 Found 335 hits with Last Name = 'sodeoka' and Initial = 'm'
Found 335 hits with Last Name = 'sodeoka' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099066
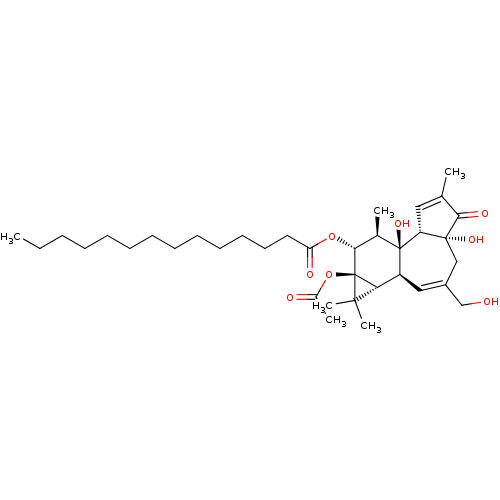
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345024

((R)-(4-(dodecyloxy)-1-(hydroxymethyl)-3-oxo-1,3-di...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(=O)C(C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C32H52O6/c1-8-9-10-11-12-13-14-15-16-17-21-36-25-20-18-19-24-26(25)28(34)38-32(24,22-33)23-37-29(35)27(30(2,3)4)31(5,6)7/h18-20,27,33H,8-17,21-23H2,1-7H3/t32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146558
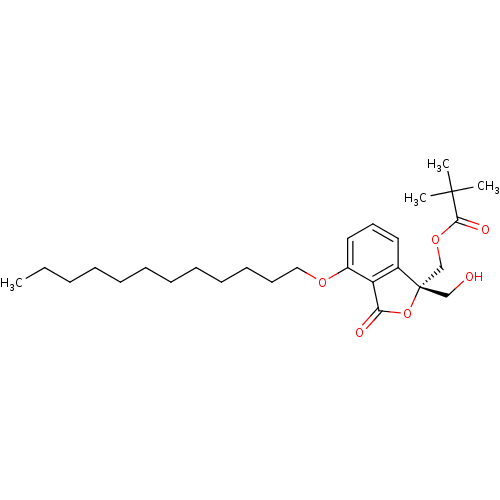
(2,2-Dimethyl-propionic acid (S)-4-dodecyloxy-1-hyd...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(=O)C(C)(C)C Show InChI InChI=1S/C27H42O6/c1-5-6-7-8-9-10-11-12-13-14-18-31-22-17-15-16-21-23(22)24(29)33-27(21,19-28)20-32-25(30)26(2,3)4/h15-17,28H,5-14,18-20H2,1-4H3/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146556

(Acetic acid (R)-4-decyloxy-1-hydroxymethyl-3-oxo-1...)Show SMILES CCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(C)=O Show InChI InChI=1S/C22H32O6/c1-3-4-5-6-7-8-9-10-14-26-19-13-11-12-18-20(19)21(25)28-22(18,15-23)16-27-17(2)24/h11-13,23H,3-10,14-16H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146559

(But-3-enoic acid (R)-1-acetoxymethyl-1-hydroxymeth...)Show SMILES CC(=O)OC[C@@]1(CO)OC(=O)c2c1cccc2OC(=O)CC=C Show InChI InChI=1S/C16H16O7/c1-3-5-13(19)22-12-7-4-6-11-14(12)15(20)23-16(11,8-17)9-21-10(2)18/h3-4,6-7,17H,1,5,8-9H2,2H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146555

(Acetic acid (S)-4-decyloxy-1-hydroxymethyl-3-oxo-1...)Show SMILES CCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(C)=O Show InChI InChI=1S/C22H32O6/c1-3-4-5-6-7-8-9-10-14-26-19-13-11-12-18-20(19)21(25)28-22(18,15-23)16-27-17(2)24/h11-13,23H,3-10,14-16H2,1-2H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146560

