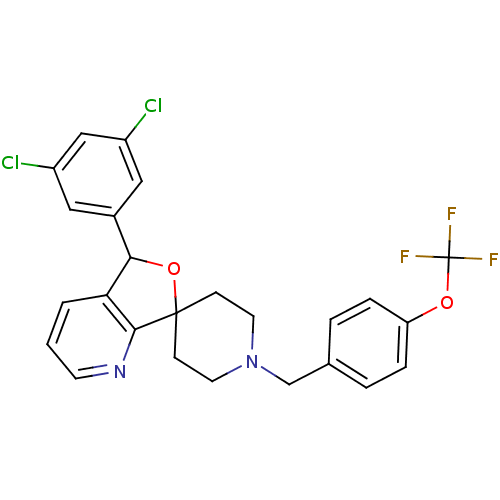
(CHEMBL1951461 | US8785634, 3)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2cccnc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C25H21Cl2F3N2O2/c26-18-12-17(13-19(27)14-18)22-21-2-1-9-31-23(21)24(34-22)7-10-32(11-8-24)15-16-3-5-20(6-4-16)33-25(28,29)30/h1-6,9,12-14,22H,7-8,10-11,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364682

(CHEMBL1951471 | US8785634, 82)Show SMILES FC(F)(F)Oc1ccc(cc1)S(=O)(=O)N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H19Cl2F3N2O4S/c25-16-12-15(13-17(26)14-16)21-20-2-1-9-30-22(20)23(35-21)7-10-31(11-8-23)36(32,33)19-5-3-18(4-6-19)34-24(27,28)29/h1-6,9,12-14,21H,7-8,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338029

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccncc1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-18-14-20-21(15-19(18)28)31-25(30-20)22-2-1-10-35(22)23(36)7-13-34-11-5-17(6-12-34)26-32-24(33-37-26)16-3-8-29-9-4-16/h3-4,8-9,14-15,17,22H,1-2,5-7,10-13H2,(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364682

(CHEMBL1951471 | US8785634, 82)Show SMILES FC(F)(F)Oc1ccc(cc1)S(=O)(=O)N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C24H19Cl2F3N2O4S/c25-16-12-15(13-17(26)14-16)21-20-2-1-9-30-22(20)23(35-21)7-10-31(11-8-23)36(32,33)19-5-3-18(4-6-19)34-24(27,28)29/h1-6,9,12-14,21H,7-8,10-11H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364666

(CHEMBL1951456)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C26H22Cl2F3NO2/c27-19-13-18(14-20(28)15-19)24-22-3-1-2-4-23(22)25(34-24)9-11-32(12-10-25)16-17-5-7-21(8-6-17)33-26(29,30)31/h1-8,13-15,24H,9-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364684

(CHEMBL1951473)Show SMILES CC(N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)cc(Cl)c1)c1ccc(F)cn1 Show InChI InChI=1S/C24H22Cl2FN3O/c1-15(21-5-4-19(27)14-29-21)30-9-6-24(7-10-30)23-20(3-2-8-28-23)22(31-24)16-11-17(25)13-18(26)12-16/h2-5,8,11-15,22H,6-7,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338033

((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 |r| Show InChI InChI=1S/C28H30Cl2N6O4S/c1-41(38,39)19-6-4-17(5-7-19)26-33-28(40-34-26)18-8-12-35(13-9-18)14-10-25(37)36-11-2-3-24(36)27-31-22-15-20(29)21(30)16-23(22)32-27/h4-7,15-16,18,24H,2-3,8-14H2,1H3,(H,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364667
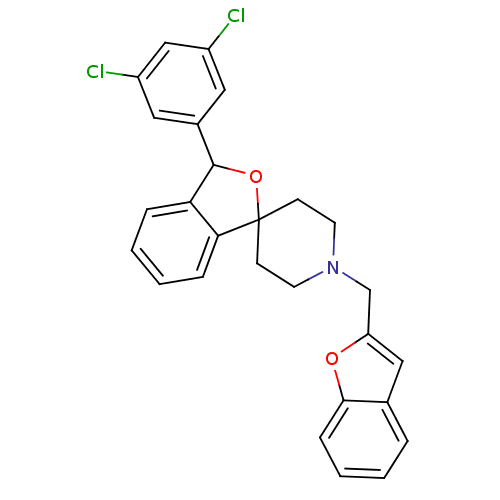
(CHEMBL1951455)Show SMILES Clc1cc(Cl)cc(c1)C1OC2(CCN(Cc3cc4ccccc4o3)CC2)c2ccccc12 Show InChI InChI=1S/C27H23Cl2NO2/c28-20-13-19(14-21(29)16-20)26-23-6-2-3-7-24(23)27(32-26)9-11-30(12-10-27)17-22-15-18-5-1-4-8-25(18)31-22/h1-8,13-16,26H,9-12,17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364668

