

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193900 (US9199976, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 86 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193910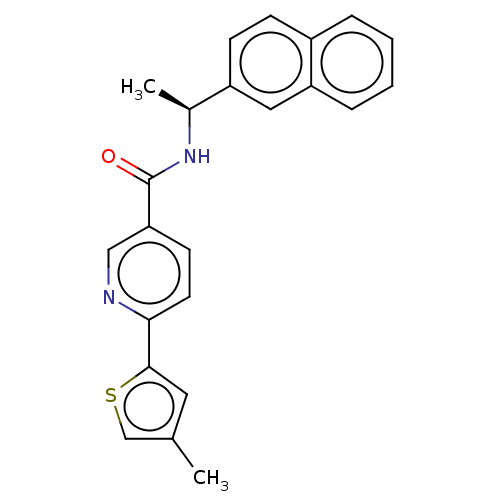 (US9199976, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300129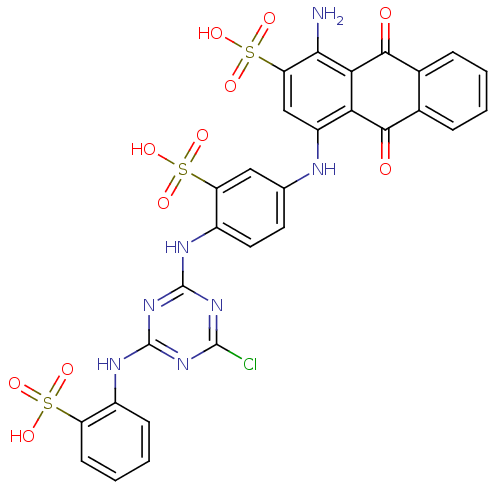 (CHEMBL572528 | CIBACRON BLUE | Cibacron Blue 3Ga) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193907 (US9199976, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193895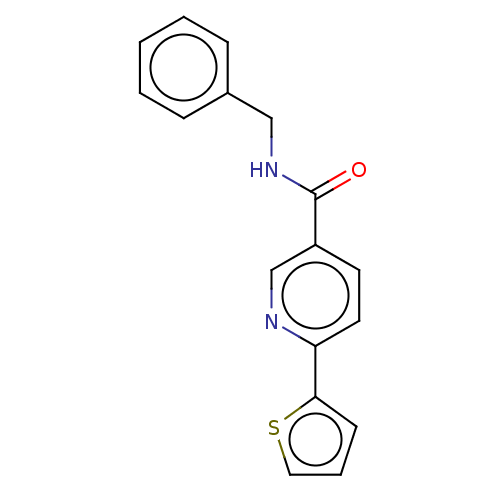 (US9199976, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 597 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193897 (US9199976, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 660 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324491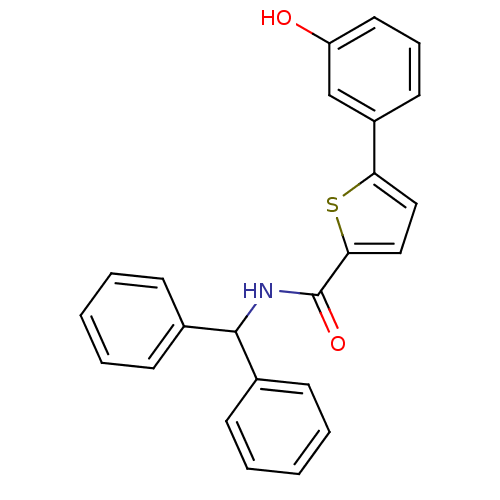 (CHEMBL1215159 | N-Benzhydryl-5-(3-hydroxyphenyl)th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM21625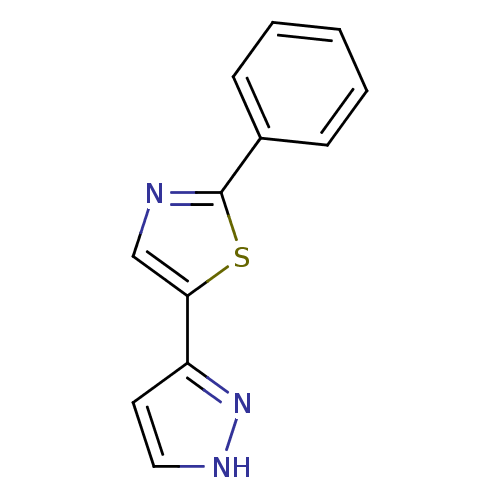 (2-phenyl-5-(1H-pyrazol-3-yl)-1,3-thiazole | 2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193909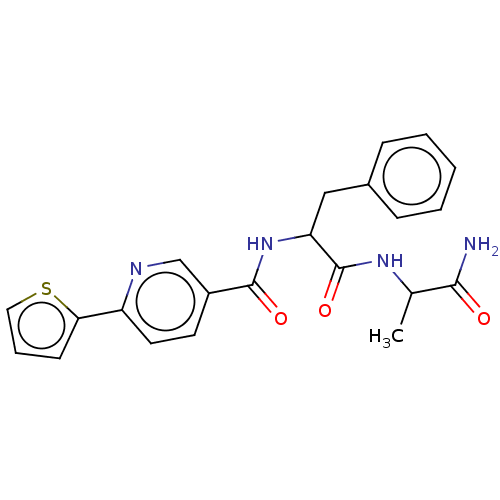 (US9199976, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324482 (CHEMBL1215370 | N-(1-Amino-3-(1H-indol-3-yl)-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193894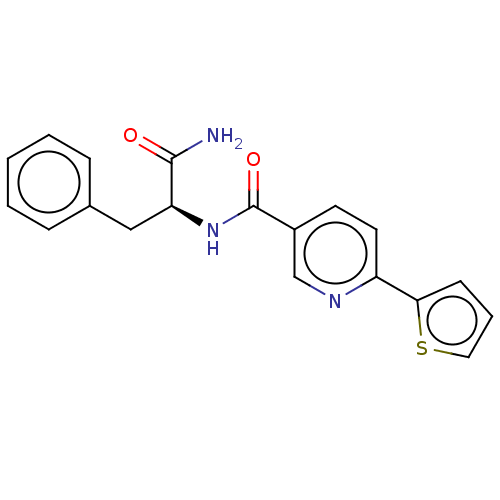 (US9199976, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324480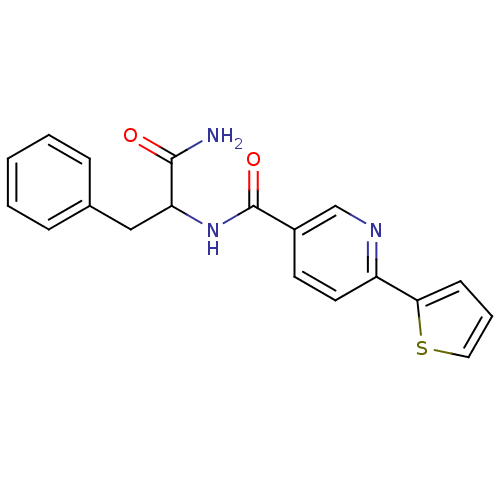 (CHEMBL1215516 | N-(1-Amino-1-oxo-3-phenylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193899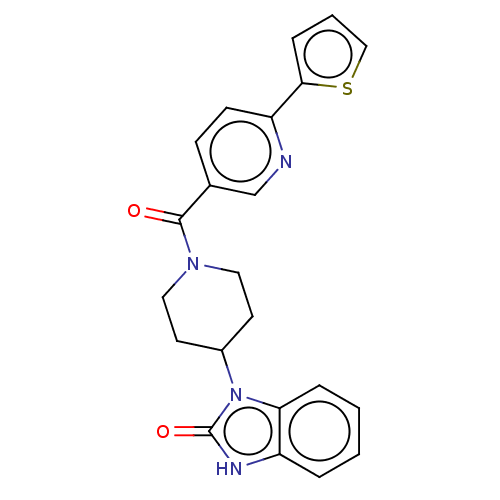 (US9199976, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193898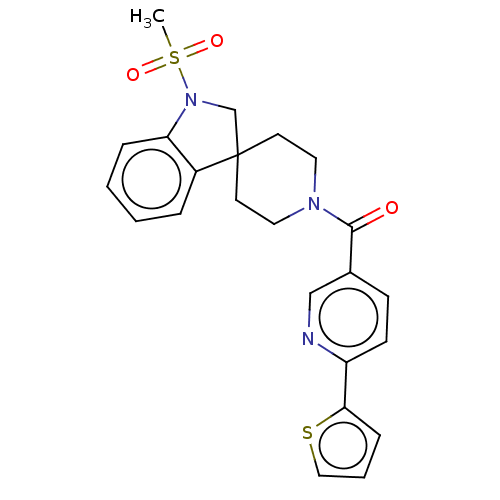 (US9199976, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324485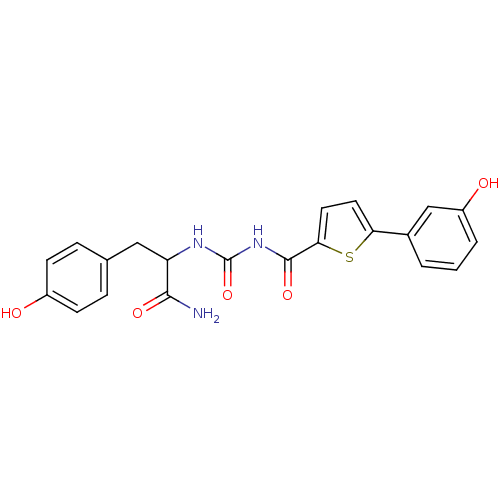 (CHEMBL1215297 | N-(1-Amino-3-(4-hydroxyphenyl)-1-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193903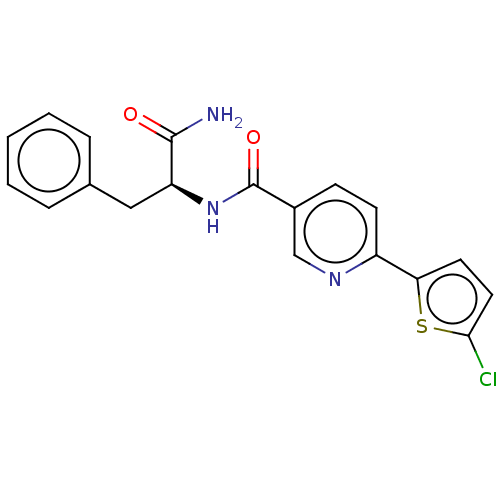 (US9199976, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM21624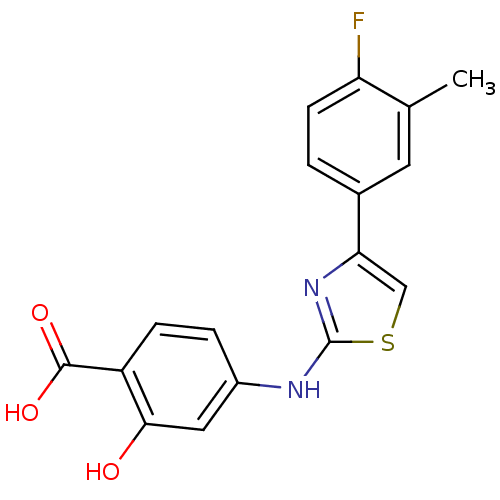 (4-{[4-(4-fluoro-3-methylphenyl)-1,3-thiazol-2-yl]a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193902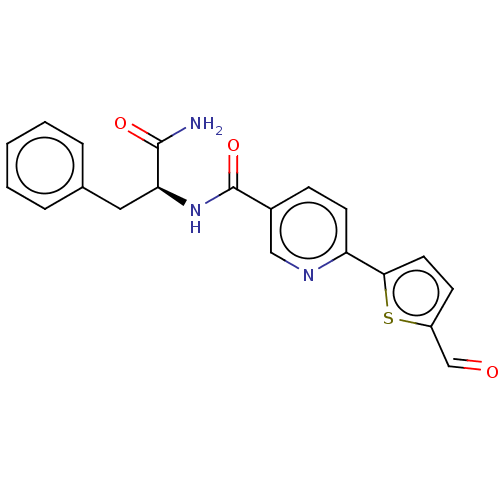 (US9199976, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300128 (1-(3-(1H-tetrazol-5-yl)propyl)-4-(benzhydryloxy)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193908 (US9199976, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324490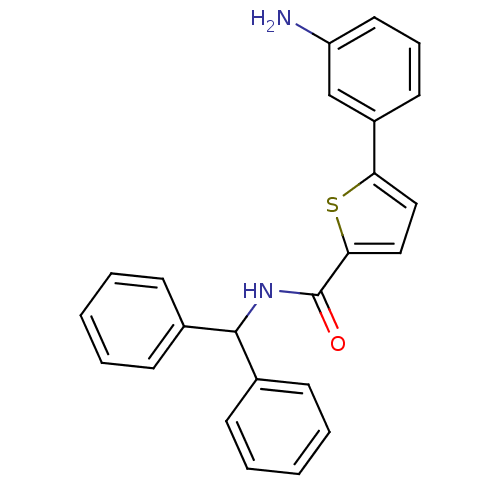 (5-(3-Aminophenyl)-N-benzhydrylthiophene-2-carboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193896 (US9199976, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193904 (US9199976, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324484 (CHEMBL1215298 | N-(1-Amino-3-(1H-indol-3-yl)-1-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193906 (US9199976, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193901 (US9199976, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324486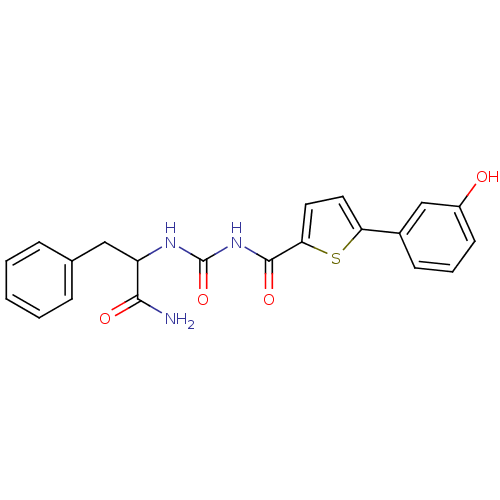 (CHEMBL1215296 | N-(1-Amino-1-oxo-3-phenylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300128 (1-(3-(1H-tetrazol-5-yl)propyl)-4-(benzhydryloxy)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324483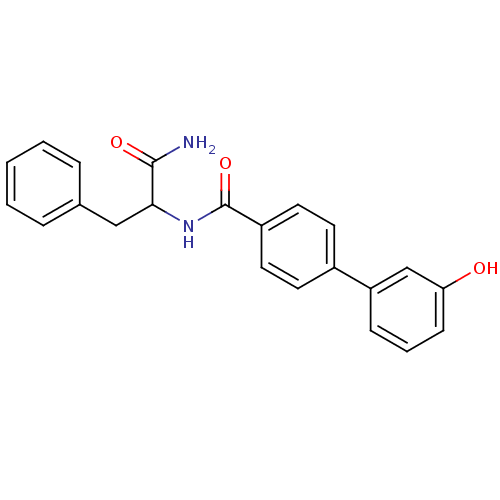 (CHEMBL1215369 | N-(1-amino-1-oxo-3-phenylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM193905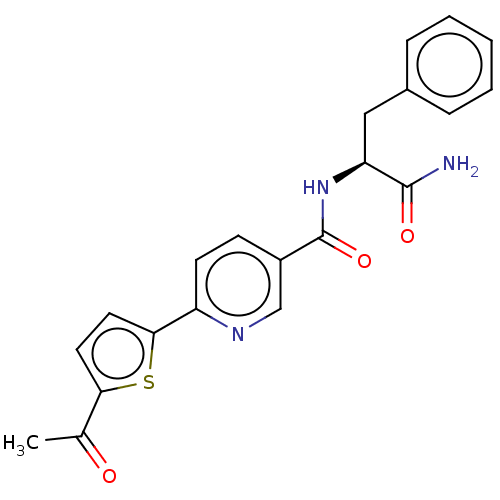 (US9199976, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
The University of Queensland US Patent | Assay Description The H-PGDS catalyzed conjugation of GSH and 1-chloro-2,4-dinitrobenzene (CDNB) was used as the biochemical assay for enzyme inhibition. Reactions wer... | US Patent US9199976 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324487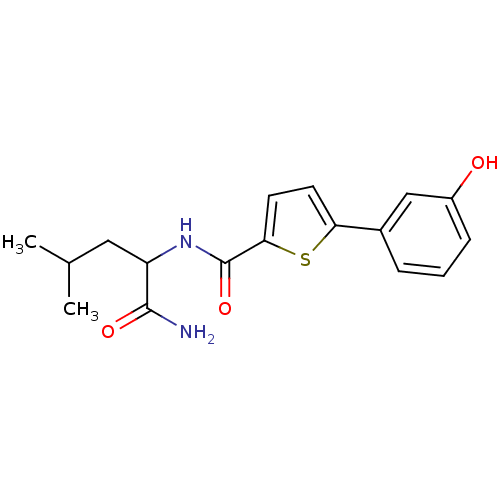 (CHEMBL1215295 | N-(1-Amino-4-methyl-1-oxopentan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324488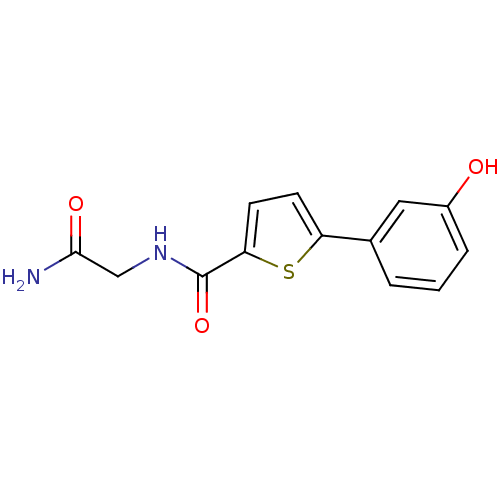 (CHEMBL1215294 | N-(2-Amino-2-oxoethyl)-5-(3-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300127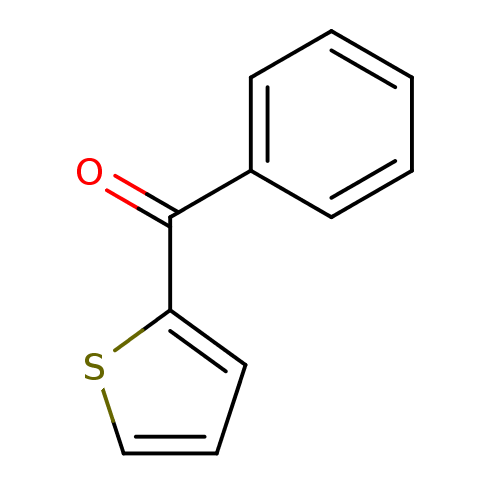 (CHEMBL567059 | NSC-4502 | phenyl(thiophen-2-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324489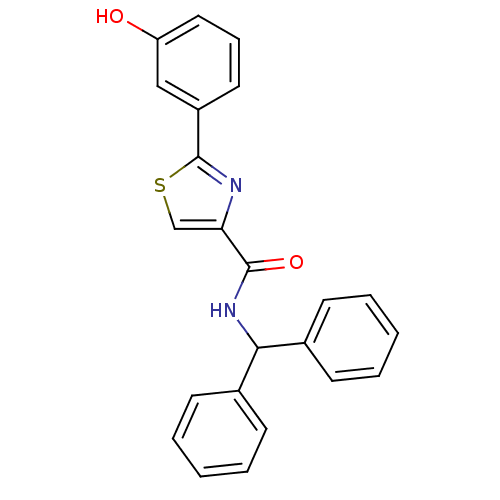 (CHEMBL1215162 | N-benzhydryl-2-(3-hydroxyphenyl)th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300127 (CHEMBL567059 | NSC-4502 | phenyl(thiophen-2-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324479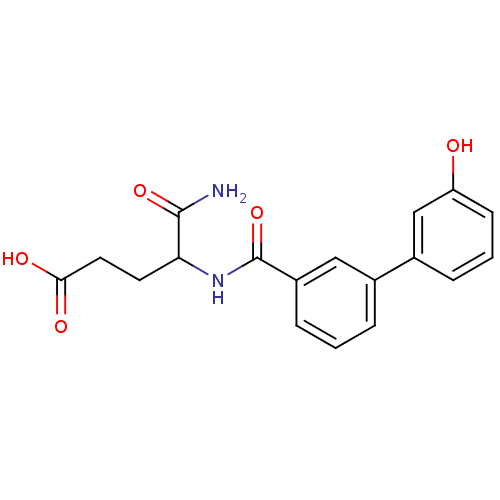 (5-amino-4-(3'-hydroxy-[1,1'-biphenyl]-4-ylcarboxam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50324481 (CHEMBL1215446 | N-(1-amino-1-oxo-3-phenylpropan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay | J Med Chem 53: 5536-48 (2010) Article DOI: 