(Acetic acid (S)-4-dodecyloxy-1-hydroxymethyl-3-oxo...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(C)=O Show InChI InChI=1S/C24H36O6/c1-3-4-5-6-7-8-9-10-11-12-16-28-21-15-13-14-20-22(21)23(27)30-24(20,17-25)18-29-19(2)26/h13-15,25H,3-12,16-18H2,1-2H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146561
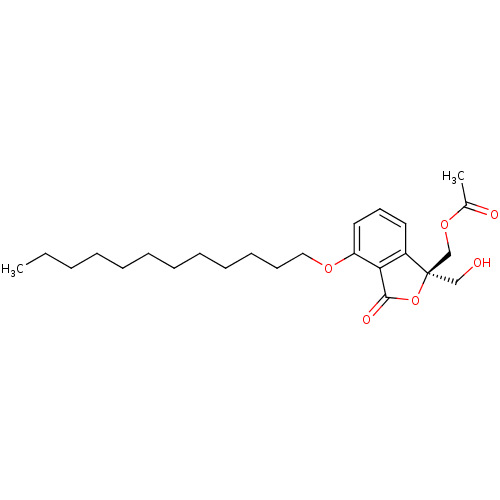
(Acetic acid (R)-4-dodecyloxy-1-hydroxymethyl-3-oxo...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(C)=O Show InChI InChI=1S/C24H36O6/c1-3-4-5-6-7-8-9-10-11-12-16-28-21-15-13-14-20-22(21)23(27)30-24(20,17-25)18-29-19(2)26/h13-15,25H,3-12,16-18H2,1-2H3/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146557
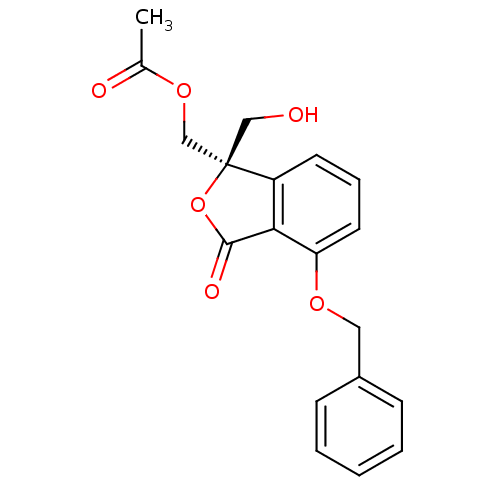
(Acetic acid (R)-4-benzyloxy-1-hydroxymethyl-3-oxo-...)Show SMILES CC(=O)OC[C@@]1(CO)OC(=O)c2c1cccc2OCc1ccccc1 Show InChI InChI=1S/C19H18O6/c1-13(21)24-12-19(11-20)15-8-5-9-16(17(15)18(22)25-19)23-10-14-6-3-2-4-7-14/h2-9,20H,10-12H2,1H3/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146554
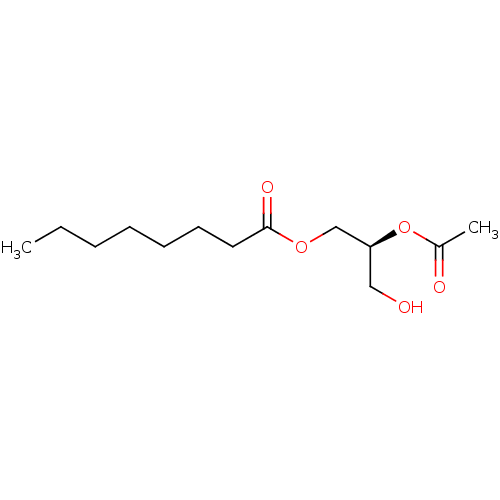
(CHEMBL102042 | Octanoic acid (S)-2-acetoxy-3-hydro...)Show InChI InChI=1S/C13H24O5/c1-3-4-5-6-7-8-13(16)17-10-12(9-14)18-11(2)15/h12,14H,3-10H2,1-2H3/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345027
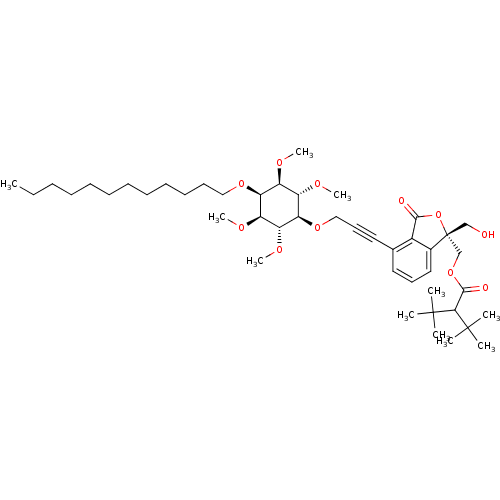
(((R)-4-(3-((2R,3R,5S,6S)-4-(dodecyloxy)-2,3,5,6-te...)Show SMILES CCCCCCCCCCCCO[C@H]1[C@@H](OC)[C@H](OC)[C@@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@H](OC)[C@H]1OC |r| Show InChI InChI=1S/C45H72O11/c1-12-13-14-15-16-17-18-19-20-21-27-53-38-34(49-8)36(51-10)39(37(52-11)35(38)50-9)54-28-23-25-31-24-22-26-32-33(31)41(47)56-45(32,29-46)30-55-42(48)40(43(2,3)4)44(5,6)7/h22,24,26,34-40,46H,12-21,27-30H2,1-11H3/t34-,35+,36-,37+,38-,39+,45-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345026
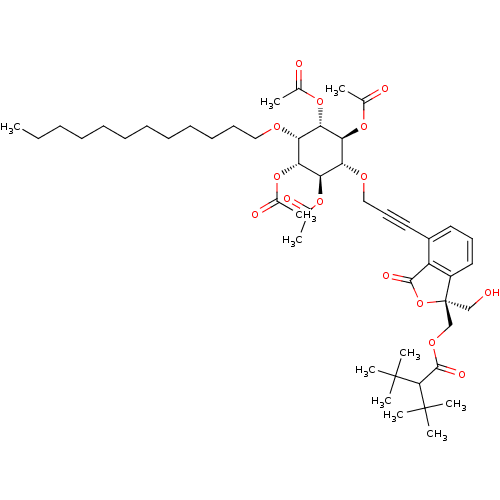
((1R,2R,4S,5S)-3-(3-((R)-1-((2-tert-butyl-3,3-dimet...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C49H72O15/c1-12-13-14-15-16-17-18-19-20-21-27-57-38-40(60-31(2)51)42(62-33(4)53)39(43(63-34(5)54)41(38)61-32(3)52)58-28-23-25-35-24-22-26-36-37(35)45(55)64-49(36,29-50)30-59-46(56)44(47(6,7)8)48(9,10)11/h22,24,26,38-44,50H,12-21,27-30H2,1-11H3/t38-,39+,40+,41-,42+,43-,49-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345025
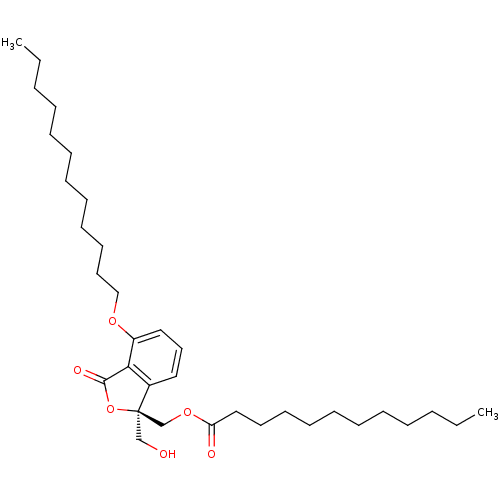
((R)-(4-(dodecyloxy)-1-(hydroxymethyl)-3-oxo-1,3-di...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(=O)CCCCCCCCCCC |r| Show InChI InChI=1S/C34H56O6/c1-3-5-7-9-11-13-15-17-19-21-26-38-30-24-22-23-29-32(30)33(37)40-34(29,27-35)28-39-31(36)25-20-18-16-14-12-10-8-6-4-2/h22-24,35H,3-21,25-28H2,1-2H3/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345030

