 Found 930 hits with Last Name = 'smith' and Initial = 'ac'
Found 930 hits with Last Name = 'smith' and Initial = 'ac' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ketohexokinase
(Homo sapiens (Human)) | BDBM319585

(US10174007, Example 4 | US10787438, Example 4 | US...)Show SMILES C[C@H]1CCN1c1nc(cc(n1)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C16H19F3N4O2/c1-8-2-3-23(8)15-20-12(16(17,18)19)5-13(21-15)22-6-10-9(4-14(24)25)11(10)7-22/h5,8-11H,2-4,6-7H2,1H3,(H,24,25)/t8-,9-,10-,11+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed noncompetitive inhibition of recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50341385
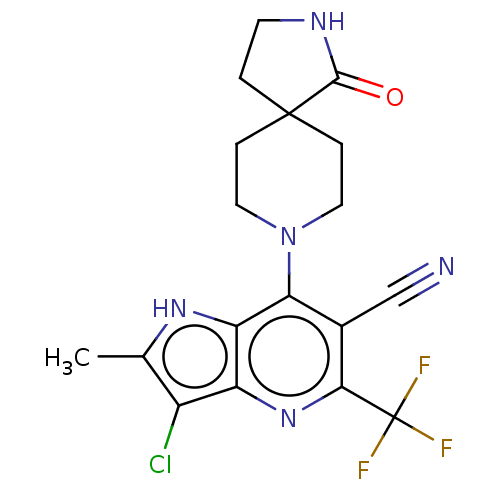
(CHEMBL4161567)Show SMILES Cc1[nH]c2c(N3CCC4(CCNC4=O)CC3)c(C#N)c(nc2c1Cl)C(F)(F)F Show InChI InChI=1S/C18H17ClF3N5O/c1-9-11(19)12-13(25-9)14(10(8-23)15(26-12)18(20,21)22)27-6-3-17(4-7-27)2-5-24-16(17)28/h25H,2-7H2,1H3,(H,24,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327358

(CHEMBL4168403)Show SMILES Cc1[nH]c2c(N3CCC(N)(CC3)C(N)=O)c(C#N)c(nc2c1Cl)C(F)(F)F Show InChI InChI=1S/C16H16ClF3N6O/c1-7-9(17)10-11(24-7)12(8(6-21)13(25-10)16(18,19)20)26-4-2-15(23,3-5-26)14(22)27/h24H,2-5,23H2,1H3,(H2,22,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327363
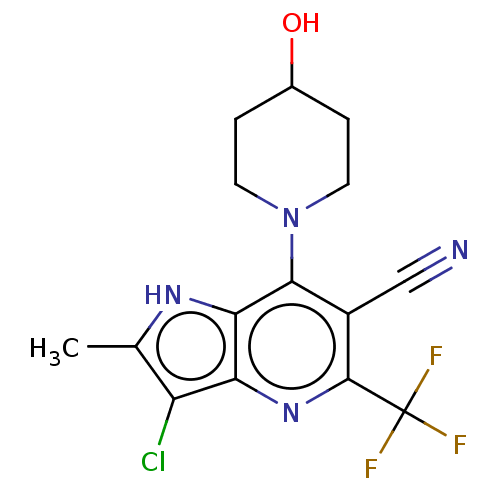
(CHEMBL4176684)Show SMILES Cc1[nH]c2c(N3CCC(O)CC3)c(C#N)c(nc2c1Cl)C(F)(F)F Show InChI InChI=1S/C25H27N3O/c1-20-9-7-10-21(19-20)13-14-24-25(27-23-12-4-3-11-22(23)26-24)29-18-8-17-28-15-5-2-6-16-28/h3-4,7,9-12,19H,2,5-6,8,15-18H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327360

(CHEMBL4168827)Show SMILES CN1CCC2(CCN(CC2)c2c(C#N)c(nc3c(Cl)c(C)[nH]c23)C(F)(F)F)C1=O Show InChI InChI=1S/C27H36N6O2/c1-27(2,3)19-8-10-20(11-9-19)29-26(34)30-21-12-13-22-23(18-21)31-24(25(32-22)35-4)28-14-17-33-15-6-5-7-16-33/h8-13,18H,5-7,14-17H2,1-4H3,(H,28,31)(H2,29,30,34) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327362

