

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091438 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(5-hydroxy-1H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233075 (3-(3,4-dichlorophenyl)-N-((1s,4s)-4-((4-(5-hydroxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233073 (CHEMBL399472 | N-((1s,4s)-4-((4-(1H-indol-3-yl)pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233071 (3-(3,4-dichlorophenyl)-N-((1r,4r)-4-((4-(5-hydroxy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091454 ((E)-3-(4-Bromo-phenyl)-N-{5-[4-(1H-indol-3-yl)-pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091458 ((E)-3-(3,4-Dichloro-phenyl)-N-{5-[4-(1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091465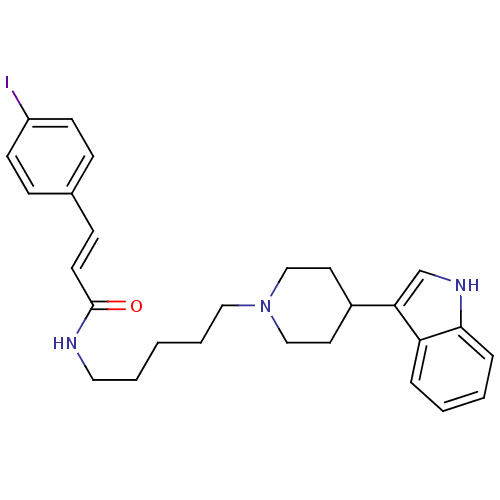 ((E)-N-(5-(4-(1H-indol-3-yl)piperidin-1-yl)pentyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091445 ((E)-3-(4-Chloro-phenyl)-N-{5-[4-(1H-indol-3-yl)-pi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091471 ((E)-N-(5-(4-(1H-indol-3-yl)piperidin-1-yl)pentyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091460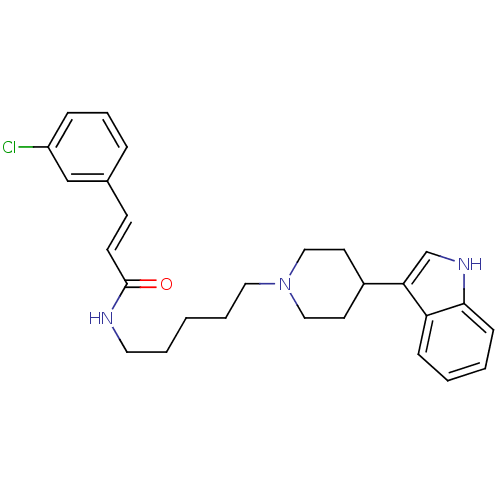 ((E)-3-(3-Chloro-phenyl)-N-{5-[4-(1H-indol-3-yl)-pi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233072 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[4-(1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091442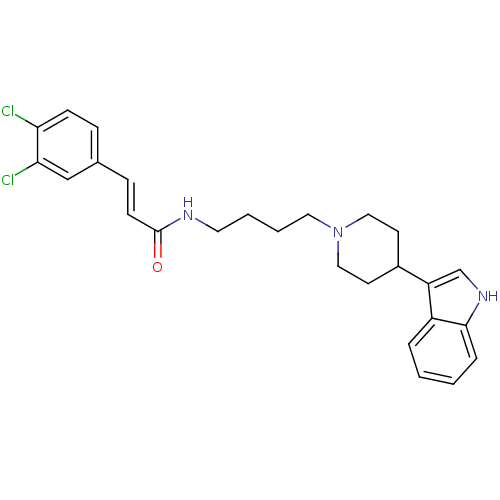 ((E)-3-(3,4-Dichloro-phenyl)-N-{4-[4-(1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091467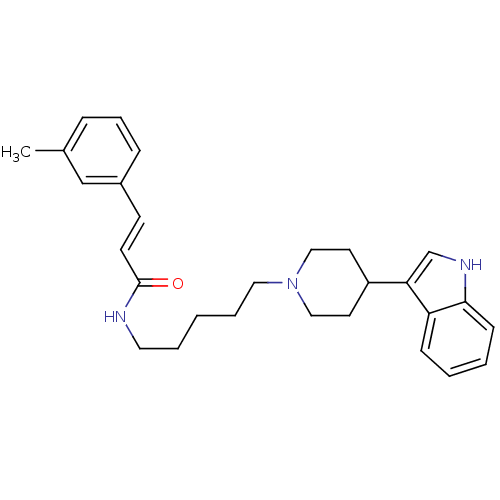 ((E)-N-(5-(4-(1H-indol-3-yl)piperidin-1-yl)pentyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091448 ((E)-N-(5-(4-(1H-indol-3-yl)piperidin-1-yl)pentyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091439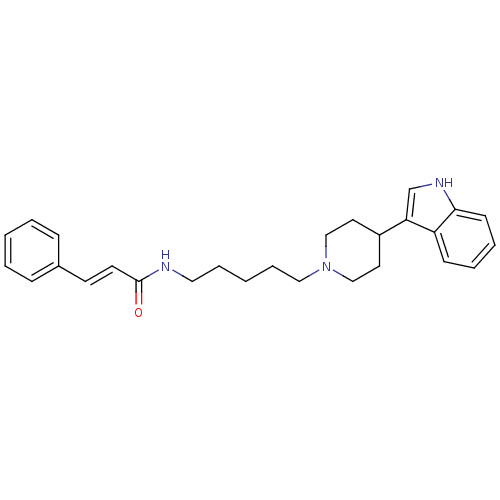 ((E)-N-(5-(4-(1H-indol-3-yl)piperidin-1-yl)pentyl)c...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091468 ((E)-3-(4-Dimethylamino-phenyl)-N-{5-[4-(1H-indol-3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091443 ((E)-3-Biphenyl-4-yl-N-{5-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091441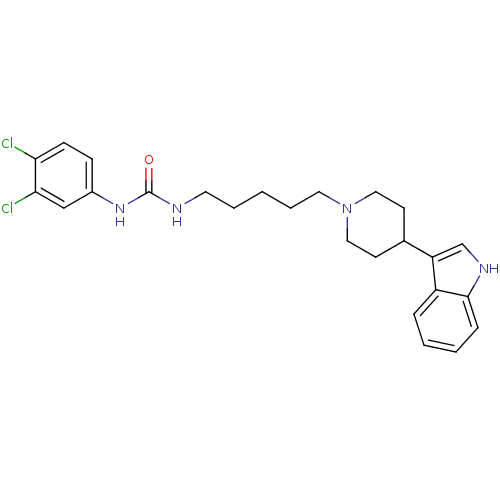 (1-(3,4-Dichloro-phenyl)-3-{5-[4-(1H-indol-3-yl)-pi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50233074 ((E)-N-(5-(4-(1H-indol-3-yl)piperidin-1-yl)pentyl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091453 ((E)-3-(3,4-Dichloro-phenyl)-N-{3-[4-(1H-indol-3-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091446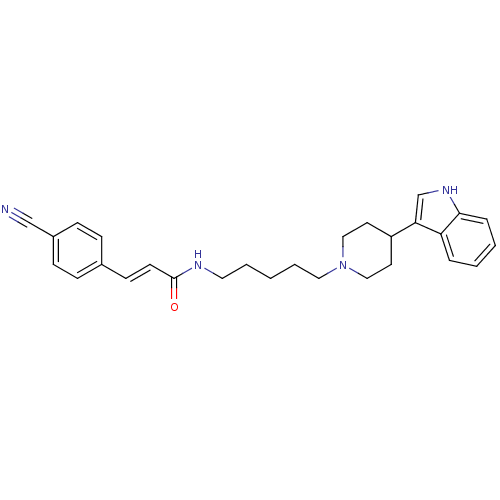 ((E)-3-(4-Cyano-phenyl)-N-{5-[4-(1H-indol-3-yl)-pip...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091466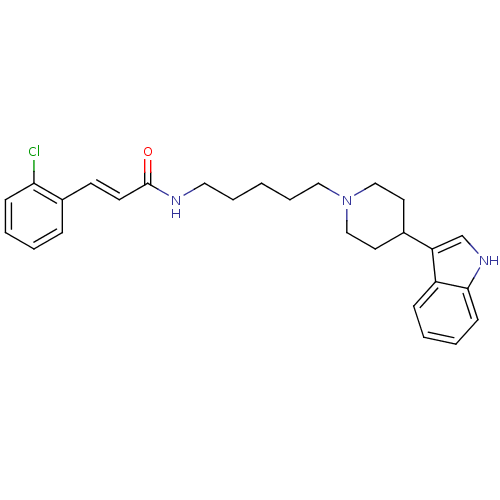 ((E)-3-(2-Chloro-phenyl)-N-{5-[4-(1H-indol-3-yl)-pi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091464 (2-(3,4-Dichloro-phenyl)-N-{5-[4-(1H-indol-3-yl)-pi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091459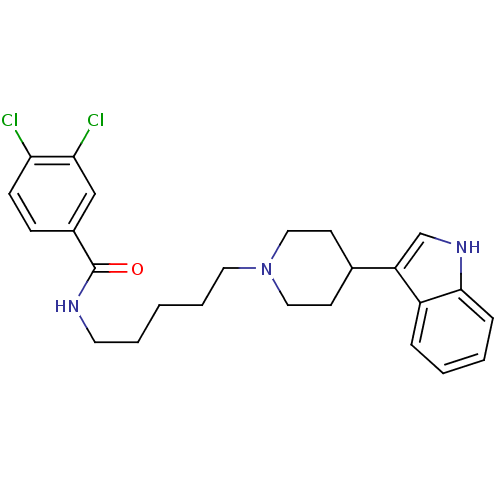 (3,4-Dichloro-N-{5-[4-(1H-indol-3-yl)-piperidin-1-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091444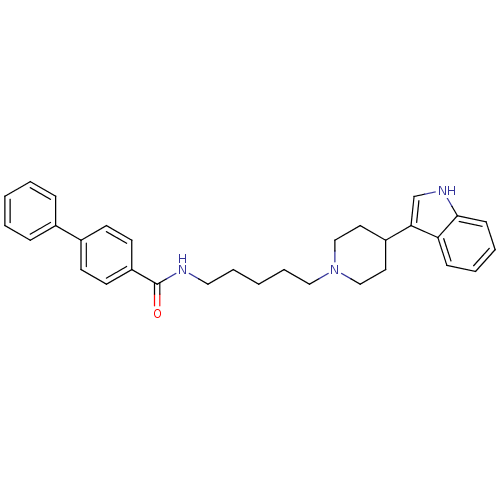 (Biphenyl-4-carboxylic acid {5-[4-(1H-indol-3-yl)-p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091450 (3-(3,4-Dichloro-phenyl)-N-{5-[4-(1H-indol-3-yl)-pi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091455 ((E)-3-(4-Hydroxy-phenyl)-N-{5-[4-(1H-indol-3-yl)-p...