 Found 166 hits with Last Name = 'soglia' and Initial = 'jr'
Found 166 hits with Last Name = 'soglia' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190788
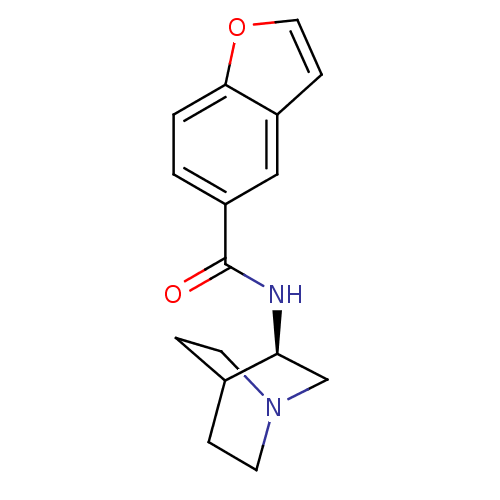
(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190785
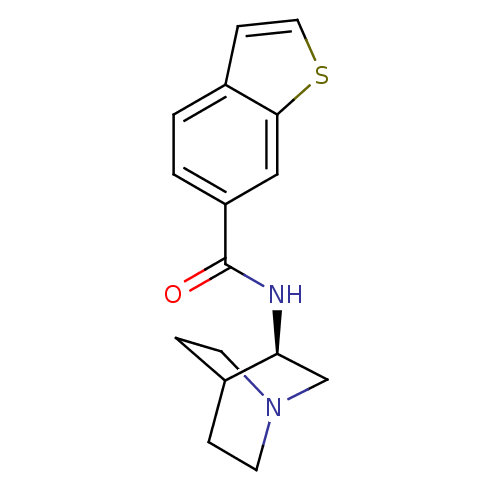
(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190793
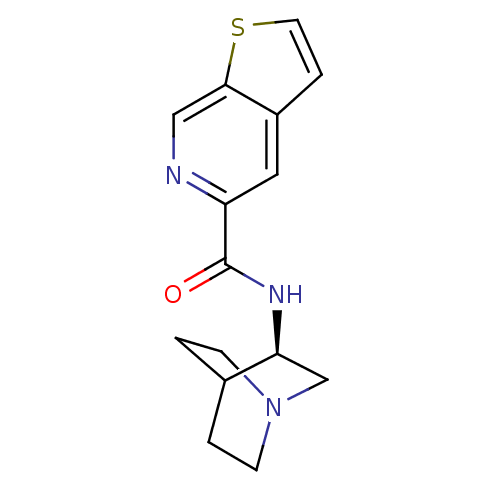
(CHEMBL268939 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2sccc2cn1 |wU:3.2,(26.77,-30.5,;26.77,-32.04,;28.11,-32.81,;29.44,-32.03,;29.43,-30.5,;30.77,-29.72,;32.11,-30.49,;32.11,-32.03,;30.78,-32.8,;29.9,-31.66,;30.72,-30.86,;25.44,-32.82,;24.1,-32.06,;22.78,-32.83,;21.31,-32.36,;20.4,-33.6,;21.31,-34.85,;22.77,-34.37,;24.1,-35.14,;25.45,-34.37,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190791
(CHEMBL210865 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccsc2cn1 |wU:3.2,(11.37,-32.25,;11.38,-33.79,;12.72,-34.55,;14.05,-33.78,;14.04,-32.24,;15.38,-31.47,;16.72,-32.24,;16.71,-33.78,;15.39,-34.55,;14.51,-33.4,;15.33,-32.61,;10.05,-34.57,;8.71,-33.8,;7.38,-34.58,;5.92,-34.1,;5.01,-35.35,;5.92,-36.59,;7.38,-36.12,;8.71,-36.89,;10.06,-36.12,)| Show InChI InChI=1S/C15H17N3OS/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190789
