

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002186 (CHEMBL440987 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002182 (CHEMBL415617 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002185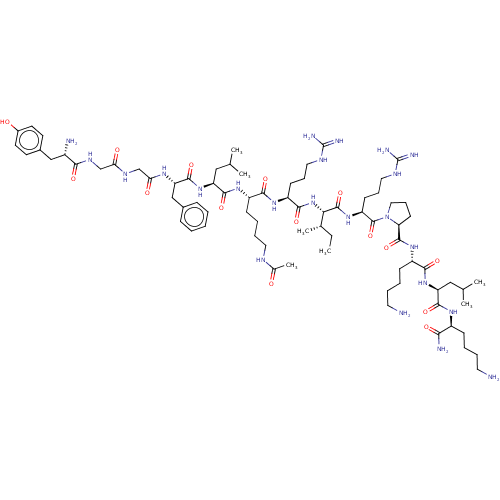 (CHEMBL438741 | Tyr-Gly-Gly-Phe-Leu-Lys(Ac)-Arg-Ile...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002181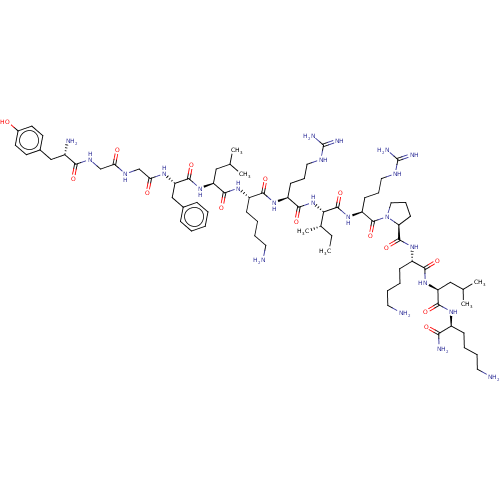 (CHEMBL266717 | Tyr-Gly-Gly-Phe-Leu-Lys-Arg-Ile-Arg...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002183 (CHEMBL266515 | Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Ile-Arg...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002184 (CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002185 (CHEMBL438741 | Tyr-Gly-Gly-Phe-Leu-Lys(Ac)-Arg-Ile...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002178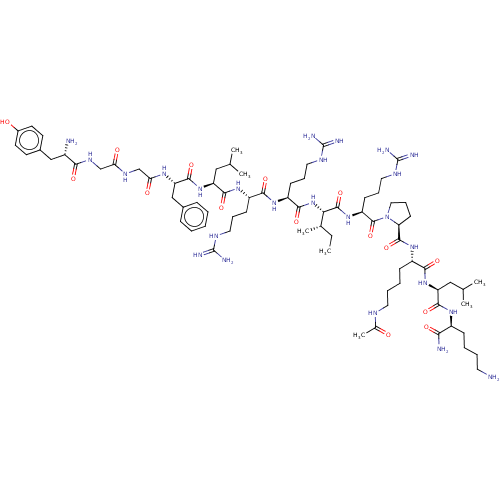 (CHEMBL440446 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002186 (CHEMBL440987 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002182 (CHEMBL415617 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002178 (CHEMBL440446 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002183 (CHEMBL266515 | Tyr-Gly-Gly-Phe-Leu-Arg-Lys-Ile-Arg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002179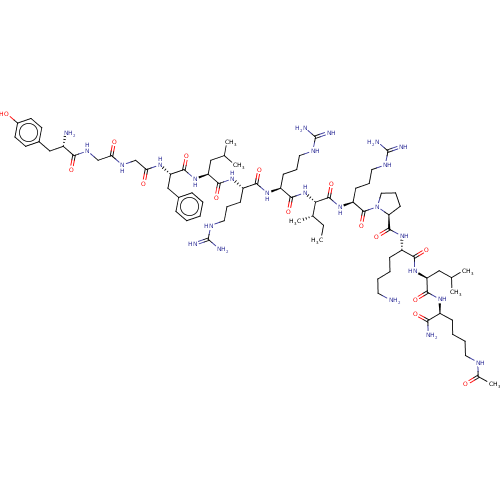 (CHEMBL263597 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002181 (CHEMBL266717 | Tyr-Gly-Gly-Phe-Leu-Lys-Arg-Ile-Arg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002180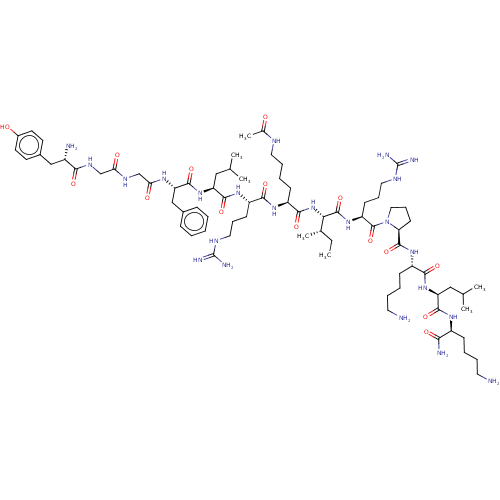 (CHEMBL263583 | Tyr-Gly-Gly-Phe-Leu-Arg-Lys(Ac)-Ile...