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091472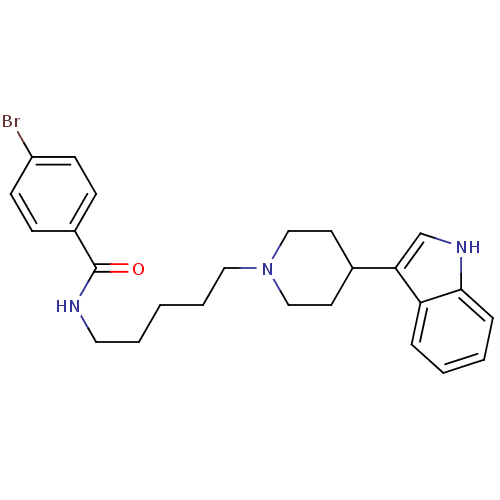 (4-Bromo-N-{5-[4-(1H-indol-3-yl)-piperidin-1-yl]-pe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50091452 ((E)-3-(4-Acetylamino-phenyl)-N-{5-[4-(1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 receptor | Bioorg Med Chem Lett 18: 1450-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.072 BindingDB Entry DOI: 10.7270/Q26T0MC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50198149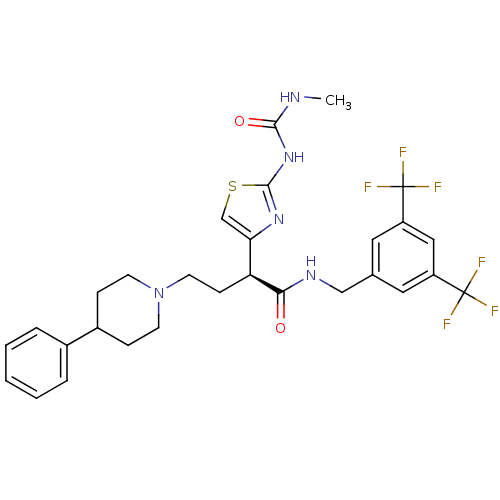 ((S)-1-(4-(1-(3,5-bis(trifluoromethyl)benzylamino)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50198109 ((S)-N-(3,5-bis(trifluoromethyl)benzyl)-2-(2-acetam...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212127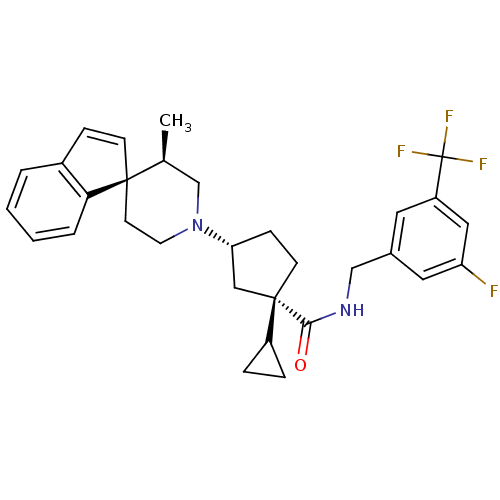 ((1S,3R)-1-cyclopropyl-N-{[3-fluoro-5-(trifluoromet...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50219407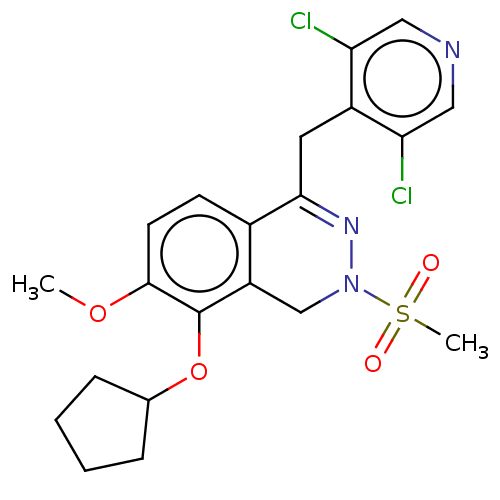 (CHEMBL84350) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description In vitro inhibitory concentration against phosphodiesterase 4. | Bioorg Med Chem Lett 13: 2473-9 (2003) BindingDB Entry DOI: 