(CHEMBL4159390)Show SMILES OCC(O)CSCc1cccc(c1)C(=O)Nc1ccc(Cl)cc1C(=O)N\N=C\c1cccc(F)c1 Show InChI InChI=1S/C23H23N3O/c1-18-8-10-19(11-9-18)12-13-22-23(27-17-16-26-14-4-5-15-26)25-21-7-3-2-6-20(21)24-22/h2-3,6-11H,4-5,14-17H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50341383
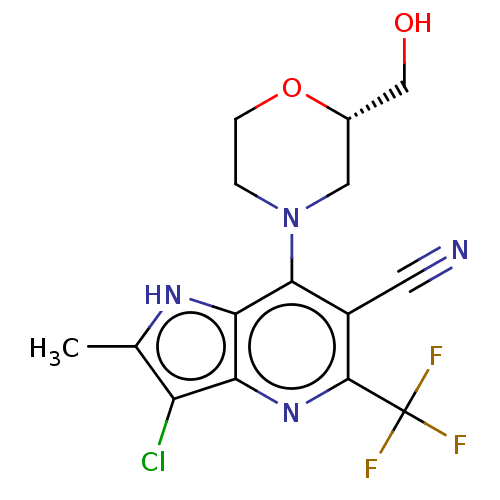
(CHEMBL4175607)Show SMILES Cc1[nH]c2c(N3CCO[C@H](CO)C3)c(C#N)c(nc2c1Cl)C(F)(F)F |r| Show InChI InChI=1S/C15H14ClF3N4O2/c1-7-10(16)11-12(21-7)13(23-2-3-25-8(5-23)6-24)9(4-20)14(22-11)15(17,18)19/h8,21,24H,2-3,5-6H2,1H3/t8-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327357
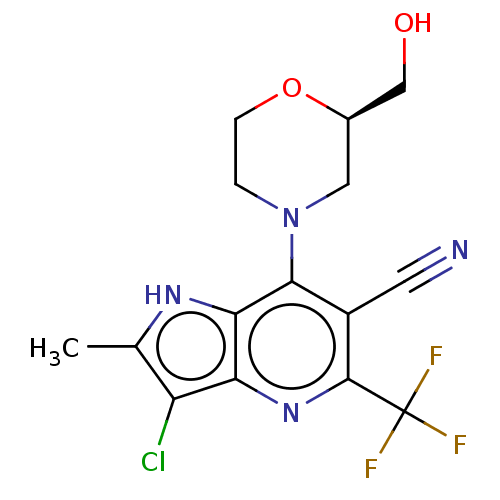
(CHEMBL4164986)Show SMILES Cc1[nH]c2c(N3CCO[C@@H](CO)C3)c(C#N)c(nc2c1Cl)C(F)(F)F |r| Show InChI InChI=1S/C15H14ClF3N4O2/c1-7-10(16)11-12(21-7)13(23-2-3-25-8(5-23)6-24)9(4-20)14(22-11)15(17,18)19/h8,21,24H,2-3,5-6H2,1H3/t8-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327355
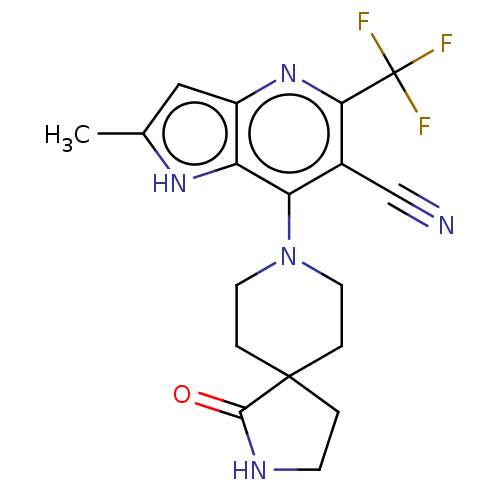
(CHEMBL4164691)Show SMILES Cc1cc2nc(c(C#N)c(N3CCC4(CCNC4=O)CC3)c2[nH]1)C(F)(F)F Show InChI InChI=1S/C18H18F3N5O/c1-10-8-12-13(24-10)14(11(9-22)15(25-12)18(19,20)21)26-6-3-17(4-7-26)2-5-23-16(17)27/h8,24H,2-7H2,1H3,(H,23,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327359
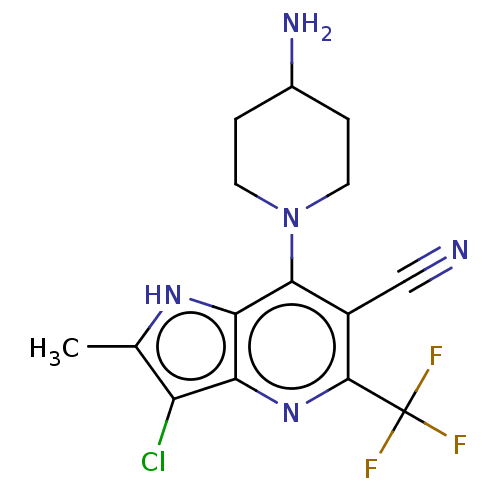
(CHEMBL4160507)Show SMILES Cc1[nH]c2c(N3CCC(N)CC3)c(C#N)c(nc2c1Cl)C(F)(F)F Show InChI InChI=1S/C15H15ClF3N5/c1-7-10(16)11-12(22-7)13(24-4-2-8(21)3-5-24)9(6-20)14(23-11)15(17,18)19/h8,22H,2-5,21H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327361

(CHEMBL4172179)Show SMILES FC(F)(F)c1nc2cc[nH]c2c(N2CCC3(CCNC3=O)CC2)c1C#N Show InChI InChI=1S/C20H19N3O/c1-23(2)14-15-24-20-19(13-12-16-8-4-3-5-9-16)21-17-10-6-7-11-18(17)22-20/h3-11H,14-15H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50327356