(((R)-4-(3-((2R,3S,5R,6S)-4-(dodecyloxy)-2,3,5,6-te...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OCc2ccc(cc2)C(C)C)[C@@H](OCc2ccc(cc2)C(C)C)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OCc2ccc(cc2)C(C)C)[C@@H]1OCc1ccc(cc1)C(C)C |r| Show InChI InChI=1S/C81H112O11/c1-16-17-18-19-20-21-22-23-24-25-47-85-70-72(87-49-59-31-39-63(40-32-59)55(2)3)74(89-51-61-35-43-65(44-36-61)57(6)7)71(75(90-52-62-37-45-66(46-38-62)58(8)9)73(70)88-50-60-33-41-64(42-34-60)56(4)5)86-48-27-29-67-28-26-30-68-69(67)77(83)92-81(68,53-82)54-91-78(84)76(79(10,11)12)80(13,14)15/h26,28,30-46,55-58,70-76,82H,16-25,47-54H2,1-15H3/t70-,71+,72+,73-,74+,75-,81-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345028

(((R)-4-(3-((1S,2S,3R,4R,5S,6R)-4-(dodecyloxy)-2,3,...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#8]-[#6@@H]-1-[#6@H](-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@@H](-[#8]-[#6]\[#6]=[#6]\[#6](-[#6])-[#6])-[#6@H](-[#8]-[#6]C#Cc2cccc3c2-[#6](=O)-[#8][C@]3([#6]-[#8])[#6]-[#8]-[#6](=O)-[#6](C([#6])([#6])[#6])C([#6])([#6])[#6])-[#6@@H](-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@@H]-1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C62H98O11/c1-16-17-18-19-20-21-22-23-24-25-36-66-53-54(69-39-33-45(4)5)51(67-37-27-29-44(2)3)52(55(70-40-34-46(6)7)56(53)71-41-35-47(8)9)68-38-28-31-48-30-26-32-49-50(48)58(64)73-62(49,42-63)43-72-59(65)57(60(10,11)12)61(13,14)15/h26-27,29-30,32-35,44,51-57,63H,16-25,36-43H2,1-15H3/b29-27+/t51-,52-,53+,54+,55+,56+,62+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345031
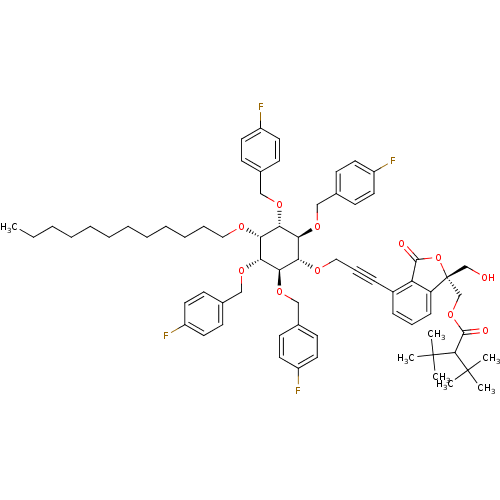
(((R)-4-(3-((2R,3S,5R,6S)-4-(dodecyloxy)-2,3,5,6-te...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OCc2ccc(F)cc2)[C@@H](OCc2ccc(F)cc2)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OCc2ccc(F)cc2)[C@@H]1OCc1ccc(F)cc1 |r| Show InChI InChI=1S/C69H84F4O11/c1-8-9-10-11-12-13-14-15-16-17-39-77-58-60(79-41-47-23-31-52(70)32-24-47)62(81-43-49-27-35-54(72)36-28-49)59(63(82-44-50-29-37-55(73)38-30-50)61(58)80-42-48-25-33-53(71)34-26-48)78-40-19-21-51-20-18-22-56-57(51)65(75)84-69(56,45-74)46-83-66(76)64(67(2,3)4)68(5,6)7/h18,20,22-38,58-64,74H,8-17,39-46H2,1-7H3/t58-,59+,60+,61-,62+,63-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345029