(CHEMBL1951457)Show SMILES Fc1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)cc(Cl)c2)nc1 Show InChI InChI=1S/C24H21Cl2FN2O/c25-17-11-16(12-18(26)13-17)23-21-3-1-2-4-22(21)24(30-23)7-9-29(10-8-24)15-20-6-5-19(27)14-28-20/h1-6,11-14,23H,7-10,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364406

(CHEMBL1950445)Show SMILES C[C@H](NC(=O)[C@@H](N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H24Cl2N4O/c1-14(16-8-10-18(11-9-16)17-6-4-3-5-7-17)23(28)25(32)29-15(2)24-30-21-12-19(26)20(27)13-22(21)31-24/h3-15,23H,28H2,1-2H3,(H,29,32)(H,30,31)/t14-,15-,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364406

(CHEMBL1950445)Show SMILES C[C@H](NC(=O)[C@@H](N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H24Cl2N4O/c1-14(16-8-10-18(11-9-16)17-6-4-3-5-7-17)23(28)25(32)29-15(2)24-30-21-12-19(26)20(27)13-22(21)31-24/h3-15,23H,28H2,1-2H3,(H,29,32)(H,30,31)/t14-,15-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364672

(CHEMBL1951461 | US8785634, 3)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2cccnc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C25H21Cl2F3N2O2/c26-18-12-17(13-19(27)14-18)22-21-2-1-9-31-23(21)24(34-22)7-10-32(11-8-24)15-16-3-5-20(6-4-16)33-25(28,29)30/h1-6,9,12-14,22H,7-8,10-11,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364669

(CHEMBL1951458)Show SMILES FC(F)(F)c1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)cc(Cl)c2)nc1 Show InChI InChI=1S/C25H21Cl2F3N2O/c26-18-11-16(12-19(27)13-18)23-21-3-1-2-4-22(21)24(33-23)7-9-32(10-8-24)15-20-6-5-17(14-31-20)25(28,29)30/h1-6,11-14,23H,7-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364673

(CHEMBL1951462)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccncc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C25H21Cl2F3N2O2/c26-18-11-17(12-19(27)13-18)23-21-5-8-31-14-22(21)24(34-23)6-9-32(10-7-24)15-16-1-3-20(4-2-16)33-25(28,29)30/h1-5,8,11-14,23H,6-7,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50338027
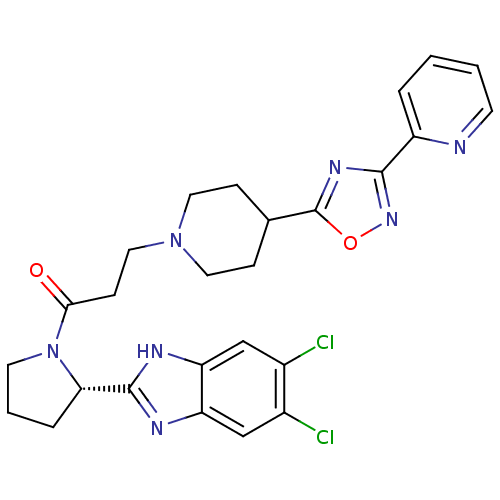
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccccn1 |r| Show InChI InChI=1S/C26H27Cl2N7O2/c27-17-14-20-21(15-18(17)28)31-25(30-20)22-5-3-10-35(22)23(36)8-13-34-11-6-16(7-12-34)26-32-24(33-37-26)19-4-1-2-9-29-19/h1-2,4,9,14-16,22H,3,5-8,10-13H2,(H,30,31)/t22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338032
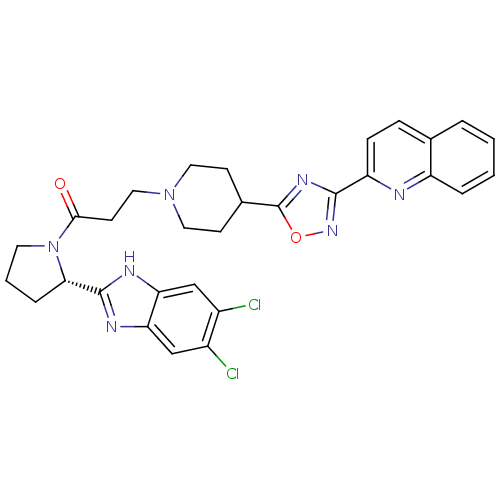
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES Clc1cc2nc([nH]c2cc1Cl)[C@@H]1CCCN1C(=O)CCN1CCC(CC1)c1nc(no1)-c1ccc2ccccc2n1 |r| Show InChI InChI=1S/C30H29Cl2N7O2/c31-20-16-24-25(17-21(20)32)35-29(34-24)26-6-3-12-39(26)27(40)11-15-38-13-9-19(10-14-38)30-36-28(37-41-30)23-8-7-18-4-1-2-5-22(18)33-23/h1-2,4-5,7-8,16-17,19,26H,3,6,9-15H2,(H,34,35)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364674