(CHEMBL4172584)Show SMILES Cc1cc2nc(c(C#N)c(N3CCC(CC3)Nc3ccccn3)c2[nH]1)C(F)(F)F Show InChI InChI=1S/C20H19F3N6/c1-12-10-15-17(26-12)18(14(11-24)19(28-15)20(21,22)23)29-8-5-13(6-9-29)27-16-4-2-3-7-25-16/h2-4,7,10,13,26H,5-6,8-9H2,1H3,(H,25,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Sodium-dependent phosphate transport protein 2A
(Homo sapiens) | BDBM50341384
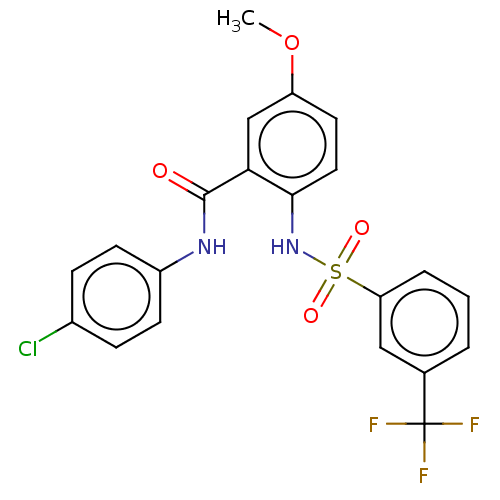
(CHEMBL4170023)Show SMILES COc1ccc(NS(=O)(=O)c2cccc(c2)C(F)(F)F)c(c1)C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C21H16ClF3N2O4S/c1-31-16-9-10-19(18(12-16)20(28)26-15-7-5-14(22)6-8-15)27-32(29,30)17-4-2-3-13(11-17)21(23,24)25/h2-12,27H,1H3,(H,26,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of (C[3H]3) from NaPi2a (unknown origin) expressed in HEK293 cell membranes coexpressing tetracyclin after 1 hr by scintillation countin... |
ACS Med Chem Lett 9: 440-445 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00013
BindingDB Entry DOI: 10.7270/Q26T0Q7K |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287519
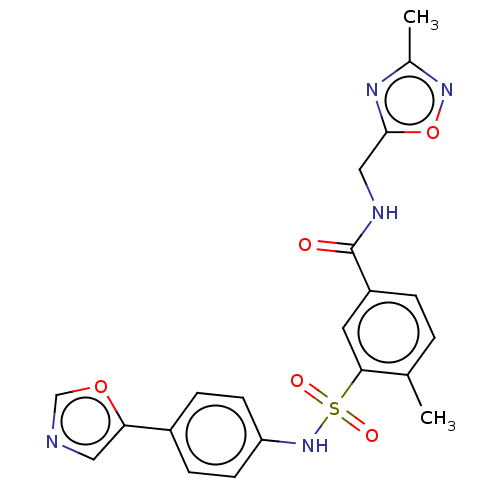
(CHEMBL4172084)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2cnco2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)23-11-20-24-14(2)25-31-20)9-19(13)32(28,29)26-17-7-5-15(6-8-17)18-10-22-12-30-18/h3-10,12,26H,11H2,1-2H3,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287556