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002179 (CHEMBL263597 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]- bremazocine to opioid receptor kappa of guinea pig cerebral membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002184 (CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards kappa opioid receptor in guinea pig cerebellar membrane using [3H]-bremazocine | J Med Chem 36: 1100-3 (1993) BindingDB Entry DOI: 10.7270/Q2639NS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002180 (CHEMBL263583 | Tyr-Gly-Gly-Phe-Leu-Arg-Lys(Ac)-Ile...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002184 (CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards mu-opioid receptor in rat forebrain membrane using [3H]-DAMGO | J Med Chem 36: 1100-3 (1993) BindingDB Entry DOI: 10.7270/Q2639NS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50521920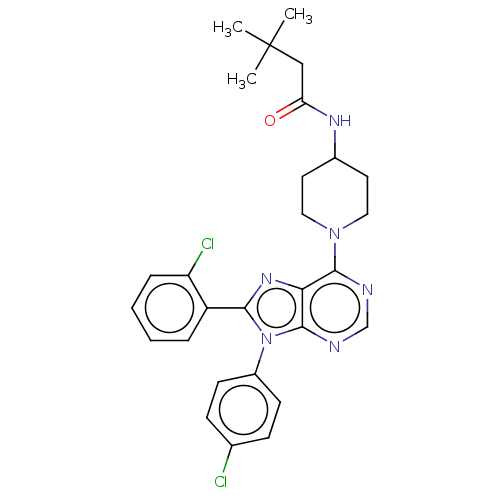 (CHEMBL4469545) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 27: 3632-3649 (2019) Article DOI: 10.1016/j.bmc.2019.07.002 BindingDB Entry DOI: 10.7270/Q2GT5RKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516264 (CHEMBL4463013) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cell membranes | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50521917 (CHEMBL4520650) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 27: 3632-3649 (2019) Article DOI: 10.1016/j.bmc.2019.07.002 BindingDB Entry DOI: 10.7270/Q2GT5RKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002184 (CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO to opioid receptor mu of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463404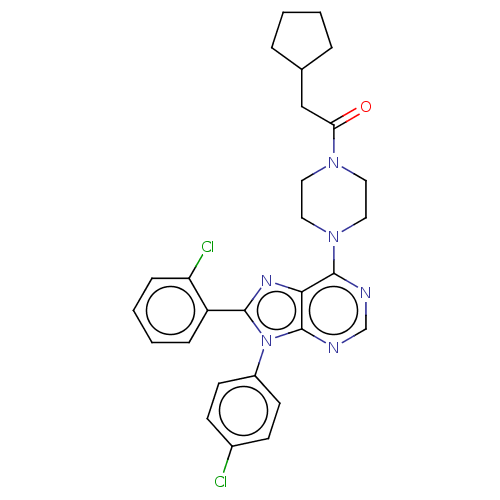 (CHEMBL4244751) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50383388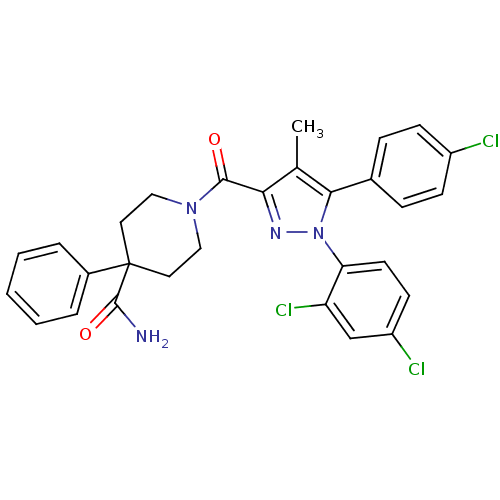 (CHEMBL2030738) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 2820-34 (2012) Article DOI: 10.1021/jm201731z BindingDB Entry DOI: 10.7270/Q2P2704Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002185 (CHEMBL438741 | Tyr-Gly-Gly-Phe-Leu-Lys(Ac)-Arg-Ile...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE to opioid receptor delta of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002178 (CHEMBL440446 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE to opioid receptor delta of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463413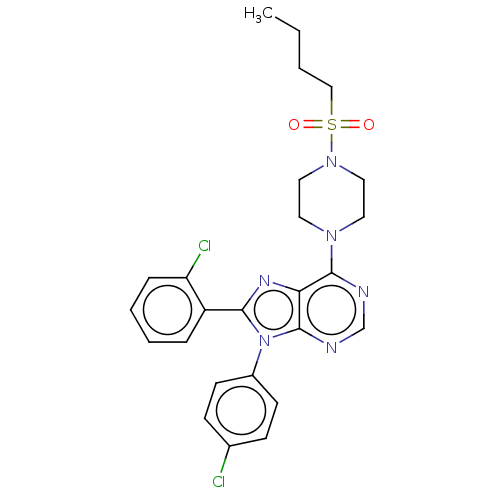 (CHEMBL4242477) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50521900 (CHEMBL4580498) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 27: 3632-3649 (2019) Article DOI: 10.1016/j.bmc.2019.07.002 BindingDB Entry DOI: 10.7270/Q2GT5RKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-SR141716A from human CB1 receptor expressed in CHO cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27337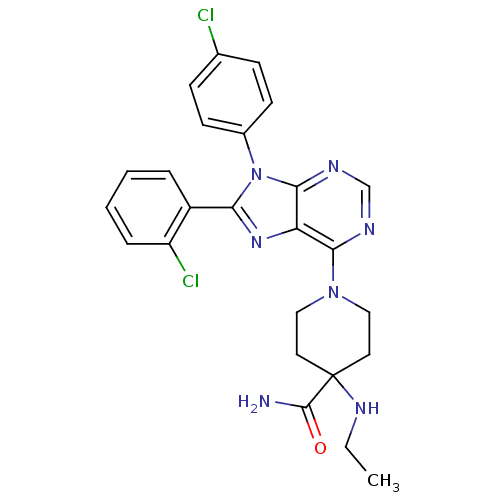 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534464 (CHEMBL4483714) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516261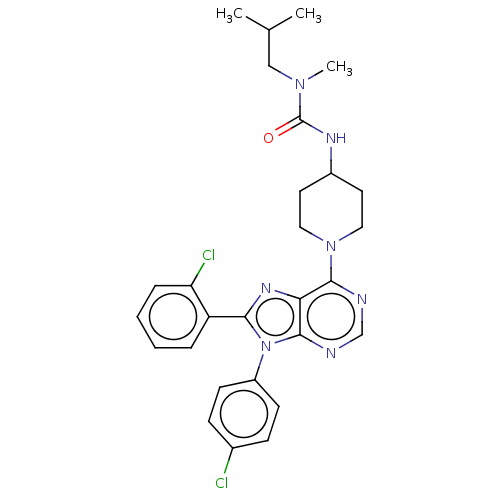 (CHEMBL4466640) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cell membranes | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534464 (CHEMBL4483714) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.859 