(CHEMBL1951463)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2cnccc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C25H21Cl2F3N2O2/c26-18-11-17(12-19(27)13-18)23-21-14-31-8-5-22(21)24(34-23)6-9-32(10-7-24)15-16-1-3-20(4-2-16)33-25(28,29)30/h1-5,8,11-14,23H,6-7,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50338035
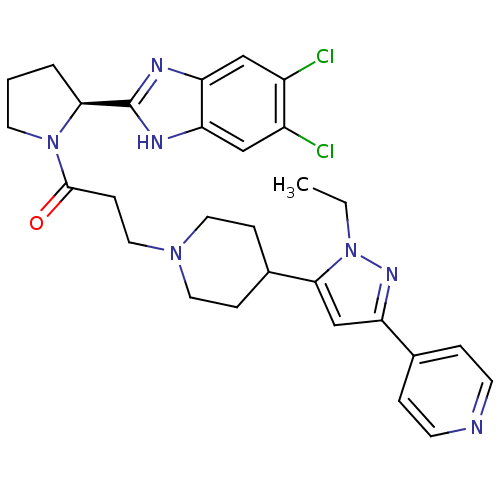
((S)-1-(2-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)py...)Show SMILES CCn1nc(cc1C1CCN(CCC(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1)-c1ccncc1 |r| Show InChI InChI=1S/C29H33Cl2N7O/c1-2-38-27(18-23(35-38)19-5-10-32-11-6-19)20-7-13-36(14-8-20)15-9-28(39)37-12-3-4-26(37)29-33-24-16-21(30)22(31)17-25(24)34-29/h5-6,10-11,16-18,20,26H,2-4,7-9,12-15H2,1H3,(H,33,34)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 21: 1299-305 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.090
BindingDB Entry DOI: 10.7270/Q2VX0GSS |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364393
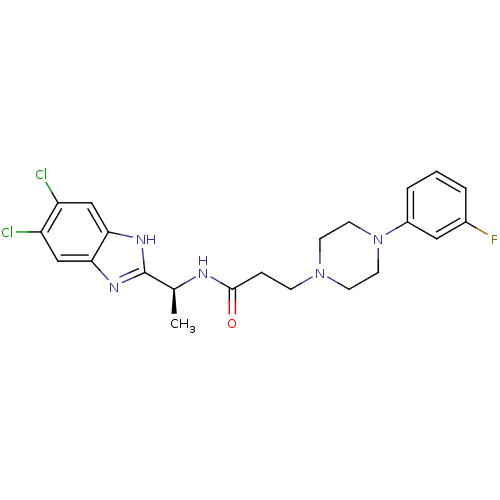
(CHEMBL1950309)Show SMILES C[C@H](NC(=O)CCN1CCN(CC1)c1cccc(F)c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C22H24Cl2FN5O/c1-14(22-27-19-12-17(23)18(24)13-20(19)28-22)26-21(31)5-6-29-7-9-30(10-8-29)16-4-2-3-15(25)11-16/h2-4,11-14H,5-10H2,1H3,(H,26,31)(H,27,28)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364674