(CHEMBL4161489)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(cc1)-c1ccno1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C21H20N6O4S/c1-14-3-4-16(21(28)22-12-18-13-27(2)26-24-18)11-20(14)32(29,30)25-17-7-5-15(6-8-17)19-9-10-23-31-19/h3-11,13,25H,12H2,1-2H3,(H,22,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287552
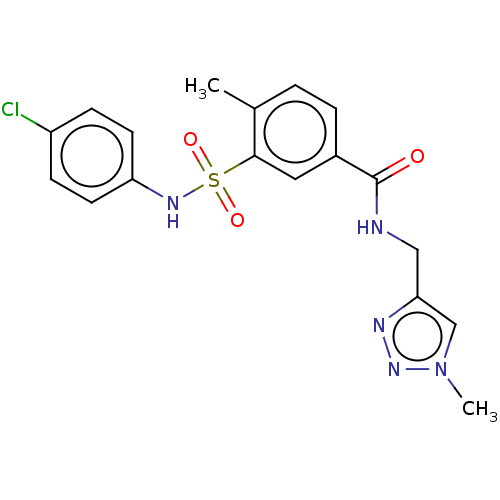
(CHEMBL4175004)Show SMILES Cc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NCc1cn(C)nn1 Show InChI InChI=1S/C18H18ClN5O3S/c1-12-3-4-13(18(25)20-10-16-11-24(2)23-21-16)9-17(12)28(26,27)22-15-7-5-14(19)6-8-15/h3-9,11,22H,10H2,1-2H3,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287558
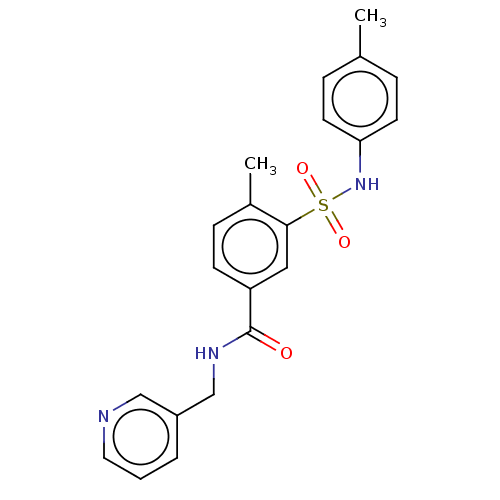
(CHEMBL1333494)Show SMILES Cc1ccc(NS(=O)(=O)c2cc(ccc2C)C(=O)NCc2cccnc2)cc1 Show InChI InChI=1S/C21H21N3O3S/c1-15-5-9-19(10-6-15)24-28(26,27)20-12-18(8-7-16(20)2)21(25)23-14-17-4-3-11-22-13-17/h3-13,24H,14H2,1-2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287559
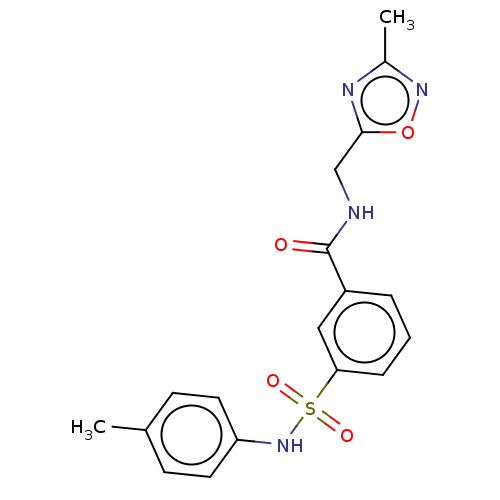
(CHEMBL4170197)Show SMILES Cc1noc(CNC(=O)c2cccc(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C18H18N4O4S/c1-12-6-8-15(9-7-12)22-27(24,25)16-5-3-4-14(10-16)18(23)19-11-17-20-13(2)21-26-17/h3-10,22H,11H2,1-2H3,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287555
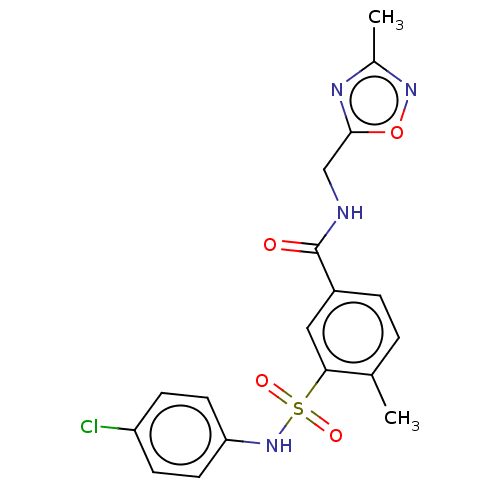
(CHEMBL4174695)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(Cl)cc2)n1 Show InChI InChI=1S/C18H17ClN4O4S/c1-11-3-4-13(18(24)20-10-17-21-12(2)22-27-17)9-16(11)28(25,26)23-15-7-5-14(19)6-8-15/h3-9,23H,10H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287553
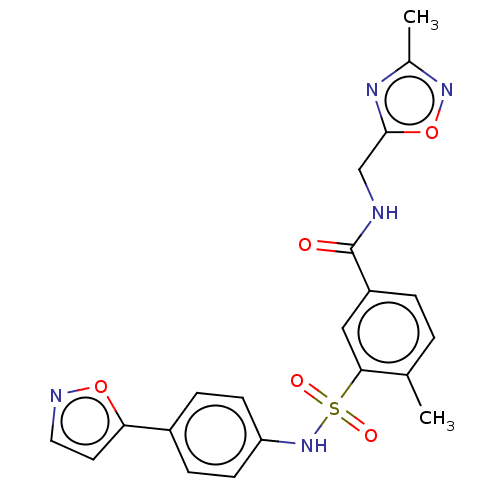
(CHEMBL4166791)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(cc2)-c2ccno2)n1 Show InChI InChI=1S/C21H19N5O5S/c1-13-3-4-16(21(27)22-12-20-24-14(2)25-31-20)11-19(13)32(28,29)26-17-7-5-15(6-8-17)18-9-10-23-30-18/h3-11,26H,12H2,1-2H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287554

(CHEMBL4162926)Show SMILES Cc1noc(CNC(=O)c2ccc(C)c(c2)S(=O)(=O)Nc2ccc(C)cc2)n1 Show InChI InChI=1S/C19H20N4O4S/c1-12-4-8-16(9-5-12)23-28(25,26)17-10-15(7-6-13(17)2)19(24)20-11-18-21-14(3)22-27-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287557