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516262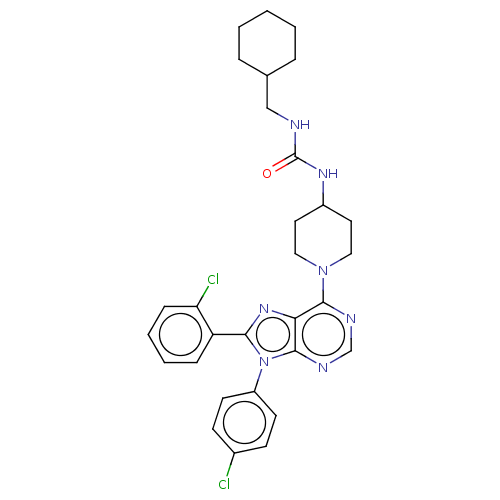 (CHEMBL4567690) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cell membranes | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516249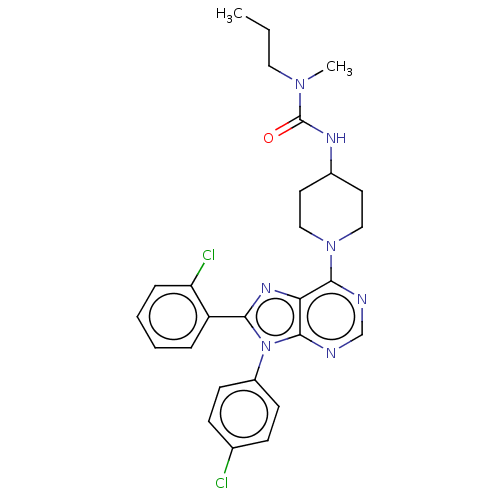 (CHEMBL4524602) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cell membranes | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461710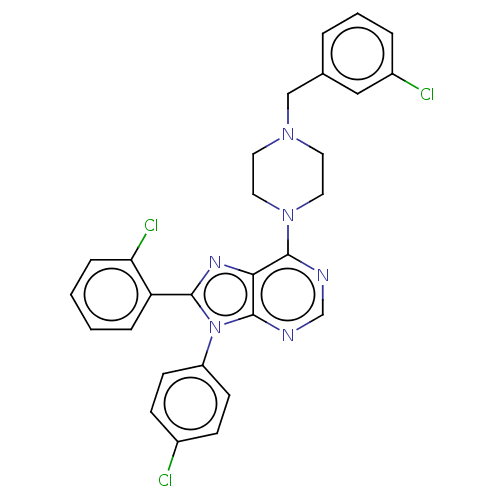 (CHEMBL4225147) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50521897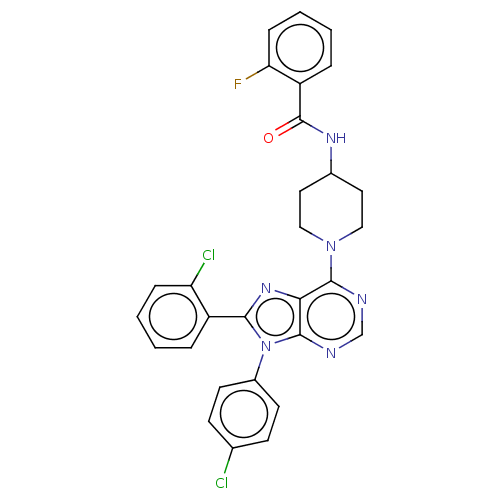 (CHEMBL4567814) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cell membranes | Bioorg Med Chem 27: 3632-3649 (2019) Article DOI: 10.1016/j.bmc.2019.07.002 BindingDB Entry DOI: 10.7270/Q2GT5RKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463410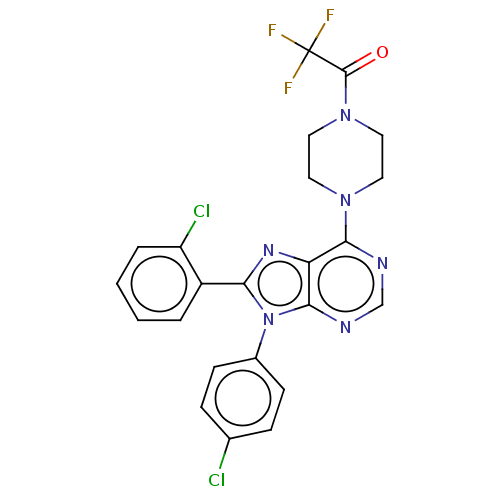 (CHEMBL4250064) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534476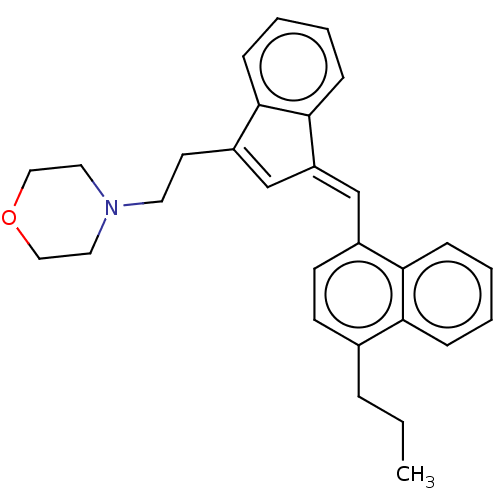 (CHEMBL4443660) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461703 (CHEMBL4225421) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516238 (CHEMBL4578966) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cell membranes | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50461698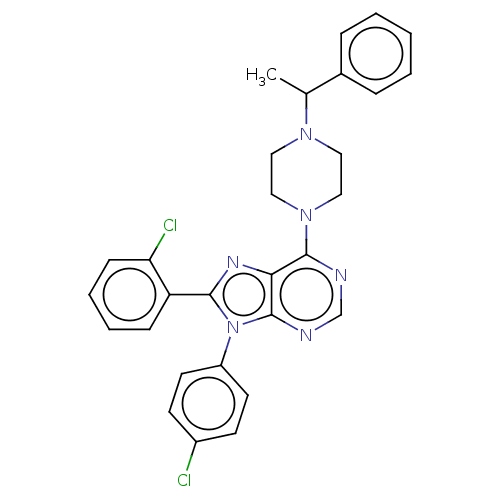 (CHEMBL4227354) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 expressed in CHOK1 cell membranes | J Med Chem 61: 4370-4385 (2018) Article DOI: 10.1021/acs.jmedchem.7b01820 BindingDB Entry DOI: 10.7270/Q20004QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002180 (CHEMBL263583 | Tyr-Gly-Gly-Phe-Leu-Arg-Lys(Ac)-Ile...