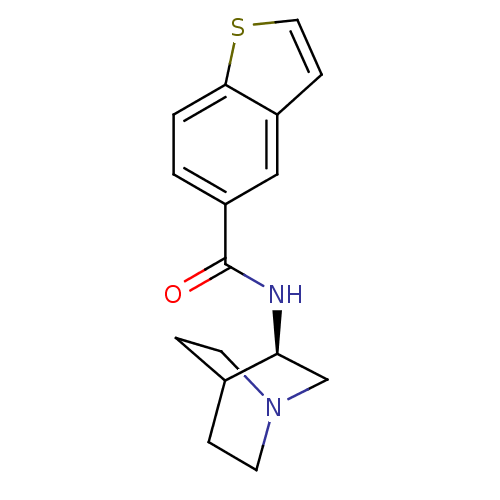
(CHEMBL208565 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2sccc2c1 |wU:3.2,(11.98,-5.28,;11.99,-6.82,;13.33,-7.58,;14.66,-6.8,;14.65,-5.27,;15.99,-4.5,;17.32,-5.26,;17.32,-6.8,;16,-7.58,;15.12,-6.43,;15.94,-5.63,;10.66,-7.59,;10.66,-9.15,;9.32,-9.92,;7.99,-9.15,;6.52,-9.62,;5.62,-8.37,;6.53,-7.13,;7.99,-7.61,;9.32,-6.83,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190786
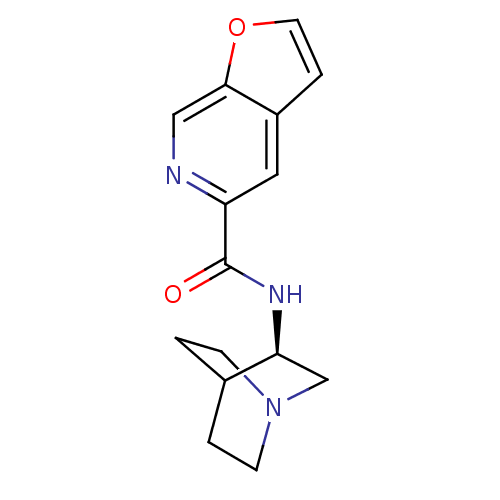
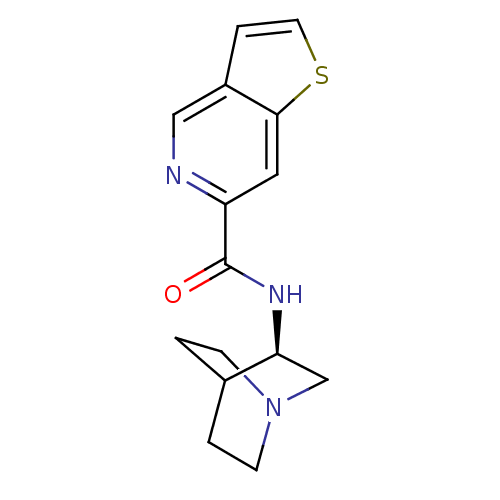
((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190783
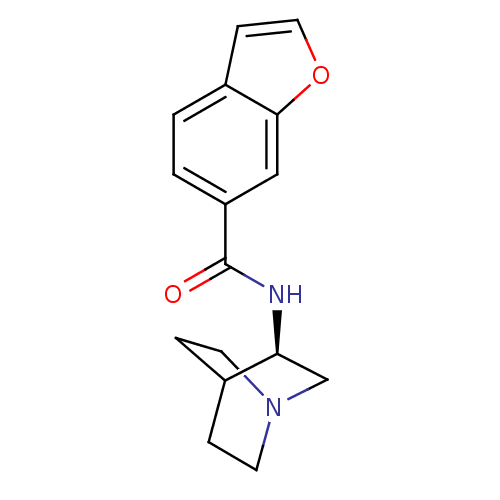
(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190784
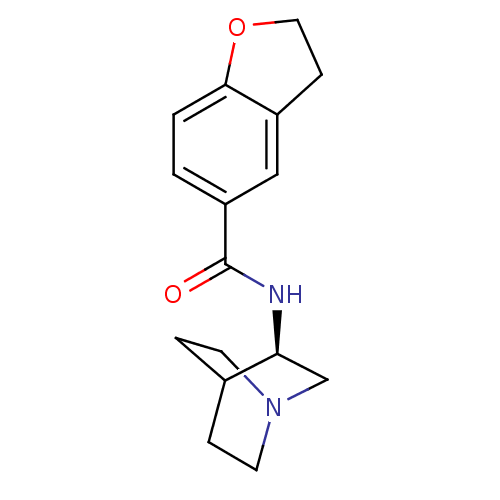
(CHEMBL378496 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2OCCc2c1 |wU:3.2,(11.67,-14.82,;11.67,-16.36,;13.01,-17.12,;14.34,-16.35,;14.33,-14.81,;15.67,-14.04,;17.01,-14.81,;17.01,-16.35,;15.68,-17.12,;14.8,-15.97,;15.62,-15.18,;10.34,-17.14,;10.35,-18.69,;9,-19.46,;7.67,-18.69,;6.21,-19.16,;5.3,-17.92,;6.21,-16.67,;7.68,-17.15,;9,-16.37,)| Show InChI InChI=1S/C16H20N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,9,11,14H,3-8,10H2,(H,17,19)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50161764
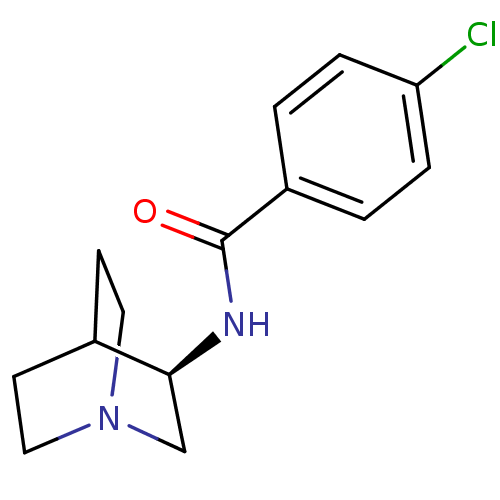