(CHEMBL4159568)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(C)cc1)C(=O)NCc1nc(C)no1 Show InChI InChI=1S/C19H20N4O5S/c1-12-4-7-15(8-5-12)23-29(25,26)17-10-14(6-9-16(17)27-3)19(24)20-11-18-21-13(2)22-28-18/h4-10,23H,11H2,1-3H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50287520

(CHEMBL4159883)Show SMILES COc1ccc(cc1S(=O)(=O)Nc1ccc(Cl)cc1)C(=O)NC(C)c1cnn(C)n1 Show InChI InChI=1S/C19H20ClN5O4S/c1-12(16-11-21-25(2)23-16)22-19(26)13-4-9-17(29-3)18(10-13)30(27,28)24-15-7-5-14(20)6-8-15/h4-12,24H,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-labelled dofetilide from human ERG expressed in HEK293 cell membrane homogenates |
ACS Med Chem Lett 9: 125-130 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00481
BindingDB Entry DOI: 10.7270/Q2KH0QVZ |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319582
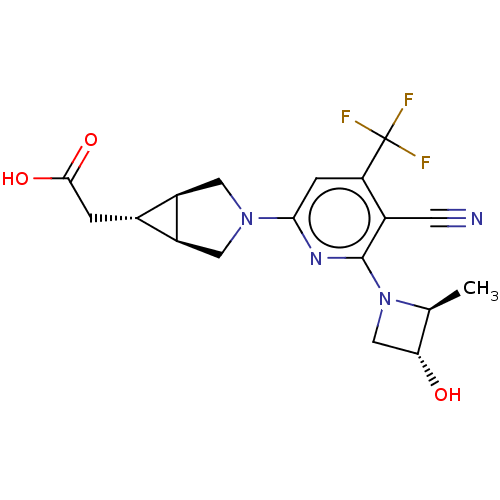
(US10174007, Example 1 | US10787438, Example 1 | US...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-8-14(26)7-25(8)17-10(4-22)13(18(19,20)21)3-15(23-17)24-5-11-9(2-16(27)28)12(11)6-24/h3,8-9,11-12,14,26H,2,5-7H2,1H3,(H,27,28)/t8-,9-,11-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 1 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated for... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439646

(CHEMBL2419600 | US8993586, 110)Show SMILES CCCNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C27H34N6O2/c1-5-12-28-22-9-8-18-6-7-19(15-21(18)29-22)25(35)32-13-10-27(11-14-32)16-20-17-33(26(2,3)4)31-23(20)24(34)30-27/h6-9,15,17H,5,10-14,16H2,1-4H3,(H,28,29)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50439646

