

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114361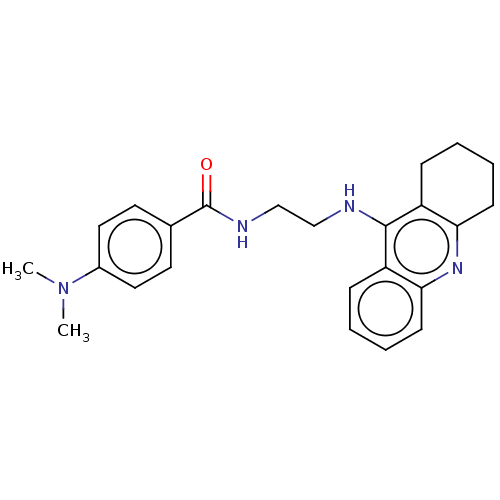 (CHEMBL3604192) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114361 (CHEMBL3604192) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114368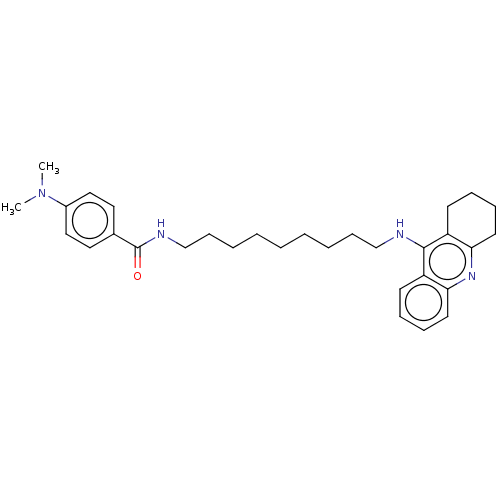 (CHEMBL3604199) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114367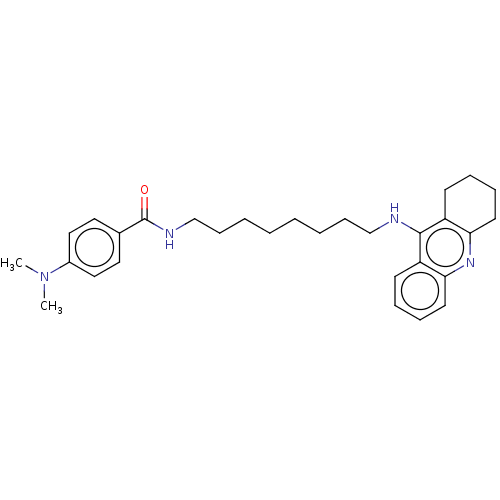 (CHEMBL3604198) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114365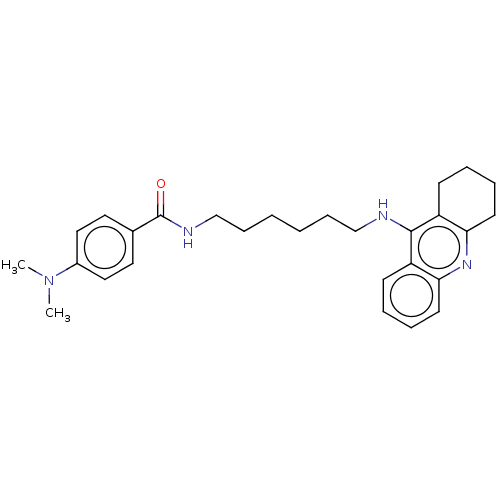 (CHEMBL3604196) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114367 (CHEMBL3604198) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114368 (CHEMBL3604199) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM227587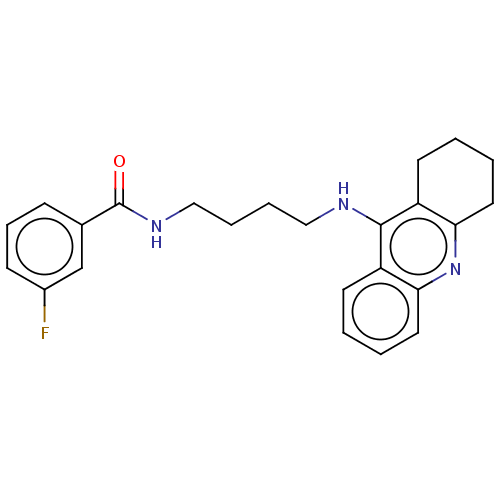 (3-fluoro-N-[4-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114365 (CHEMBL3604196) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM227584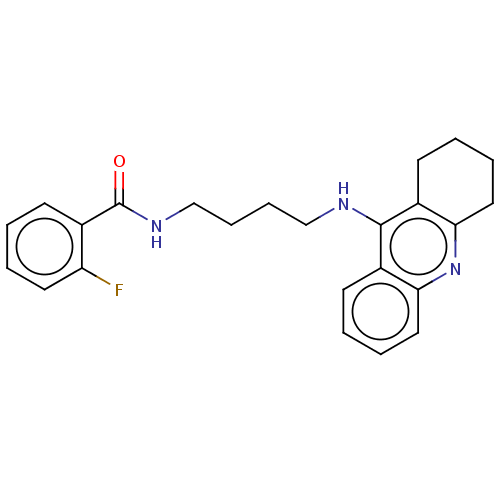 (2-Fluoro-N-[4-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114364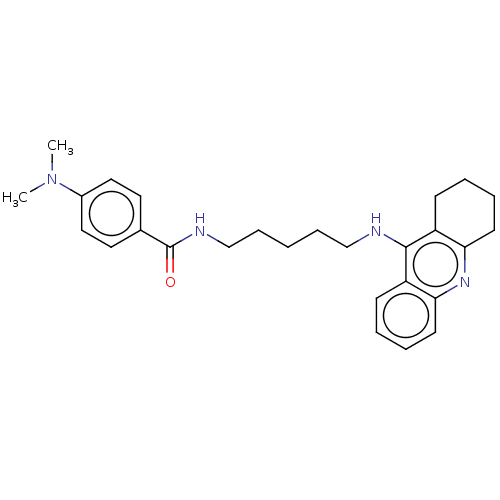 (CHEMBL3604195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM227586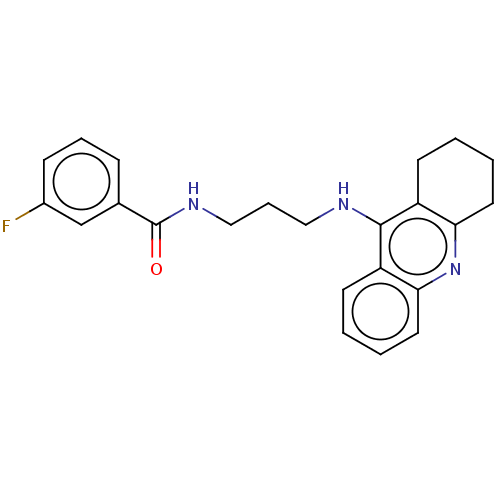 (3-Fluoro-N-[3-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.79 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM227585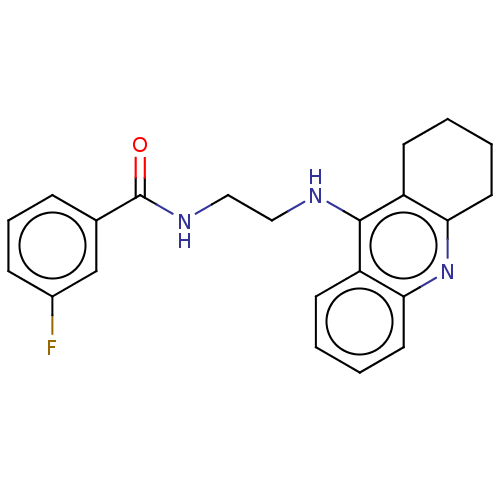 (3-Fluoro-N-[2-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.76 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114363 (CHEMBL3604194) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM227583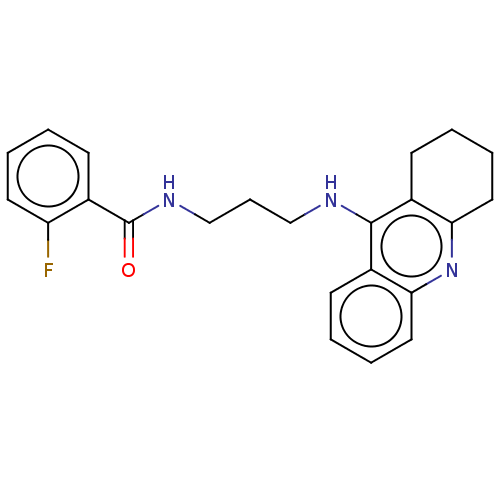 (2-Fluoro-N-[3-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114362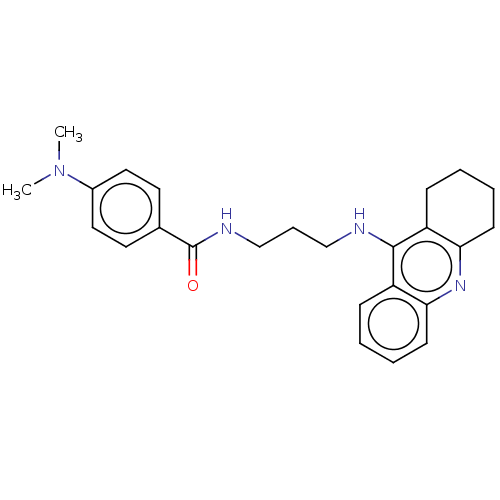 (CHEMBL3604193) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM227584 (2-Fluoro-N-[4-(1,2,3,4-tetrahydroacridin-9-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41.4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114365 (CHEMBL3604196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114362 (CHEMBL3604193) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114366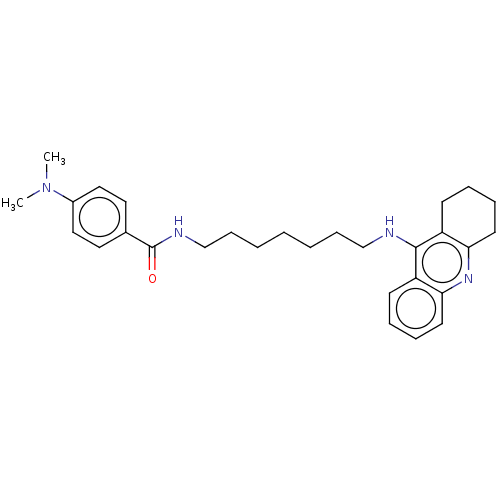 (CHEMBL3604197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114367 (CHEMBL3604198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80.8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114364 (CHEMBL3604195) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM227587 (3-fluoro-N-[4-(1,2,3,4-tetrahydroacridin-9-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97.6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114368 (CHEMBL3604199) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM227583 (2-Fluoro-N-[3-(1,2,3,4-tetrahydroacridin-9-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114361 (CHEMBL3604192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 142 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114366 (CHEMBL3604197) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM227582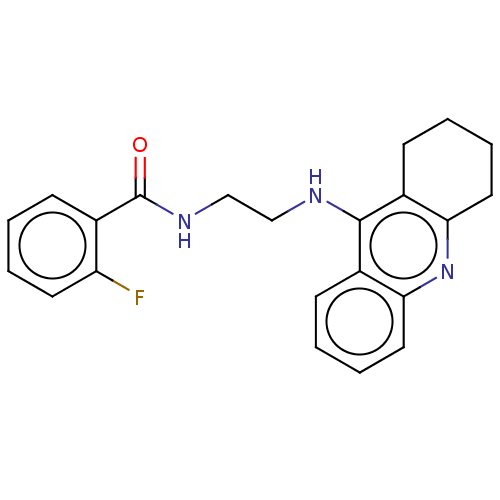 (2-Fluoro-N-[2-(1,2,3,4-tetrahydroacridin-9-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 198 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114362 (CHEMBL3604193) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50114363 (CHEMBL3604194) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50114366 (CHEMBL3604197) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114363 (CHEMBL3604194) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 295 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114364 (CHEMBL3604195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase in human erythrocytes using acetylthiocholine iodide substrate by spectrophotometric Ellman's method | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM227586 (3-Fluoro-N-[3-(1,2,3,4-tetrahydroacridin-9-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 515 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Equus caballus (Horse)) | BDBM227582 (2-Fluoro-N-[2-(1,2,3,4-tetrahydroacridin-9-ylamino...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM227585 (3-Fluoro-N-[2-(1,2,3,4-tetrahydroacridin-9-ylamino...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Medical University of Lodz | Assay Description The test was run as described in the following procedure: stock solution of the test compounds was diluted in phosphate buffer pH 7.4 to give final c... | Bioorg Chem 72: 315-322 (2017) Article DOI: 10.1016/j.bioorg.2017.05.003 BindingDB Entry DOI: 10.7270/Q2RX99ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10620 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using ATC iodide substrate by Ellman's assay | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50114361 (CHEMBL3604192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) assessed as enzyme-mediated Amyloid beta peptide (1 to 42) aggregation incubated for 5 hrs by thioflavin-T based ... | Bioorg Med Chem 23: 5610-8 (2015) Article DOI: 10.1016/j.bmc.2015.07.029 BindingDB Entry DOI: 10.7270/Q25T3N9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||