((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190790
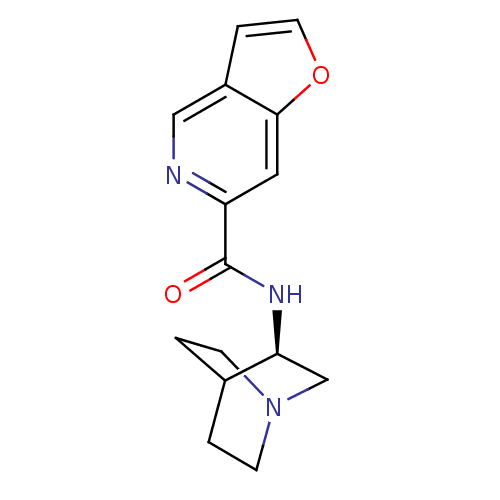
(CHEMBL214195 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2occc2cn1 |wU:3.2,(27.35,-21.97,;27.36,-23.51,;28.69,-24.27,;30.02,-23.5,;30.01,-21.96,;31.35,-21.19,;32.69,-21.96,;32.69,-23.5,;31.36,-24.27,;30.48,-23.12,;31.3,-22.32,;26.02,-24.29,;24.68,-23.52,;23.36,-24.3,;21.89,-23.82,;20.99,-25.07,;21.89,-26.31,;23.36,-25.84,;24.69,-26.61,;26.03,-25.84,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-14-11(8-16-12)3-6-20-14)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50190794
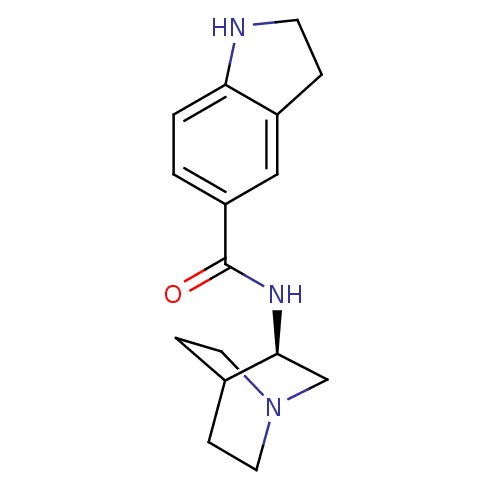
(CHEMBL211572 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2NCCc2c1 |wU:3.2,(-3.98,-5.4,;-3.98,-6.95,;-2.64,-7.71,;-1.31,-6.93,;-1.32,-5.4,;.02,-4.62,;1.36,-5.39,;1.36,-6.93,;.03,-7.7,;-.85,-6.56,;-.03,-5.76,;-5.31,-7.72,;-5.3,-9.27,;-6.64,-10.05,;-7.97,-9.28,;-9.44,-9.75,;-10.34,-8.5,;-9.44,-7.26,;-7.97,-7.74,;-6.65,-6.96,)| Show InChI InChI=1S/C16H21N3O/c20-16(13-1-2-14-12(9-13)3-6-17-14)18-15-10-19-7-4-11(15)5-8-19/h1-2,9,11,15,17H,3-8,10H2,(H,18,20)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]MLA from alpha7 nAChR in Sprague-Dawley rat brain homogenates |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190785