(CHEMBL2419600 | US8993586, 110)Show SMILES CCCNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C27H34N6O2/c1-5-12-28-22-9-8-18-6-7-19(15-21(18)29-22)25(35)32-13-10-27(11-14-32)16-20-17-33(26(2,3)4)31-23(20)24(34)30-27/h6-9,15,17H,5,10-14,16H2,1-4H3,(H,28,29)(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439642
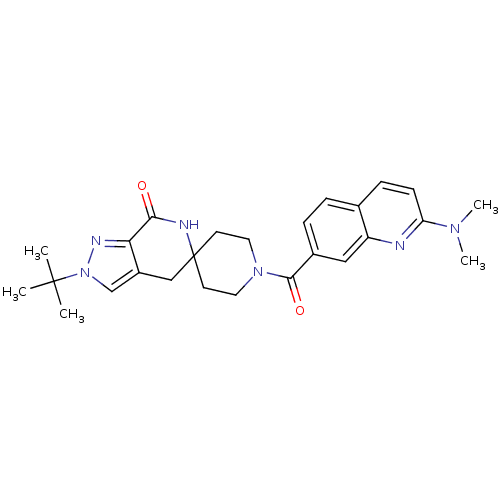
(CHEMBL2419589 | US8993586, 105)Show SMILES CN(C)c1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C26H32N6O2/c1-25(2,3)32-16-19-15-26(28-23(33)22(19)29-32)10-12-31(13-11-26)24(34)18-7-6-17-8-9-21(30(4)5)27-20(17)14-18/h6-9,14,16H,10-13,15H2,1-5H3,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Phospholipase A2, major isoenzyme
(Sus scrofa (pig)) | BDBM50055367
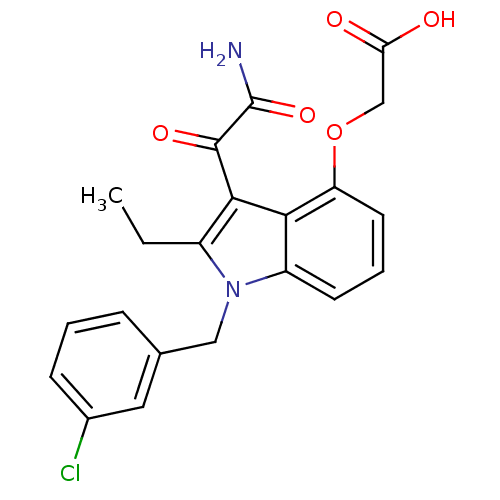
(CHEMBL345986 | [3-Aminooxalyl-1-(3-chloro-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1cccc(Cl)c1 Show InChI InChI=1S/C21H19ClN2O5/c1-2-14-19(20(27)21(23)28)18-15(7-4-8-16(18)29-11-17(25)26)24(14)10-12-5-3-6-13(22)9-12/h3-9H,2,10-11H2,1H3,(H2,23,28)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Compound wastested for inhibition of porcine secreted pancreatic PLA2 |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439644
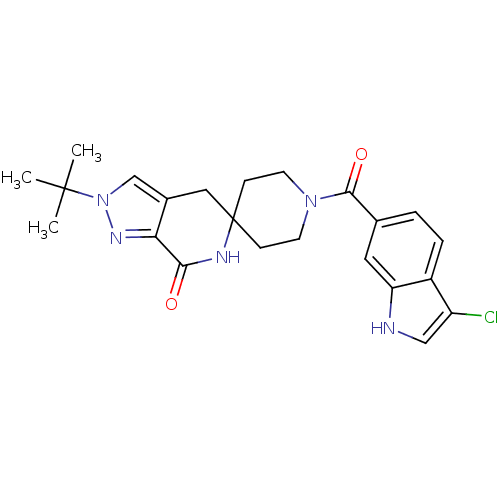
(CHEMBL2419593 | US8993586, 86)Show SMILES CC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3ccc4c(Cl)c[nH]c4c3)NC(=O)c2n1 Show InChI InChI=1S/C23H26ClN5O2/c1-22(2,3)29-13-15-11-23(26-20(30)19(15)27-29)6-8-28(9-7-23)21(31)14-4-5-16-17(24)12-25-18(16)10-14/h4-5,10,12-13,25H,6-9,11H2,1-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371
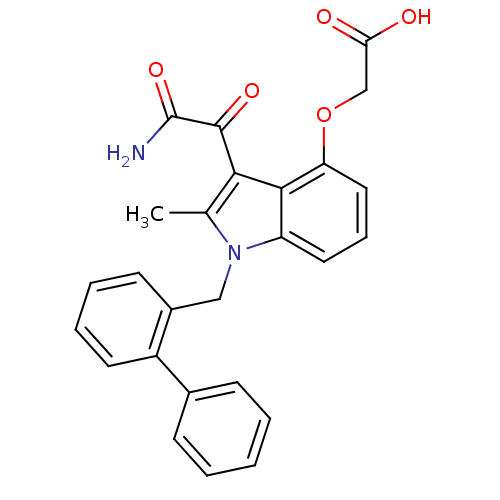
((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50134771
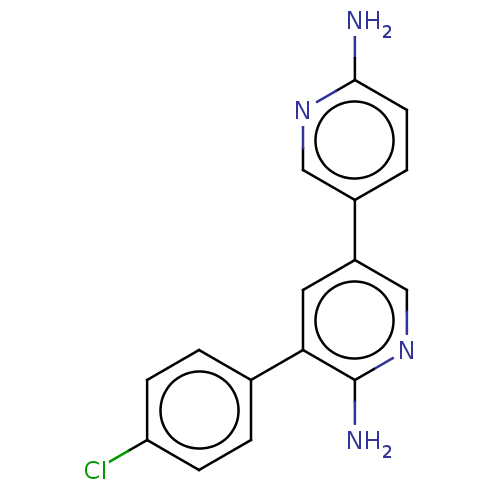
(CHEMBL3754515)Show InChI InChI=1S/C16H13ClN4/c17-13-4-1-10(2-5-13)14-7-12(9-21-16(14)19)11-3-6-15(18)20-8-11/h1-9H,(H2,18,20)(H2,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MAP4K4 catalytic domain in presence of 10 uM ATP (Km) by FRET assay |
ACS Med Chem Lett 6: 1128-33 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00215
BindingDB Entry DOI: 10.7270/Q2028TC6 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374
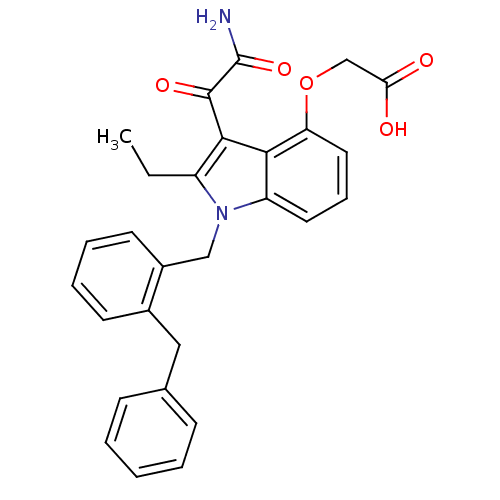
(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055374