(((R)-1-(hydroxymethyl)-3-oxo-4-(3-((2R,3S,5R,6S)-2...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C69H88O11/c1-8-9-10-11-12-13-14-15-16-29-43-73-58-60(75-45-51-32-21-17-22-33-51)62(77-47-53-36-25-19-26-37-53)59(63(78-48-54-38-27-20-28-39-54)61(58)76-46-52-34-23-18-24-35-52)74-44-31-41-55-40-30-42-56-57(55)65(71)80-69(56,49-70)50-79-66(72)64(67(2,3)4)68(5,6)7/h17-28,30,32-40,42,58-64,70H,8-16,29,43-50H2,1-7H3/t58-,59+,60+,61-,62+,63-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Influenza A virus A/PR/8/34(H1N1) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human influenza A virus A/Aichi/2/1968(H3N2) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human influenza A virus A/Aichi/2/1968(H3N2) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217841
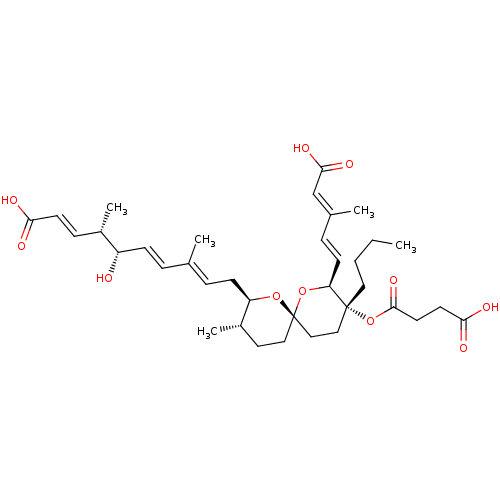
(REVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H52O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.46 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Influenza A virus A/PR/8/34(H1N1) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479281
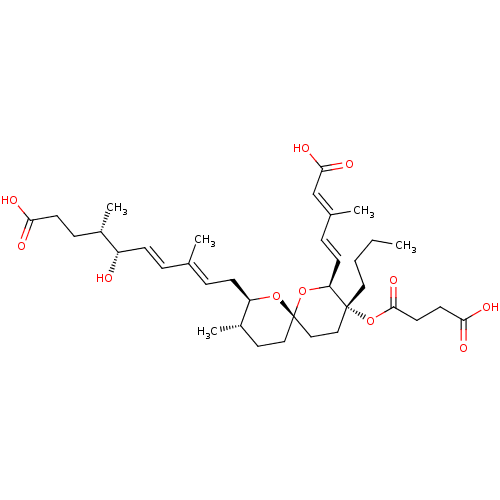
(2,3-DIHYDROREVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)CCC(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H54O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-10,12,14,23,26-30,37H,6-7,11,13,15-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479274

(4-HYDROXY REVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](O)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C35H50O12/c1-5-6-18-34(47-33(44)16-15-31(40)41)20-21-35(46-29(34)13-9-24(3)22-32(42)43)19-17-25(4)28(45-35)12-8-23(2)7-10-26(36)27(37)11-14-30(38)39/h7-11,13-14,22,25-29,36-37H,5-6,12,15-21H2,1-4H3,(H,38,39)(H,40,41)(H,42,43)/b10-7+,13-9+,14-11+,23-8+,24-22+/t25-,26-,27-,28+,29-,34+,35-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479284

(CHEMBL442945)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)CO)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C34H52O10/c1-6-7-17-33(44-32(41)15-14-30(37)38)19-20-34(43-29(33)13-10-24(3)21-31(39)40)18-16-25(4)28(42-34)12-9-23(2)8-11-27(36)26(5)22-35/h8-11,13,21,25-29,35-36H,6-7,12,14-20,22H2,1-5H3,(H,37,38)(H,39,40)/b11-8+,13-10+,23-9+,24-21+/t25-,26-,27-,28+,29-,33+,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36.9 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479277

