

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209097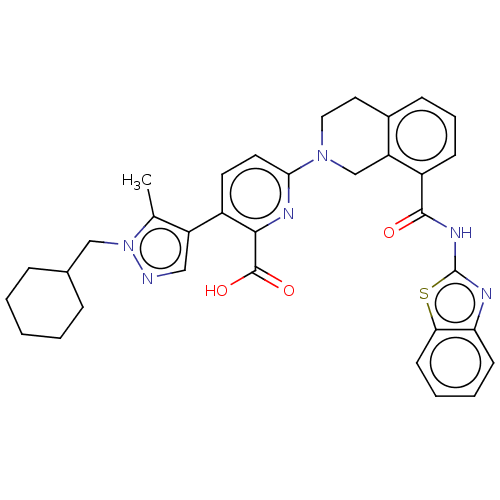 (US9266877, 43) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030752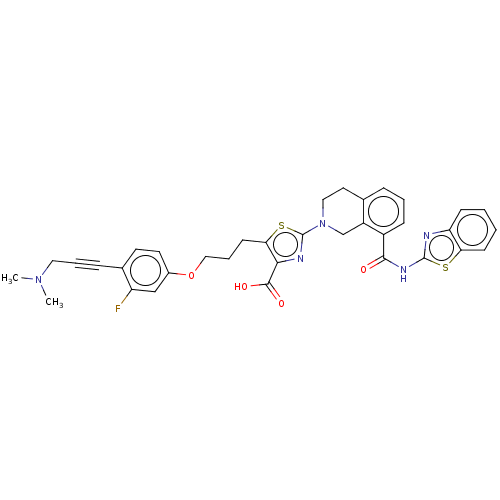 (CHEMBL3342333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030758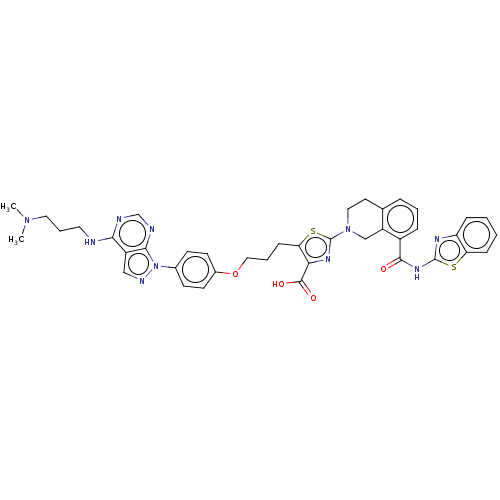 (CHEMBL3342195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030757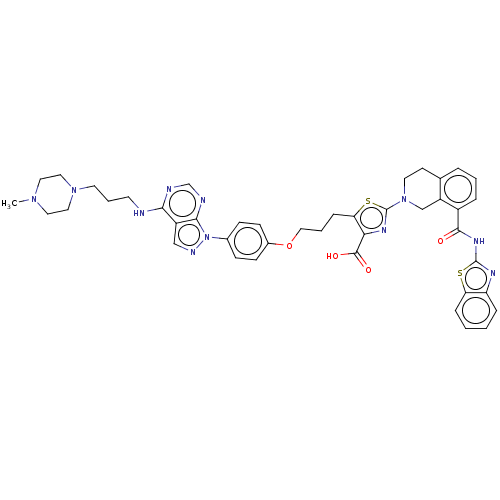 (CHEMBL3342196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030759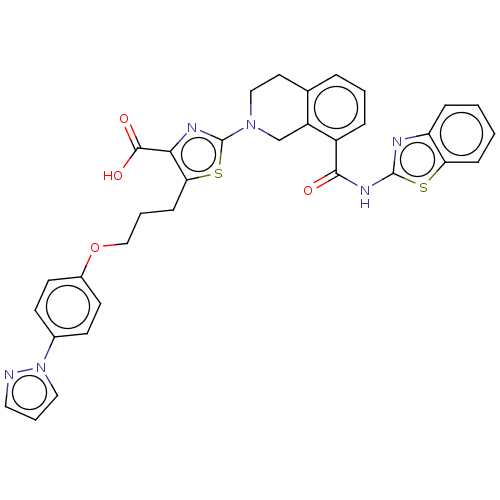 (CHEMBL3342194) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50561528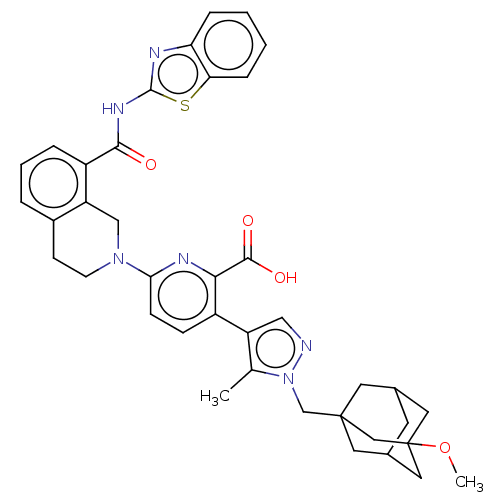 (CHEMBL4762875) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030754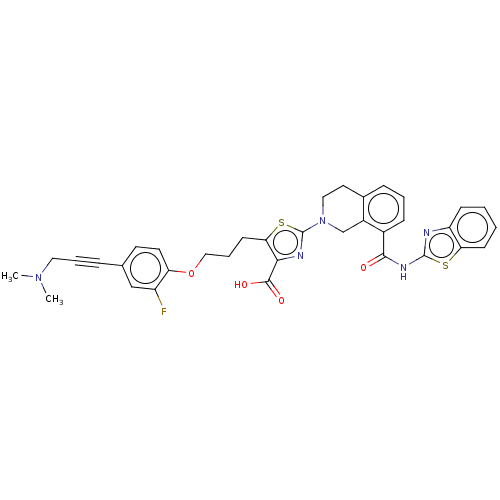 (CHEMBL3342332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50162797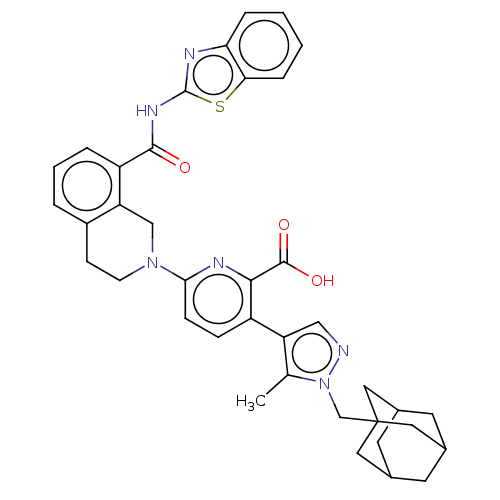 (CHEMBL3793424) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50448785 (CHEMBL3128069) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by