(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190785

(CHEMBL378349 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccsc2c1 |wU:3.2,(28.02,-3.67,;28.02,-5.21,;29.36,-5.97,;30.69,-5.19,;30.68,-3.66,;32.02,-2.89,;33.36,-3.65,;33.36,-5.2,;32.03,-5.97,;31.15,-4.82,;31.97,-4.02,;26.69,-5.98,;26.7,-7.54,;25.35,-8.31,;24.02,-7.54,;22.56,-8.01,;21.65,-6.76,;22.56,-5.52,;24.03,-6,;25.35,-5.22,)| Show InChI InChI=1S/C16H18N2OS/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190783

(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 511 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190788

(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190786

((R)-N-(quinuclidin-3-yl)furo[2,3-c]pyridine-5-carb...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1cc2ccoc2cn1 |wU:3.2,(-5.16,-23.46,;-5.16,-25,;-3.82,-25.76,;-2.49,-24.99,;-2.5,-23.45,;-1.16,-22.68,;.18,-23.45,;.18,-24.99,;-1.15,-25.76,;-2.03,-24.61,;-1.21,-23.81,;-6.49,-25.78,;-7.83,-25.01,;-9.15,-25.79,;-10.62,-25.31,;-11.52,-26.55,;-10.62,-27.8,;-9.16,-27.33,;-7.82,-28.1,;-6.48,-27.33,)| Show InChI InChI=1S/C15H17N3O2/c19-15(12-7-11-3-6-20-14(11)8-16-12)17-13-9-18-4-1-10(13)2-5-18/h3,6-8,10,13H,1-2,4-5,9H2,(H,17,19)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 628 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190788

(CHEMBL378471 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2occc2c1 |wU:3.2,(-3.91,-15.15,;-3.91,-16.69,;-2.57,-17.45,;-1.24,-16.67,;-1.25,-15.14,;.09,-14.37,;1.43,-15.13,;1.43,-16.67,;.1,-17.45,;-.78,-16.3,;.04,-15.5,;-5.24,-17.46,;-5.23,-19.02,;-6.57,-19.79,;-7.91,-19.02,;-9.37,-19.49,;-10.28,-18.24,;-9.37,-17,;-7.9,-17.48,;-6.58,-16.7,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-1-2-15-12(9-13)5-8-20-15)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 663 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50190783

(CHEMBL379302 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...)Show SMILES O=C(N[C@H]1CN2CCC1CC2)c1ccc2ccoc2c1 |wU:3.2,(27.6,-12.86,;27.61,-14.4,;28.95,-15.16,;30.28,-14.39,;30.27,-12.85,;31.61,-12.08,;32.95,-12.85,;32.94,-14.39,;31.62,-15.16,;30.74,-14.01,;31.56,-13.22,;26.28,-15.18,;26.29,-16.73,;24.94,-17.5,;23.61,-16.73,;22.15,-17.2,;21.24,-15.96,;22.15,-14.71,;23.61,-15.19,;24.94,-14.41,)| Show InChI InChI=1S/C16H18N2O2/c19-16(13-2-1-12-5-8-20-15(12)9-13)17-14-10-18-6-3-11(14)4-7-18/h1-2,5,8-9,11,14H,3-4,6-7,10H2,(H,17,19)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT3 receptor |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B/3C/3D/3E
(Homo sapiens (Human)) | BDBM50161764