(CHEMBL1951463)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2cnccc32)c2cc(Cl)cc(Cl)c2)cc1 Show InChI InChI=1S/C25H21Cl2F3N2O2/c26-18-11-17(12-19(27)13-18)23-21-14-31-8-5-22(21)24(34-23)6-9-32(10-7-24)15-16-1-3-20(4-2-16)33-25(28,29)30/h1-5,8,11-14,23H,6-7,9-10,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364688
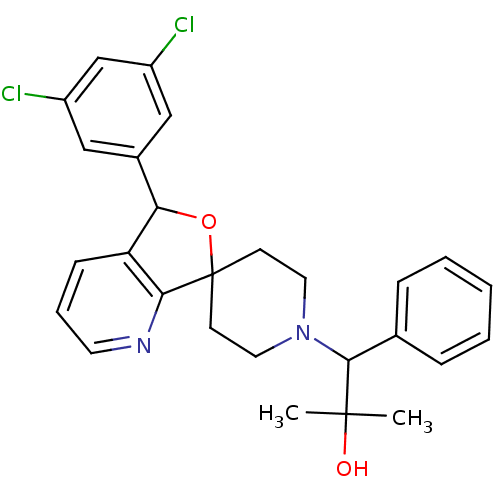
(CHEMBL1951477)Show SMILES CC(C)(O)C(N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)cc(Cl)c1)c1ccccc1 Show InChI InChI=1S/C27H28Cl2N2O2/c1-26(2,32)25(18-7-4-3-5-8-18)31-13-10-27(11-14-31)24-22(9-6-12-30-24)23(33-27)19-15-20(28)17-21(29)16-19/h3-9,12,15-17,23,25,32H,10-11,13-14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364388

(CHEMBL1950314)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)O)[C@@H](C)c1ccc(cc1)-c1cn[nH]c1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C26H28Cl2N6O3/c1-13(15-5-7-16(8-6-15)17-11-29-30-12-17)22(34-25(36)26(3,4)37)24(35)31-14(2)23-32-20-9-18(27)19(28)10-21(20)33-23/h5-14,22,37H,1-4H3,(H,29,30)(H,31,35)(H,32,33)(H,34,36)/t13-,14-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364680

(CHEMBL1951469)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2cccnc32)c2cc(Cl)ccn2)cc1 Show InChI InChI=1S/C24H21ClF3N3O2/c25-17-7-11-29-20(14-17)21-19-2-1-10-30-22(19)23(33-21)8-12-31(13-9-23)15-16-3-5-18(6-4-16)32-24(26,27)28/h1-7,10-11,14,21H,8-9,12-13,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50365013

(CHEMBL1949687)Show SMILES COC(=O)N[C@H]1CC[C@@H](CC1)Nc1cc(N[C@@H](C)c2ccc(Cl)cc2)ncn1 |r,wU:16.17,8.11,wD:5.4,(30.92,-23.64,;30.91,-25.18,;32.24,-25.96,;32.23,-27.5,;33.58,-25.2,;34.91,-25.98,;34.9,-27.52,;36.23,-28.31,;37.56,-27.53,;37.57,-26,;36.25,-25.22,;38.89,-28.31,;40.23,-27.54,;41.56,-28.32,;42.9,-27.55,;44.24,-28.32,;45.57,-27.54,;45.57,-26,;46.91,-28.31,;46.9,-29.85,;48.24,-30.62,;49.57,-29.85,;50.91,-30.62,;49.57,-28.3,;48.23,-27.54,;42.9,-26.01,;41.57,-25.24,;40.23,-26,)| Show InChI InChI=1S/C20H26ClN5O2/c1-13(14-3-5-15(21)6-4-14)24-18-11-19(23-12-22-18)25-16-7-9-17(10-8-16)26-20(27)28-2/h3-6,11-13,16-17H,7-10H2,1-2H3,(H,26,27)(H2,22,23,24,25)/t13-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate after 30 mins by fluorescence analysis |
Bioorg Med Chem Lett 22: 1727-30 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.098
BindingDB Entry DOI: 10.7270/Q2GT5NNH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data