10.7270/Q24F1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50219407 (CHEMBL84350) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 4. | Bioorg Med Chem Lett 13: 2473-9 (2003) BindingDB Entry DOI: 10.7270/Q24F1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212136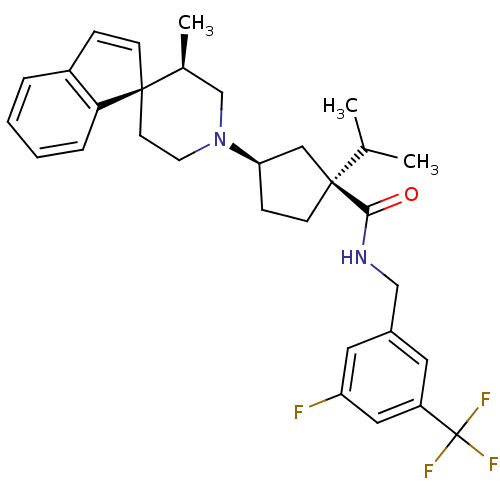 ((1S,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212122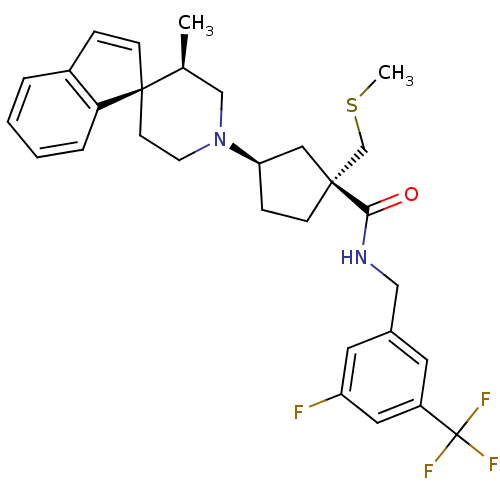 ((1R,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50198123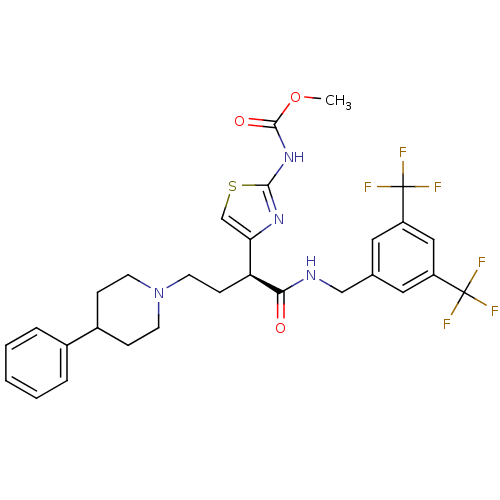 ((S)-methyl 4-(1-(3,5-bis(trifluoromethyl)benzylami...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212128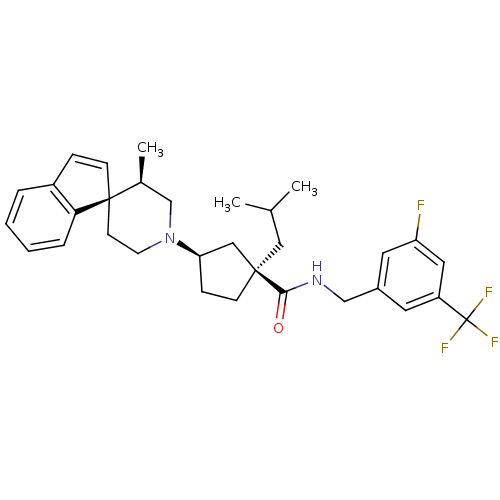 ((1R,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50212140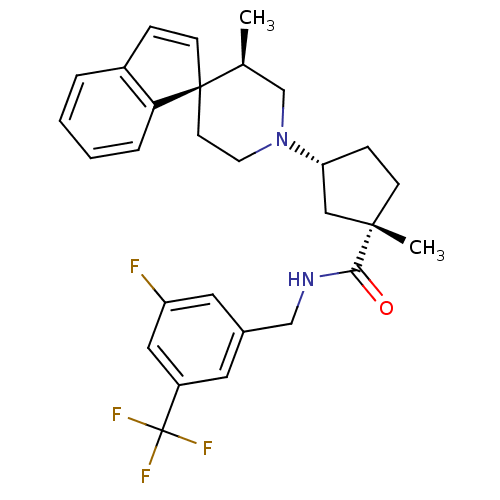 ((1S,3R)-N-{[3-fluoro-5-(trifluoromethyl)phenyl]met...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Antagonist activity at human CCR2b receptor | Bioorg Med Chem Lett 18: 1323-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.023 BindingDB Entry DOI: 10.7270/Q2SN08Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50218578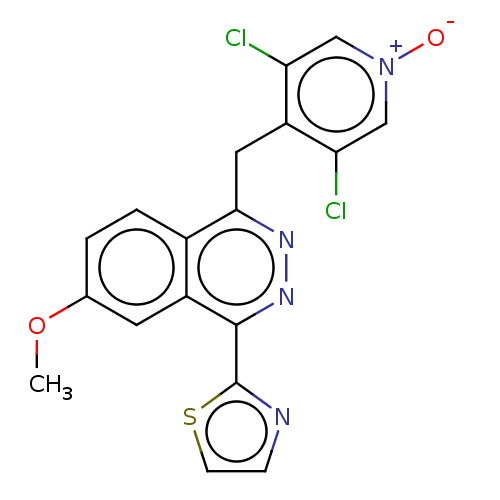 (CHEMBL79902) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description In vitro inhibitory concentration against phosphodiesterase 4. | Bioorg Med Chem Lett 13: 2473-9 (2003) BindingDB Entry DOI: 10.7270/Q24F1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D (Homo sapiens (Human)) | BDBM50218578 (CHEMBL79902) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research Curated by ChEMBL | Assay Description Inhibitory concentration against phosphodiesterase 4. | Bioorg Med Chem Lett 13: 2473-9 (2003) BindingDB Entry DOI: 10.7270/Q24F1SXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM18372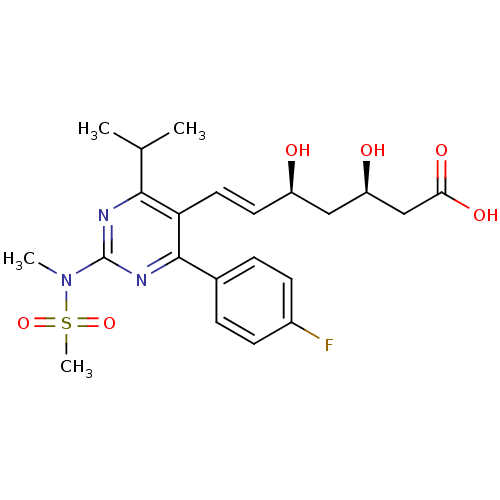 ((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50160770 ((E)-(3R,5R)-7-[4,5-Bis-(4-fluoro-phenyl)-2-isoprop...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM22164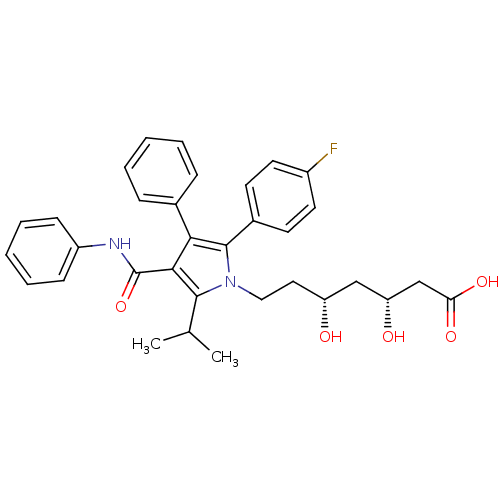 ((3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylca...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50160782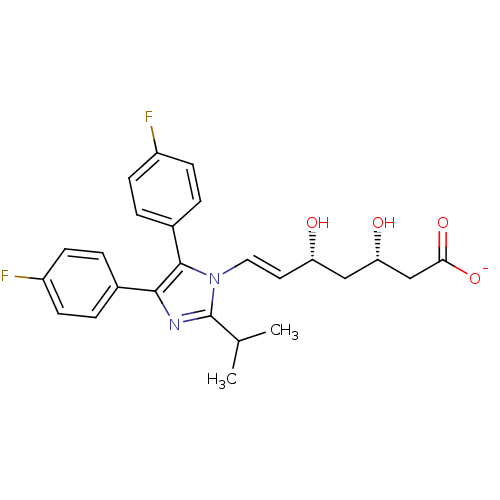 ((E)-(3S,5R)-7-[4,5-Bis-(4-fluoro-phenyl)-2-isoprop...