((R)-4-chloro-N-(quinuclidin-3-yl)benzamide | (R)-4...)Show SMILES Clc1ccc(cc1)C(=O)N[C@H]1CN2CCC1CC2 |r,wU:10.10,TLB:9:10:14.13:16.17,(9.34,-31.61,;10.67,-32.38,;10.67,-33.92,;12,-34.69,;13.35,-33.92,;13.34,-32.37,;12,-31.6,;14.68,-34.69,;16.01,-33.92,;14.68,-36.23,;16.02,-37,;16.46,-35.89,;16.53,-37.53,;15.18,-38.13,;14.9,-39.53,;16.27,-38.89,;17.81,-39.55,;18,-38.17,)| Show InChI InChI=1S/C14H17ClN2O/c15-12-3-1-11(2-4-12)14(18)16-13-9-17-7-5-10(13)6-8-17/h1-4,10,13H,5-9H2,(H,16,18)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Activity at 5HT3 receptor expressed in SHEP1 cells by FLIPR assay |
J Med Chem 49: 4425-36 (2006)
Article DOI: 10.1021/jm0602413
BindingDB Entry DOI: 10.7270/Q25B023S |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246239
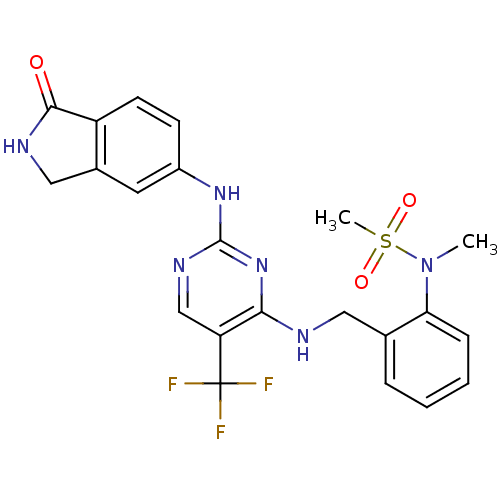
(CHEMBL487229 | N-methyl-N-(2-((2-(1-oxoisoindolin-...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3C(=O)NCc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)10-26-19-17(22(23,24)25)12-28-21(30-19)29-15-7-8-16-14(9-15)11-27-20(16)32/h3-9,12H,10-11H2,1-2H3,(H,27,32)(H2,26,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246286

(CHEMBL488247 | N-methyl-N-(2-((2-(4-(methylsulfony...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc(cc2)S(C)(=O)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H22F3N5O4S2/c1-29(35(3,32)33)18-7-5-4-6-14(18)12-25-19-17(21(22,23)24)13-26-20(28-19)27-15-8-10-16(11-9-15)34(2,30)31/h4-11,13H,12H2,1-3H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246378
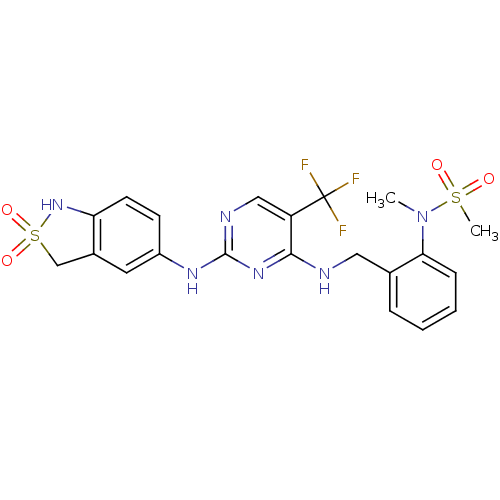
(CHEMBL452341 | N-(2-{[2-(2,2-Dioxo-2,3-dihydro-1H-...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NS(=O)(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H21F3N6O4S2/c1-30(35(2,31)32)18-6-4-3-5-13(18)10-25-19-16(21(22,23)24)11-26-20(28-19)27-15-7-8-17-14(9-15)12-36(33,34)29-17/h3-9,11,29H,10,12H2,1-2H3,(H2,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Mus musculus (Mouse)) | BDBM50192880
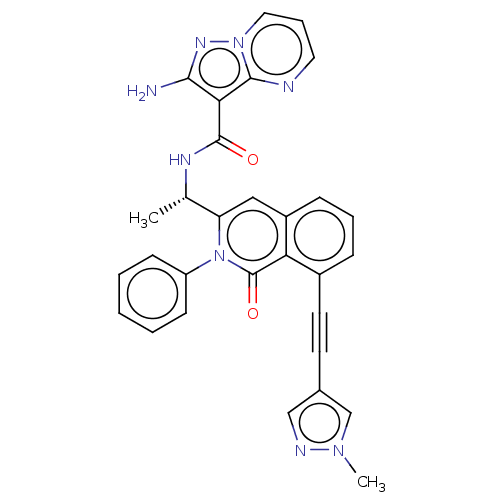
(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma in C5a-stimulated mouse RAW264.7 cells assessed as reduction in AKT phosphorylation at S473 incubated for 30 mins followed by... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060
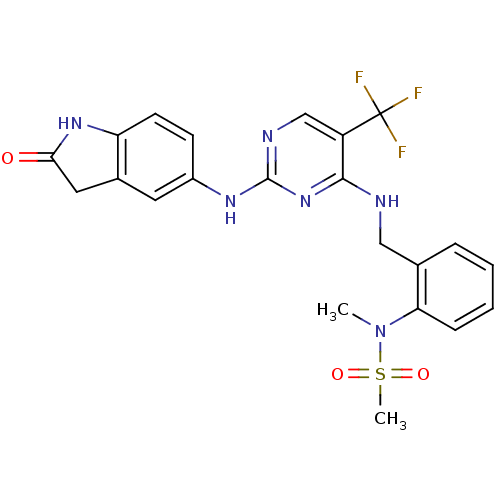
(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246140