(2,3-DIHYDRO-5-EPIREVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@@H](O)[C@@H](C)CCC(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H54O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-10,12,14,23,26-30,37H,6-7,11,13,15-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,24-9+,25-23+/t26-,27-,28+,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217935
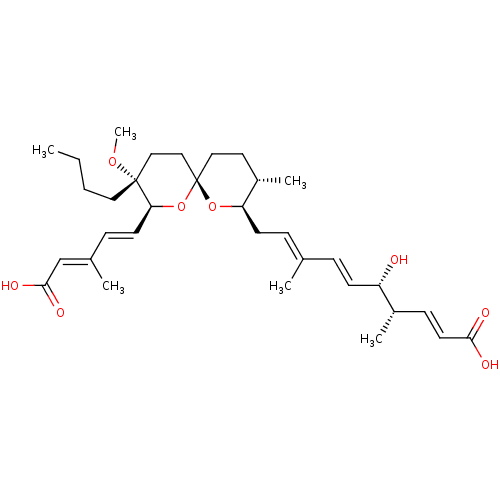
(CHEMBL114859)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C33H50O8/c1-7-8-18-32(39-6)20-21-33(41-29(32)15-11-24(3)22-31(37)38)19-17-26(5)28(40-33)14-10-23(2)9-13-27(34)25(4)12-16-30(35)36/h9-13,15-16,22,25-29,34H,7-8,14,17-21H2,1-6H3,(H,35,36)(H,37,38)/b13-9+,15-11+,16-12+,23-10+,24-22+/t25-,26-,27-,28+,29-,32+,33-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217844
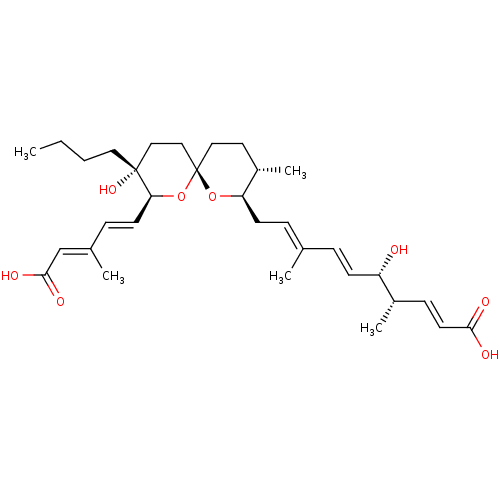
(CHEMBL115953)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@](O)(CCCC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C32H48O8/c1-6-7-17-31(38)19-20-32(40-28(31)14-10-23(3)21-30(36)37)18-16-25(5)27(39-32)13-9-22(2)8-12-26(33)24(4)11-15-29(34)35/h8-12,14-15,21,24-28,33,38H,6-7,13,16-20H2,1-5H3,(H,34,35)(H,36,37)/b12-8+,14-10+,15-11+,22-9+,23-21+/t24-,25-,26-,27+,28-,31+,32-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 168 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479282

(CHEMBL505238)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(=O)OC)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-35(44)18-16-32(39)40)22-23-37(47-31(36)15-11-26(3)24-33(41)42)21-19-28(5)30(46-37)14-10-25(2)9-13-29(38)27(4)12-17-34(43)45-6/h9-13,15,17,24,27-31,38H,7-8,14,16,18-23H2,1-6H3,(H,39,40)(H,41,42)/b13-9+,15-11+,17-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104684

((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...)Show SMILES CCCCCCCCCCCCCCCC(=O)c1c(O)oc(CC(=O)Oc2cccc(c2)C(=O)c2ccccc2)c1O Show InChI InChI=1S/C35H44O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-23-29(36)32-34(39)30(42-35(32)40)25-31(37)41-28-22-18-21-27(24-28)33(38)26-19-15-14-16-20-26/h14-16,18-22,24,39-40H,2-13,17,23,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104691
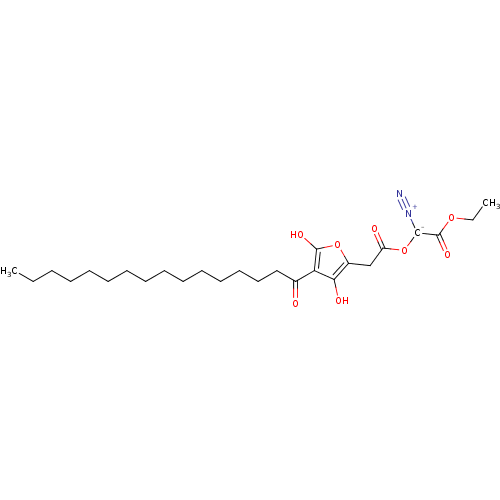
(CHEMBL423508 | Diazo-[2-(4-hexadecanoyl-3-hydroxy-...)Show SMILES CCCCCCCCCCCCCCCC(=O)c1c(O)oc(CC(=O)O[C-]([N+]#N)C(=O)OCC)c1O Show InChI InChI=1S/C26H40N2O8/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-19(29)22-23(31)20(35-25(22)32)18-21(30)36-24(28-27)26(33)34-4-2/h31-32H,3-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479276
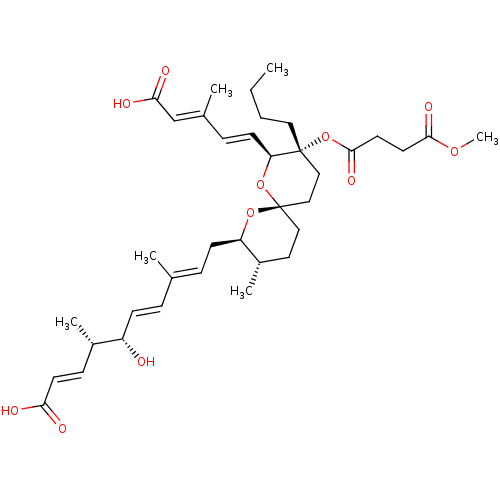
(CHEMBL461144)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(=O)OC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-35(44)18-17-34(43)45-6)22-23-37(47-31(36)15-11-26(3)24-33(41)42)21-19-28(5)30(46-37)14-10-25(2)9-13-29(38)27(4)12-16-32(39)40/h9-13,15-16,24,27-31,38H,7-8,14,17-23H2,1-6H3,(H,39,40)(H,41,42)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104690