10.1021/jm100194a BindingDB Entry DOI: 10.7270/Q21J99Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300126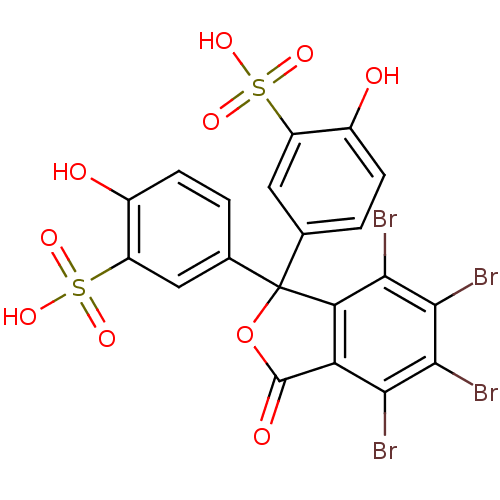 (CHEMBL574431 | Sulfobromophthalein) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300125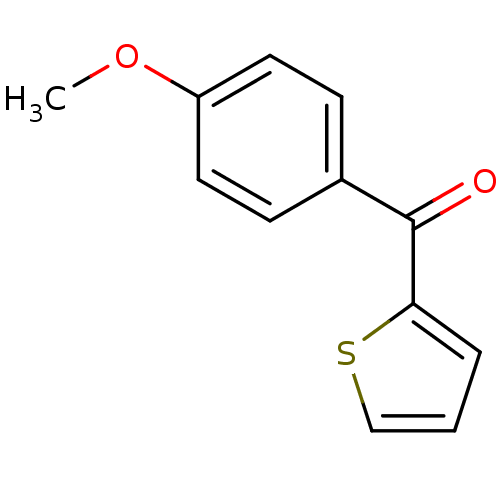 ((4-methoxyphenyl)(thiophen-2-yl)methanone | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50186231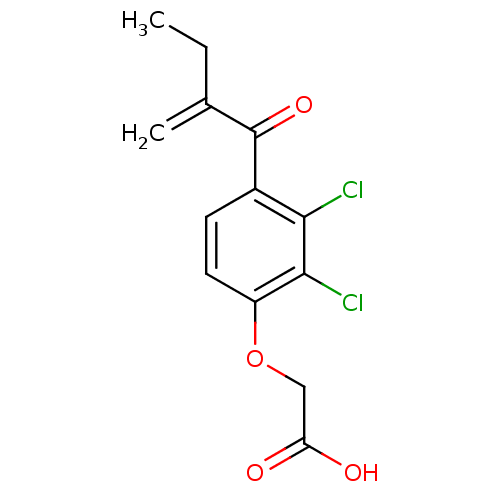 ((2,3-Dichloro-4-(2-methylene-1-oxobutyl)phenoxy)ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300124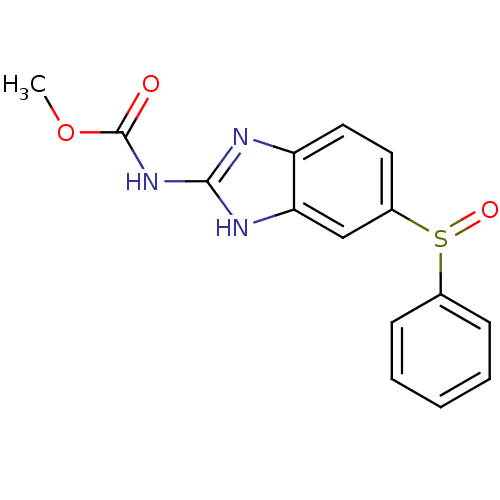 ((5-(phenylsulfinyl)-1H-benzimidazol-2-yl)carbamic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of human H-PGDS expressed in Escherichia coli BL21 assessed as rate of glutathione-chloro-dinitro benzene conjugation | Eur J Med Chem 45: 447-54 (2010) Article DOI: 10.1016/j.ejmech.2009.10.025 BindingDB Entry DOI: 10.7270/Q2HD7VQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||