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50042633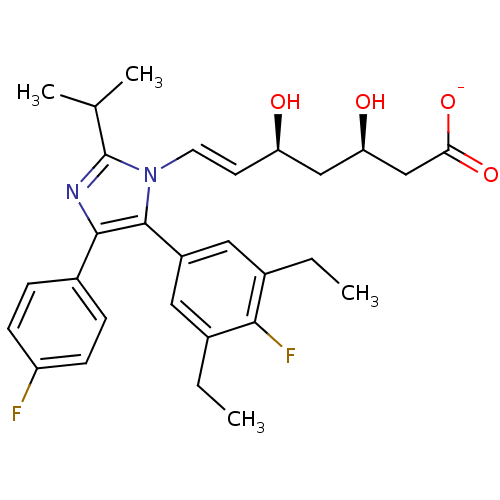 ((E)-(3R,5S)-7-[5-(3,5-Diethyl-4-fluoro-phenyl)-4-(...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50160774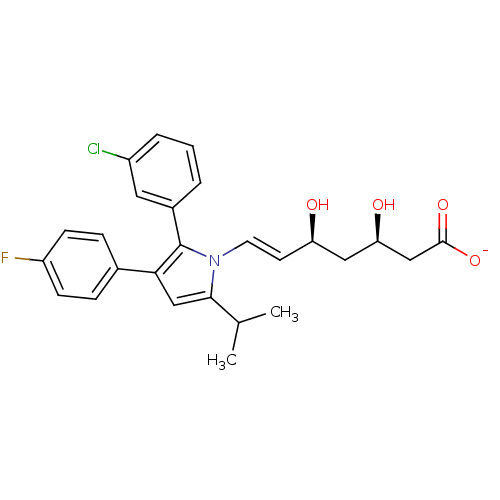 ((E)-(3R,5S)-7-[2-(3-Chloro-phenyl)-3-(4-fluoro-phe...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.15 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50160792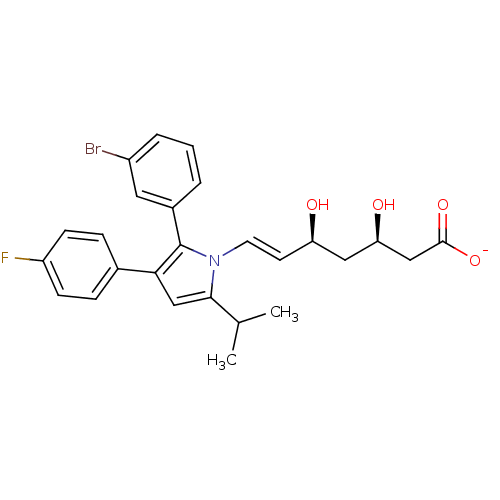 ((E)-(3R,5S)-7-[2-(3-Bromo-phenyl)-3-(4-fluoro-phen...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50042638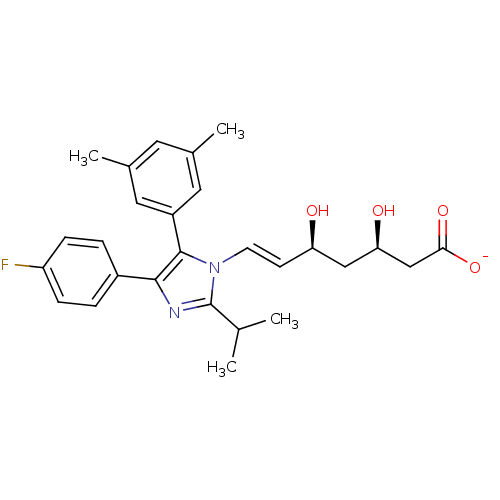 ((E)-(3R,5S)-7-[5-(3,5-Dimethyl-phenyl)-4-(4-fluoro...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.25 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Homo sapiens (Human)) | BDBM50042624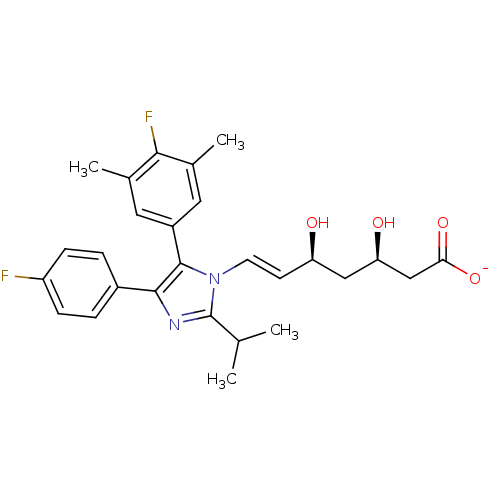 ((E)-(3R,5S)-7-[5-(4-Fluoro-3,5-dimethyl-phenyl)-4-...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductase | Bioorg Med Chem Lett 15: 1027-32 (2005) Article DOI: 10.1016/j.bmcl.2004.12.042 BindingDB Entry DOI: 10.7270/Q2TB17N9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 430 total ) | Next | Last >> |