((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...)Show SMILES CCCCCCCCCCCCCCCC(=O)c1c(O)oc(CC(=O)Oc2ccc(cc2)C(=O)c2ccccc2)c1O Show InChI InChI=1S/C35H44O7/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-29(36)32-34(39)30(42-35(32)40)25-31(37)41-28-23-21-27(22-24-28)33(38)26-18-15-14-16-19-26/h14-16,18-19,21-24,39-40H,2-13,17,20,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217849
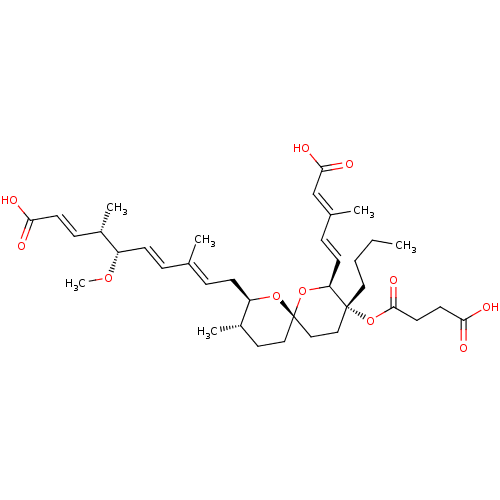
(CHEMBL115317)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](OC)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-35(44)18-17-33(40)41)22-23-37(47-31(36)15-11-26(3)24-34(42)43)21-19-28(5)30(46-37)14-10-25(2)9-13-29(45-6)27(4)12-16-32(38)39/h9-13,15-16,24,27-31H,7-8,14,17-23H2,1-6H3,(H,38,39)(H,40,41)(H,42,43)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479275
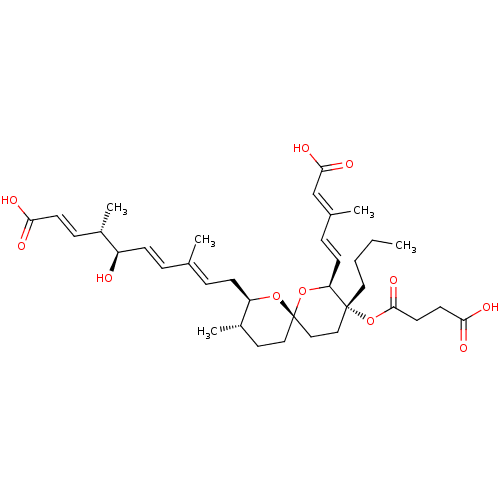
(5-EPIREVEROMYCIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C36H52O11/c1-6-7-19-35(47-34(44)17-16-32(40)41)21-22-36(46-30(35)14-10-25(3)23-33(42)43)20-18-27(5)29(45-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28+,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 572 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104674
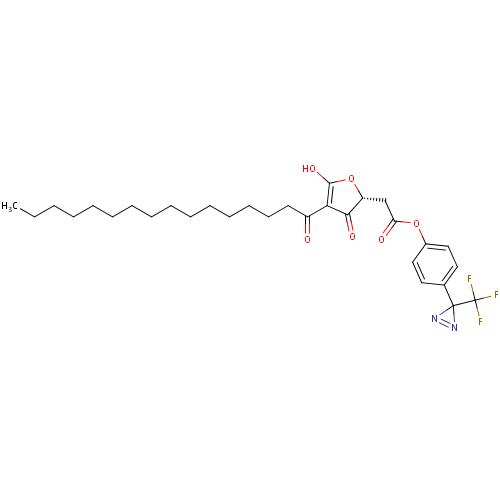
((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(O)O[C@H](CC(=O)Oc2ccc(cc2)C2(N=N2)C(F)(F)F)C1=O |c:17,34| Show InChI InChI=1S/C30H39F3N2O6/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-23(36)26-27(38)24(41-28(26)39)20-25(37)40-22-18-16-21(17-19-22)29(34-35-29)30(31,32)33/h16-19,24,39H,2-15,20H2,1H3/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388826

(CHEMBL2062715)Show SMILES Oc1c(Cl)c(N=CCN2CCCCC2)c(O)c2ncccc12 |w:5.4| Show InChI InChI=1S/C16H18ClN3O2/c17-12-14(19-7-10-20-8-2-1-3-9-20)16(22)13-11(15(12)21)5-4-6-18-13/h4-7,21-22H,1-3,8-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B in presence of 2 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50388826

(CHEMBL2062715)Show SMILES Oc1c(Cl)c(N=CCN2CCCCC2)c(O)c2ncccc12 |w:5.4| Show InChI InChI=1S/C16H18ClN3O2/c17-12-14(19-7-10-20-8-2-1-3-9-20)16(22)13-11(15(12)21)5-4-6-18-13/h4-7,21-22H,1-3,8-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25A in presence of 2 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217839
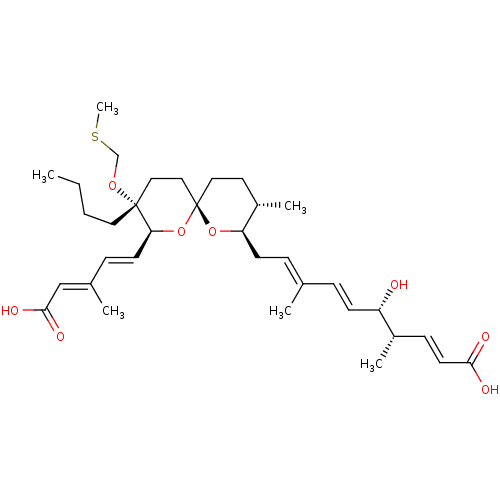
(CHEMBL327131)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OCSC)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C34H52O8S/c1-7-8-18-33(40-23-43-6)20-21-34(42-30(33)15-11-25(3)22-32(38)39)19-17-27(5)29(41-34)14-10-24(2)9-13-28(35)26(4)12-16-31(36)37/h9-13,15-16,22,26-30,35H,7-8,14,17-21,23H2,1-6H3,(H,36,37)(H,38,39)/b13-9+,15-11+,16-12+,24-10+,25-22+/t26-,27-,28-,29+,30-,33+,34-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 801 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104693