(CHEMBL436456 | [3-Aminooxalyl-1-(2-benzyl-benzyl)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1Cc1ccccc1 Show InChI InChI=1S/C28H26N2O5/c1-2-21-26(27(33)28(29)34)25-22(13-8-14-23(25)35-17-24(31)32)30(21)16-20-12-7-6-11-19(20)15-18-9-4-3-5-10-18/h3-14H,2,15-17H2,1H3,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439634
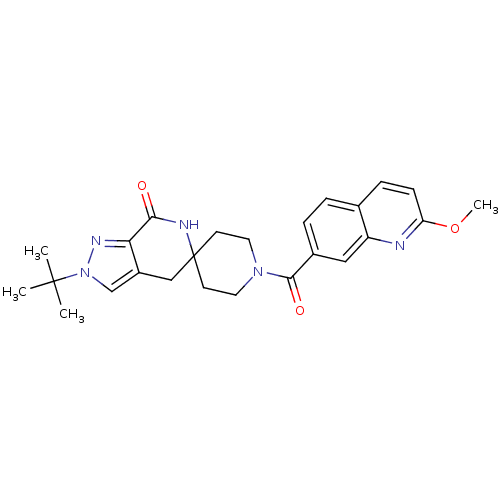
(CHEMBL2419596 | US8993586, 71)Show SMILES COc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H29N5O3/c1-24(2,3)30-15-18-14-25(27-22(31)21(18)28-30)9-11-29(12-10-25)23(32)17-6-5-16-7-8-20(33-4)26-19(16)13-17/h5-8,13,15H,9-12,14H2,1-4H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439643
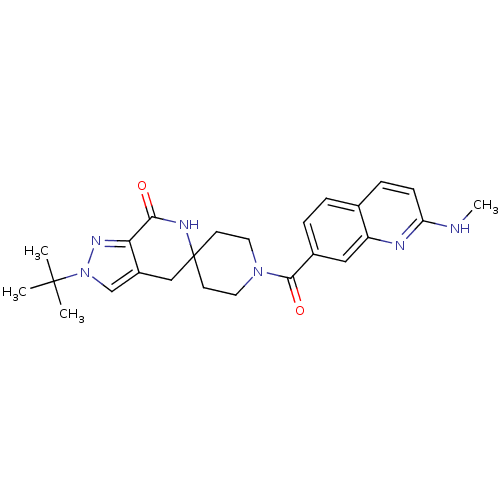
(CHEMBL2419598 | US8993586, 76)Show SMILES CNc1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H30N6O2/c1-24(2,3)31-15-18-14-25(28-22(32)21(18)29-31)9-11-30(12-10-25)23(33)17-6-5-16-7-8-20(26-4)27-19(16)13-17/h5-8,13,15H,9-12,14H2,1-4H3,(H,26,27)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439641
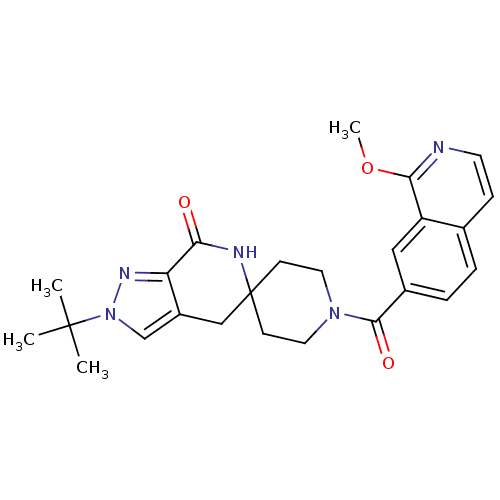
(CHEMBL2419597 | US8993586, 55)Show SMILES COc1nccc2ccc(cc12)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C25H29N5O3/c1-24(2,3)30-15-18-14-25(27-21(31)20(18)28-30)8-11-29(12-9-25)23(32)17-6-5-16-7-10-26-22(33-4)19(16)13-17/h5-7,10,13,15H,8-9,11-12,14H2,1-4H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439645
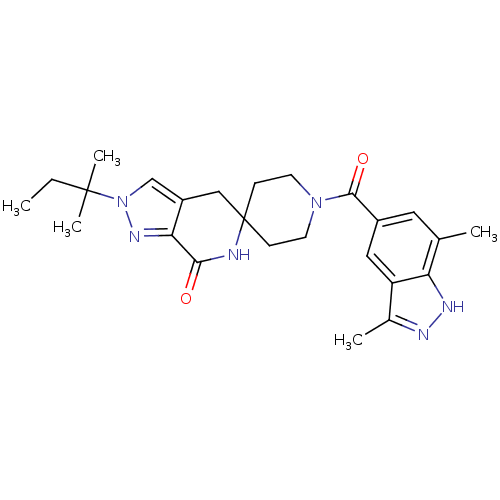
(CHEMBL2419607)Show SMILES CCC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3cc(C)c4[nH]nc(C)c4c3)NC(=O)c2n1 Show InChI InChI=1S/C25H32N6O2/c1-6-24(4,5)31-14-18-13-25(26-22(32)21(18)29-31)7-9-30(10-8-25)23(33)17-11-15(2)20-19(12-17)16(3)27-28-20/h11-12,14H,6-10,13H2,1-5H3,(H,26,32)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50439635
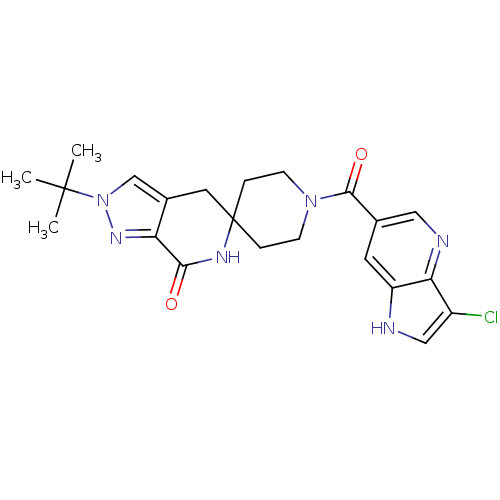
(CHEMBL2419594 | US8993586, 88)Show SMILES CC(C)(C)n1cc2CC3(CCN(CC3)C(=O)c3cnc4c(Cl)c[nH]c4c3)NC(=O)c2n1 Show InChI InChI=1S/C22H25ClN6O2/c1-21(2,3)29-12-14-9-22(26-19(30)17(14)27-29)4-6-28(7-5-22)20(31)13-8-16-18(25-10-13)15(23)11-24-16/h8,10-12,24H,4-7,9H2,1-3H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 1
(Homo sapiens (Human)) | BDBM50439642