Pfizer mobility shift assay | J Med Chem 57: 1170-87 (2014) Article DOI: 10.1021/jm401805h BindingDB Entry DOI: 10.7270/Q29C6ZX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030756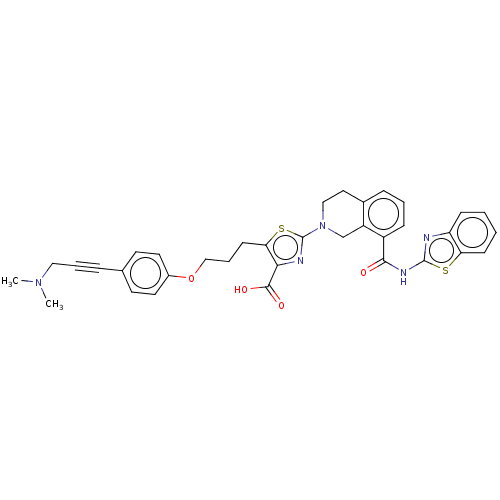 (CHEMBL3342197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50018830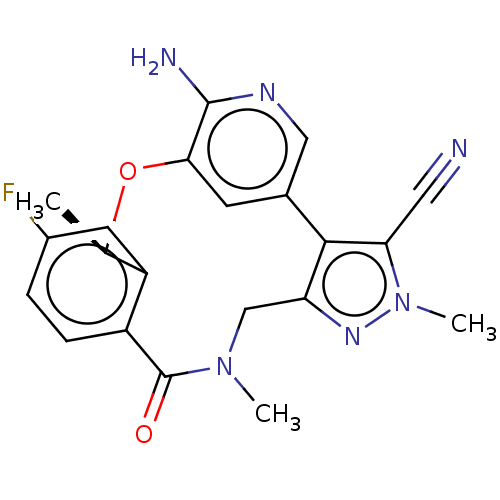 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ROS1 (unknown origin) by off-chip mobility shift assay | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836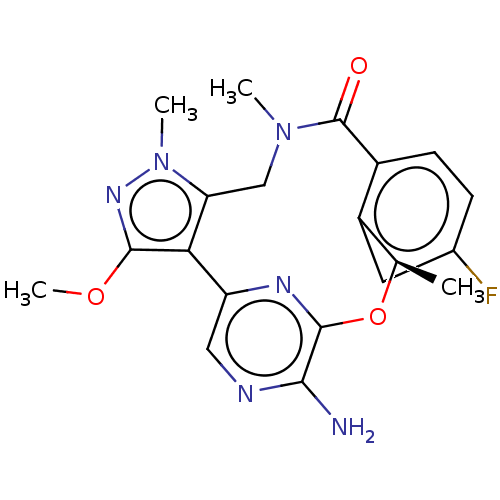 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant ALK L1196M mutant kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209074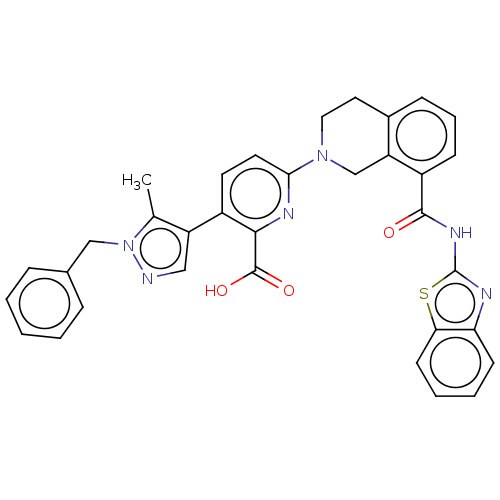 (US9266877, 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030751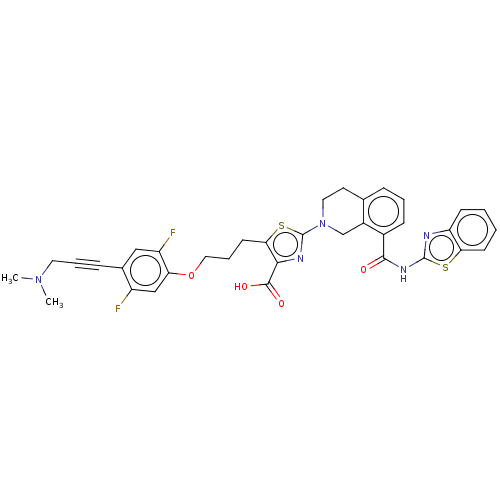 (CHEMBL3342334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241371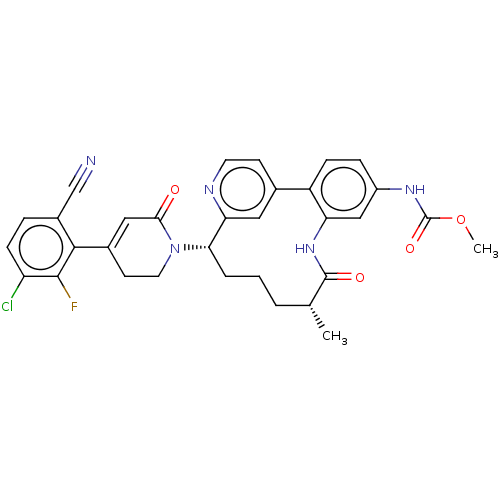 (US9409908, 3 | US9951071, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241371 (US9409908, 3 | US9951071, Example 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030757 (CHEMBL3342196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030755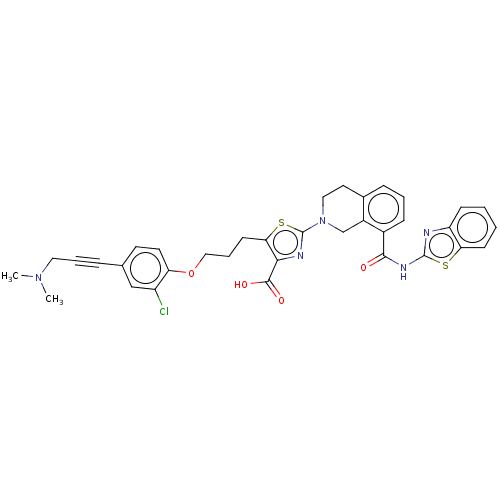 (CHEMBL3342198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241399 (US9409908, 31 | US9951071, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241399 (US9409908, 31 | US9951071, Example 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030761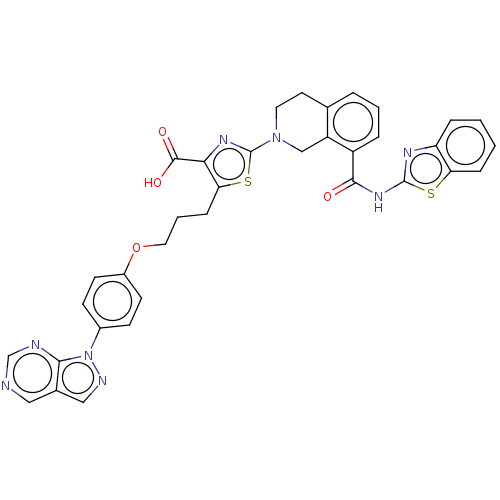 (CHEMBL3342192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM209066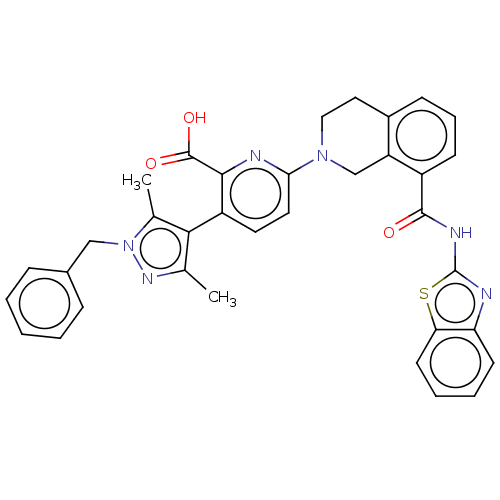 (US9266877, 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide probe binding to BCL-xl (unknown origin) incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00568 BindingDB Entry DOI: 10.7270/Q2542S8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030758 (CHEMBL3342195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241503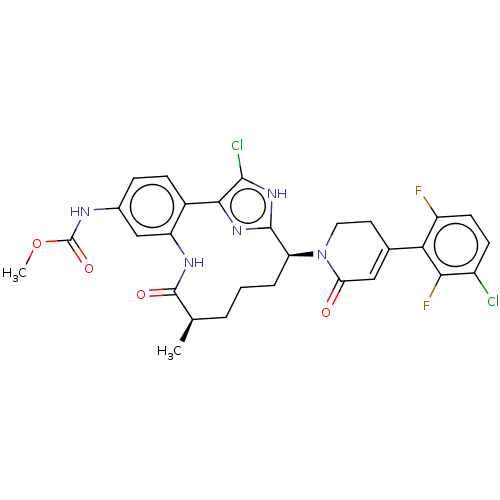 (US9409908, 135 | US9951071, Example 135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241567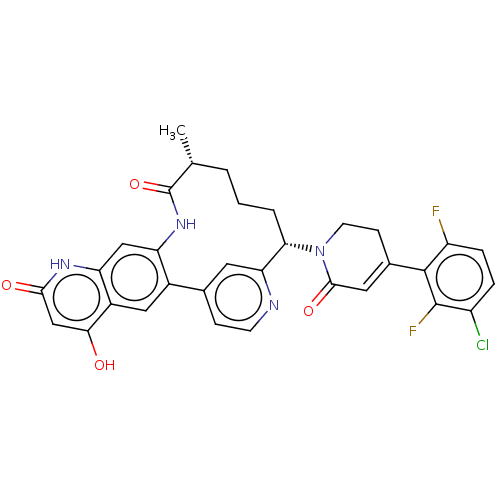 (US9409908, 199 | US9951071, Example 199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241566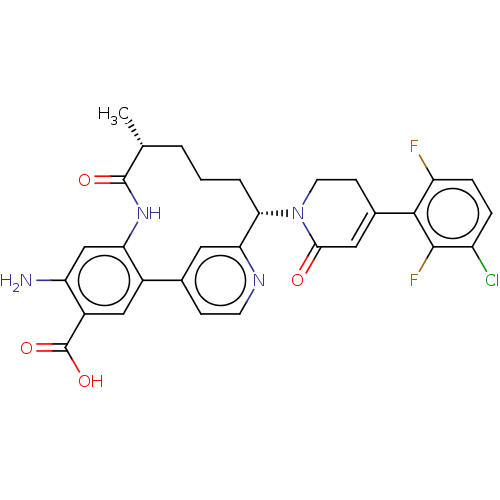 (US9409908, 198 | US9951071, Example 198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241552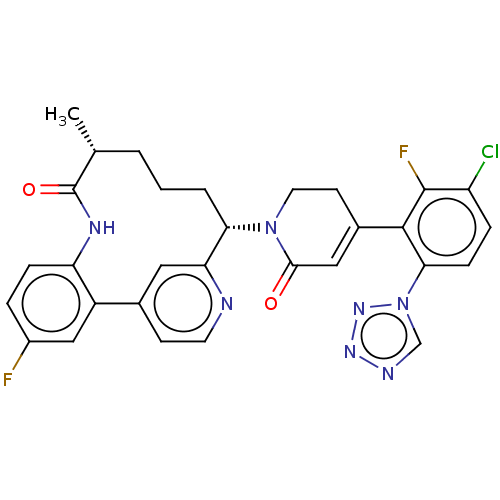 (US9409908, 184 | US9951071, Example 184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241436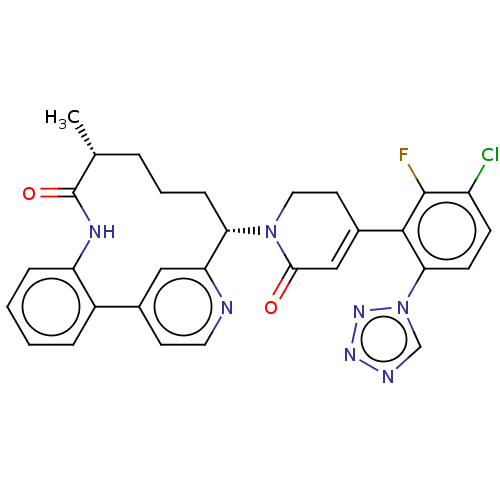 (US9409908, 68 | US9951071, Example 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241436 (US9409908, 68 | US9951071, Example 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241503 (US9409908, 135 | US9951071, Example 135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241567 (US9409908, 199 | US9951071, Example 199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241566 (US9409908, 198 | US9951071, Example 198) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241552 (US9409908, 184 | US9951071, Example 184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241451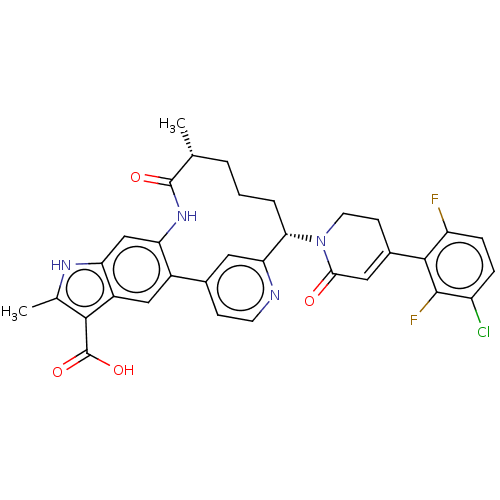 (US9409908, 83 | US9951071, Example 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241451 (US9409908, 83 | US9951071, Example 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241400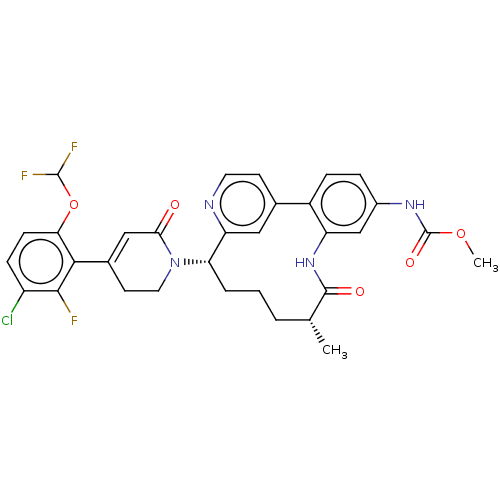 (US9409908, 32 | US9951071, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241400 (US9409908, 32 | US9951071, Example 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018830 (CHEMBL3286830 | US10543199, Compound PF-06463922 |...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241583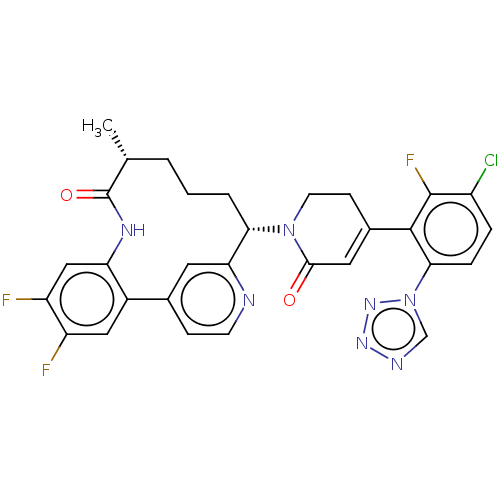 (US9409908, 215 | US9951071, Example 215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50250493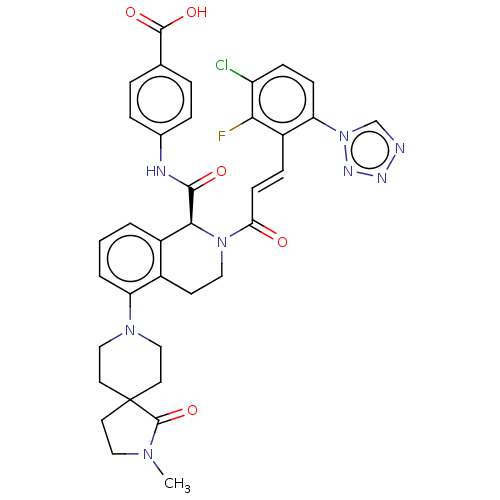 (CHEMBL4068445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method | J Med Chem 60: 9703-9723 (2017) Article DOI: 10.1021/acs.jmedchem.7b01171 BindingDB Entry DOI: 