(4-Hydroxy-5-hydroxymethyl-3-octadec-9-enoyl-5H-fur...)Show InChI InChI=1S/C23H38O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19(25)21-22(26)20(18-24)28-23(21)27/h9-10,24,26-27H,2-8,11-18H2,1H3/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479273

(CHEMBL498825)Show SMILES [H][C@]1(C\C=C(/C)\C=C\CO)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C31H46O9/c1-5-6-16-30(40-29(37)14-13-27(33)34)18-19-31(39-26(30)12-10-23(3)21-28(35)36)17-15-24(4)25(38-31)11-9-22(2)8-7-20-32/h7-10,12,21,24-26,32H,5-6,11,13-20H2,1-4H3,(H,33,34)(H,35,36)/b8-7+,12-10+,22-9+,23-21+/t24-,25+,26-,30+,31-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 995 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104677

(3-Hexadecanoyl-4-hydroxy-5-methylene-5H-furan-2-on...)Show InChI InChI=1S/C21H34O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18(22)19-20(23)17(2)25-21(19)24/h19H,2-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479280
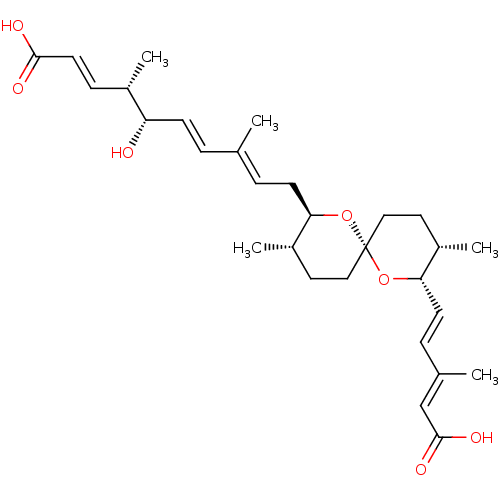
(SPIROFUNGIN A)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@H](C)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C29H42O7/c1-19(6-10-24(30)21(3)9-13-27(31)32)7-11-25-22(4)14-16-29(35-25)17-15-23(5)26(36-29)12-8-20(2)18-28(33)34/h6-10,12-13,18,21-26,30H,11,14-17H2,1-5H3,(H,31,32)(H,33,34)/b10-6+,12-8+,13-9+,19-7+,20-18+/t21-,22-,23-,24-,25+,26-,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104676

((4-Hexadecanoyl-3-hydroxy-5-oxo-2,5-dihydro-furan-...)Show SMILES CCCCCCCCCCCCCCCC(=O)c1c(O)oc(CC(=O)OCCCCCCCCC)c1O Show InChI InChI=1S/C31H54O6/c1-3-5-7-9-11-12-13-14-15-16-17-19-21-23-26(32)29-30(34)27(37-31(29)35)25-28(33)36-24-22-20-18-10-8-6-4-2/h34-35H,3-25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479272

(CHEMBL455272)Show SMILES [H][C@]1([#6]\[#6]=[#6](/[#6])\[#6]=[#6]\[#6@H](-[#8][Si;v4]([#6])([#6])C([#6])([#6])[#6])-[#6@@H](-[#6])\[#6]=[#6]\[#6](-[#8])=O)[#8][C@]2([#6]-[#6]-[#6@@H]1-[#6])[#6]-[#6][C@@]([#6]-[#6]-[#6]-[#6])([#8]-[#6](=O)-[#6]-[#6]-[#6](-[#8])=O)[C@@]([H])([#8]2)\[#6]=[#6]\[#6](\[#6])=[#6]\[#6](-[#8])=O |r| Show InChI InChI=1S/C42H66O11Si/c1-11-12-24-41(52-39(49)22-21-37(45)46)26-27-42(51-35(41)19-15-30(3)28-38(47)48)25-23-32(5)33(50-42)17-13-29(2)14-18-34(31(4)16-20-36(43)44)53-54(9,10)40(6,7)8/h13-16,18-20,28,31-35H,11-12,17,21-27H2,1-10H3,(H,43,44)(H,45,46)(H,47,48)/b18-14+,19-15+,20-16+,29-13+,30-28+/t31-,32-,33+,34-,35-,41+,42-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Mus musculus) | BDBM50104681