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE to opioid receptor delta of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463403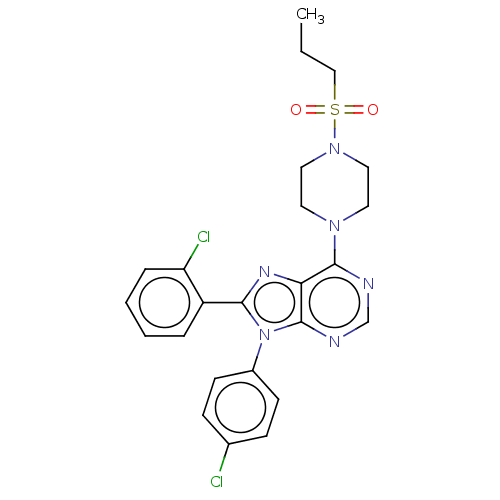 (CHEMBL4248795) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534476 (CHEMBL4443660) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50463398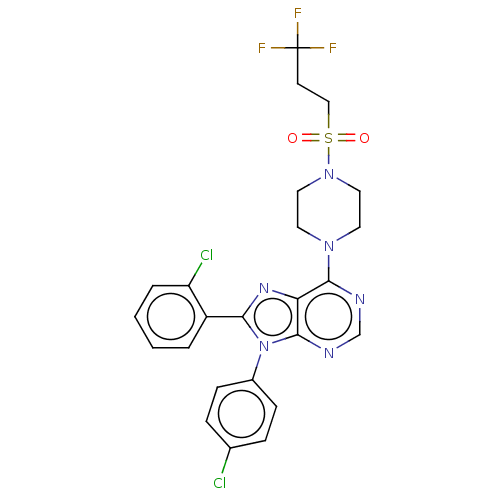 (CHEMBL4238756) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in HEK293 cell membranes after 1 hr by liquid scintillation spectrometry | Bioorg Med Chem 26: 4518-4531 (2018) Article DOI: 10.1016/j.bmc.2018.07.043 BindingDB Entry DOI: 10.7270/Q2VM4FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50399518 (CHEMBL2180214) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cells | J Med Chem 55: 10022-32 (2012) Article DOI: 10.1021/jm301181r BindingDB Entry DOI: 10.7270/Q2GB256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50516250 (CHEMBL4450173) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK293 cell membranes | J Med Chem 62: 6330-6345 (2019) Article DOI: 10.1021/acs.jmedchem.9b00727 BindingDB Entry DOI: 10.7270/Q24F1V3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002186 (CHEMBL440987 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Lys...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Oregon State University Curated by ChEMBL | Assay Description Inhibition of [3H]-DPDPE to opioid receptor delta of rat fore brain membranes was determined | J Med Chem 35: 4330-3 (1992) BindingDB Entry DOI: 10.7270/Q2FB51W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 822 total ) | Next | Last >> |