10.7270/Q2K64MHN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018837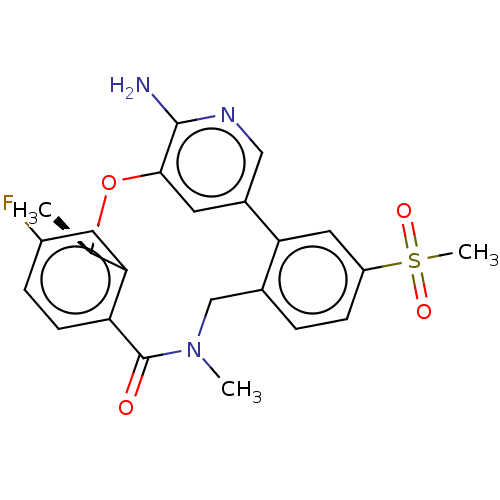 (CHEMBL3286827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241583 (US9409908, 215 | US9951071, Example 215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241422 (US9409908, 54 | US9951071, Example 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM241421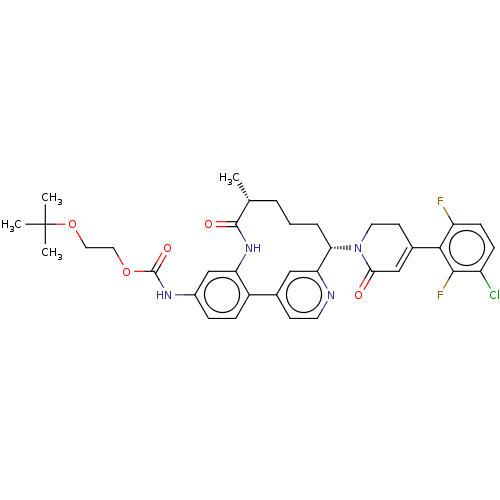 (US9409908, 53 | US9951071, Example 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | J Med Chem 51: 487-501 (2008) BindingDB Entry DOI: 10.7270/Q2R78HKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50018836 (CHEMBL3286826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | <0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of wild type human recombinant ALK kinase domain (amino acids 1093 to 1141) expressed in baculovirus system using 5'FAM-KKSRGDYMTMQIG-CONH... | J Med Chem 57: 4720-44 (2014) Article DOI: 10.1021/jm500261q BindingDB Entry DOI: 