(CHEMBL2419589 | US8993586, 105)Show SMILES CN(C)c1ccc2ccc(cc2n1)C(=O)N1CCC2(CC1)Cc1cn(nc1C(=O)N2)C(C)(C)C Show InChI InChI=1S/C26H32N6O2/c1-25(2,3)32-16-19-15-26(28-23(33)22(19)29-32)10-12-31(13-11-26)24(34)18-7-6-17-8-9-21(30(4)5)27-20(17)14-18/h6-9,14,16H,10-13,15H2,1-5H3,(H,28,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... |
J Med Chem 56: 7110-9 (2013)
Article DOI: 10.1021/jm401033t
BindingDB Entry DOI: 10.7270/Q2JW8G9D |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Acetyl-CoA carboxylase 2
(Homo sapiens (Human)) | BDBM50546612

(CHEMBL4753907)Show SMILES CC(C)n1ncc2CC3(CCN(CC3)C(=O)c3cc(nc(c3)-c3ccc(cc3)C(O)=O)N(C)C)CC(=O)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACC2 using acetyl coA as substrate incubated for 1 hr by transcreener fluorescence polarization assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00640
BindingDB Entry DOI: 10.7270/Q2RR22VT |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055387

((R)-2-(3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-...)Show SMILES C[C@@H](Oc1cccc2n(Cc3ccccc3)c(C)c(C(=O)C(N)=O)c12)C(O)=O Show InChI InChI=1S/C21H20N2O5/c1-12-17(19(24)20(22)25)18-15(23(12)11-14-7-4-3-5-8-14)9-6-10-16(18)28-13(2)21(26)27/h3-10,13H,11H2,1-2H3,(H2,22,25)(H,26,27)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055371

((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055379

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-cyclopropyl...)Show SMILES NC(=O)C(=O)c1c(C2CC2)n(Cc2ccccc2-c2ccccc2)c2cccc(OCC(O)=O)c12 Show InChI InChI=1S/C28H24N2O5/c29-28(34)27(33)25-24-21(11-6-12-22(24)35-16-23(31)32)30(26(25)18-13-14-18)15-19-9-4-5-10-20(19)17-7-2-1-3-8-17/h1-12,18H,13-16H2,(H2,29,34)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through chromogenic assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055401

((3-Aminooxalyl-1-biphenyl-2-ylmethyl-2-ethyl-1H-in...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H24N2O5/c1-2-20-25(26(32)27(28)33)24-21(13-8-14-22(24)34-16-23(30)31)29(20)15-18-11-6-7-12-19(18)17-9-4-3-5-10-17/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human nonpancreatic secretory Phospholipase A2 through DOC/PC assay |
J Med Chem 39: 5159-75 (1997)
Article DOI: 10.1021/jm960487f
BindingDB Entry DOI: 10.7270/Q22B8X54 |
More data for this
Ligand-Target Pair | |
Ketohexokinase
(Homo sapiens (Human)) | BDBM319582

(US10174007, Example 1 | US10787438, Example 1 | US...)Show SMILES C[C@H]1[C@H](O)CN1c1nc(cc(c1C#N)C(F)(F)F)N1C[C@H]2[C@H](CC(O)=O)[C@H]2C1 |r| Show InChI InChI=1S/C18H19F3N4O3/c1-8-14(26)7-25(8)17-10(4-22)13(18(19,20)21)3-15(23-17)24-5-11-9(2-16(27)28)12(11)6-24/h3,8-9,11-12,14,26H,2,5-7H2,1H3,(H,27,28)/t8-,9-,11-,12+,14+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 10 nM recombinant human N-terminal His-tagged KHKC expressed in Escherichia coli BL21 (DE3) using fructose as substrate preincubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00944
BindingDB Entry DOI: 10.7270/Q2QC074M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data