(CHEMBL109357 | Methanesulfonic acid 4-hexadecanoyl...)Show SMILES CCCCCCCCCCCCCCCC(=O)c1c(O)oc(COS(C)(=O)=O)c1O Show InChI InChI=1S/C22H38O7S/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18(23)20-21(24)19(29-22(20)25)17-28-30(2,26)27/h24-25H,3-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25B |
J Med Chem 44: 3216-22 (2001)
BindingDB Entry DOI: 10.7270/Q2WD3ZVR |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217937
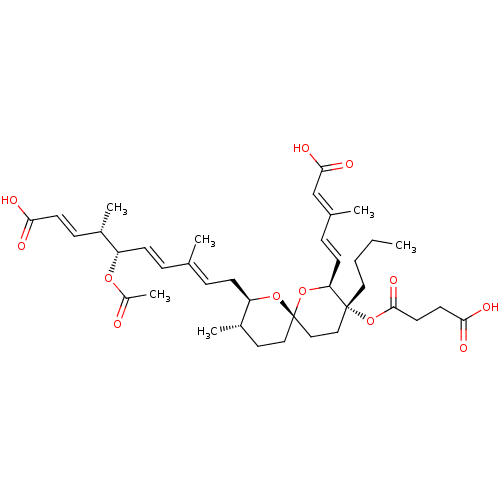
(CHEMBL114949)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](OC(C)=O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(O)=O |r| Show InChI InChI=1S/C38H54O12/c1-7-8-20-37(50-36(46)18-17-34(42)43)22-23-38(49-32(37)15-11-26(3)24-35(44)45)21-19-28(5)31(48-38)14-10-25(2)9-13-30(47-29(6)39)27(4)12-16-33(40)41/h9-13,15-16,24,27-28,30-32H,7-8,14,17-23H2,1-6H3,(H,40,41)(H,42,43)(H,44,45)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,30-,31+,32-,37+,38-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388822
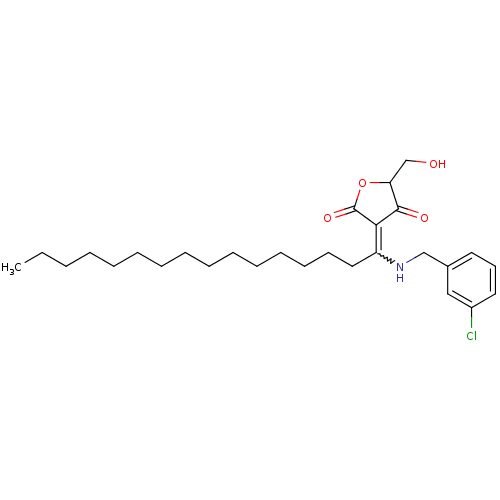
(CHEMBL2062592)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(Cl)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42ClNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-15-17-23(29)19-22/h15-17,19,25,30-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50479279

(CHEMBL510665)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@]2(CC[C@@H]1C)CC[C@@](CCCC)(OC(=O)CCC(O)=O)[C@@]([H])(O2)\C=C\C(\C)=C\C(=O)OC |r| Show InChI InChI=1S/C37H54O11/c1-7-8-20-36(48-34(43)18-17-33(41)42)22-23-37(47-31(36)15-11-26(3)24-35(44)45-6)21-19-28(5)30(46-37)14-10-25(2)9-13-29(38)27(4)12-16-32(39)40/h9-13,15-16,24,27-31,38H,7-8,14,17-23H2,1-6H3,(H,39,40)(H,41,42)/b13-9+,15-11+,16-12+,25-10+,26-24+/t27-,28-,29-,30+,31-,36+,37-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |
Isoleucine--tRNA ligase, cytoplasmic
(Homo sapiens (Human)) | BDBM50217842
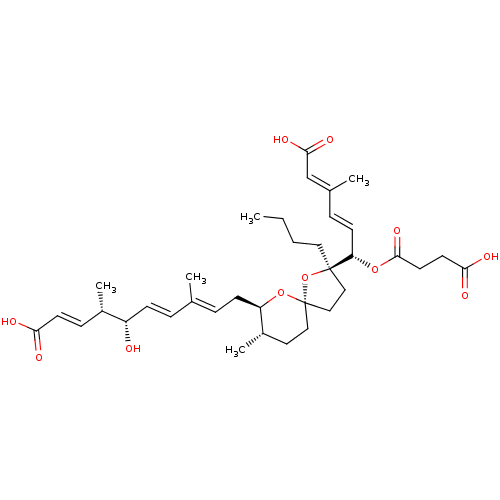
(REVEROMYCIN B)Show SMILES [H][C@]1(C\C=C(/C)\C=C\[C@H](O)[C@@H](C)\C=C\C(O)=O)O[C@@]2(CC[C@@](CCCC)(O2)[C@@H](OC(=O)CCC(O)=O)\C=C\C(\C)=C\C(O)=O)CC[C@@H]1C |r| Show InChI InChI=1S/C36H52O11/c1-6-7-19-35(30(14-10-25(3)23-33(42)43)45-34(44)17-16-32(40)41)21-22-36(47-35)20-18-27(5)29(46-36)13-9-24(2)8-12-28(37)26(4)11-15-31(38)39/h8-12,14-15,23,26-30,37H,6-7,13,16-22H2,1-5H3,(H,38,39)(H,40,41)(H,42,43)/b12-8+,14-10+,15-11+,24-9+,25-23+/t26-,27-,28-,29+,30-,35+,36-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN (Institute of Physical and Chemical Research)
Curated by ChEMBL
| Assay Description
Inhibition of IleRS |
Bioorg Med Chem Lett 18: 3756-60 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.054
BindingDB Entry DOI: 10.7270/Q2BK1G6F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data