10.7270/Q2K35W68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241422 (US9409908, 54 | US9951071, Example 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50095041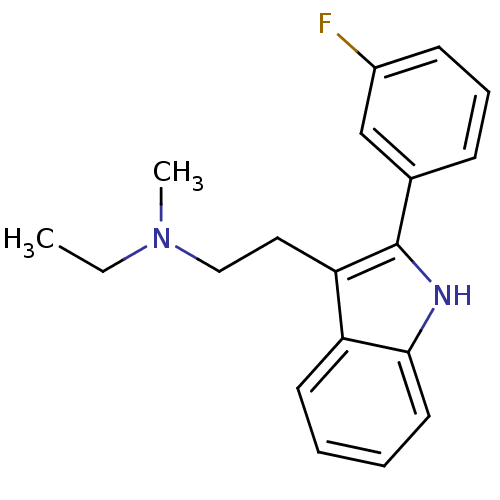 (CHEMBL328595 | Ethyl-{2-[2-(3-fluoro-phenyl)-1H-in...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacent of [H]-ketanserin from CHO cells expressing human 5-hydroxytryptamine 2A receptor. | Bioorg Med Chem Lett 10: 2697-9 (2000) BindingDB Entry DOI: 10.7270/Q27943XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241421 (US9409908, 53 | US9951071, Example 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241468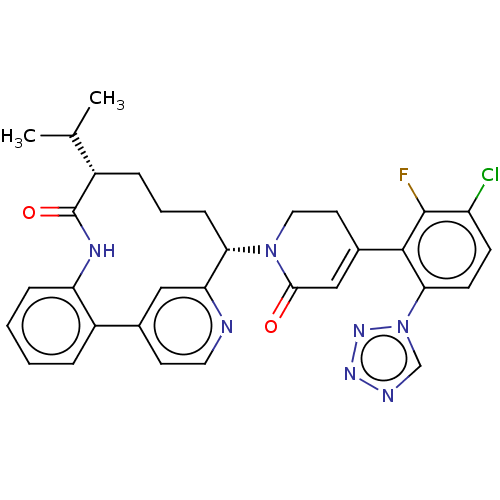 (US9409908, 100 | US9951071, Example 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM241472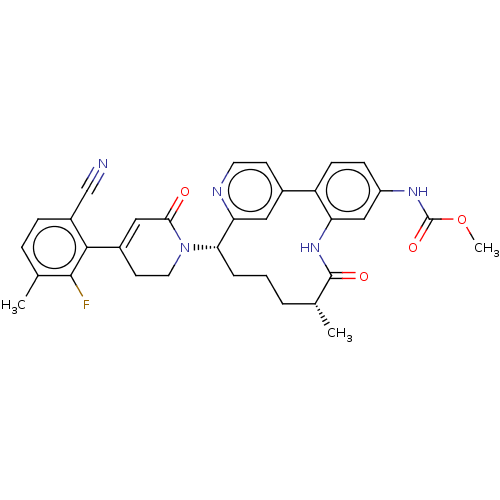 (US9409908, 104 | US9951071, Example 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake... | US Patent US9409908 (2016) BindingDB Entry DOI: 10.7270/Q27943K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 12949 total ) | Next | Last >> |