(CHEMBL513744 | D3RKN_5 | N-methyl-N-(3-((2-(2-oxoi...)Show SMILES CN(c1cccc(CNc2nc(Nc3ccc4NC(=O)Cc4c3)ncc2C(F)(F)F)c1)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)16-5-3-4-13(8-16)11-26-20-17(22(23,24)25)12-27-21(30-20)28-15-6-7-18-14(9-15)10-19(32)29-18/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246378

(CHEMBL452341 | N-(2-{[2-(2,2-Dioxo-2,3-dihydro-1H-...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NS(=O)(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H21F3N6O4S2/c1-30(35(2,31)32)18-6-4-3-5-13(18)10-25-19-16(21(22,23)24)11-26-20(28-19)27-15-7-8-17-14(9-15)12-36(33,34)29-17/h3-9,11,29H,10,12H2,1-2H3,(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246239

(CHEMBL487229 | N-methyl-N-(2-((2-(1-oxoisoindolin-...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3C(=O)NCc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)10-26-19-17(22(23,24)25)12-28-21(30-19)29-15-7-8-16-14(9-15)11-27-20(16)32/h3-9,12H,10-11H2,1-2H3,(H,27,32)(H2,26,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246330
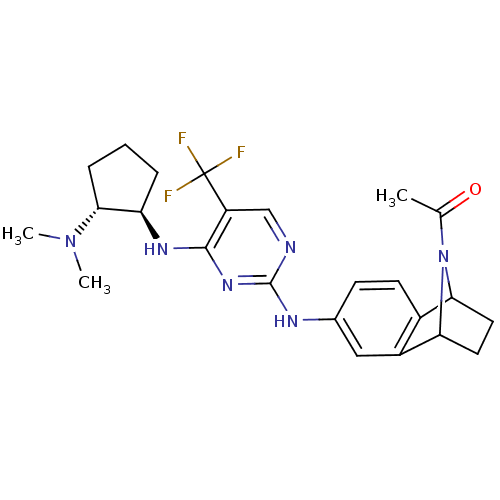
(1-{6-[4-((1R,2R)-2-Dimethylamino-cyclopentylamino)...)Show SMILES CN(C)[C@@H]1CCC[C@H]1Nc1nc(Nc2ccc3C4CCC(N4C(C)=O)c3c2)ncc1C(F)(F)F |r,TLB:26:25:19.18:21,THB:15:16:19.18:21,22:21:19.18:16.25| Show InChI InChI=1S/C24H29F3N6O/c1-13(34)33-19-9-10-20(33)16-11-14(7-8-15(16)19)29-23-28-12-17(24(25,26)27)22(31-23)30-18-5-4-6-21(18)32(2)3/h7-8,11-12,18-21H,4-6,9-10H2,1-3H3,(H2,28,29,30,31)/t18-,19?,20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246187

(5-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1cccc(CNc2nc(Nc3ccc4NC(=O)Cc4c3)ncc2C(F)(F)F)c1 Show InChI InChI=1S/C21H18F3N5O3S/c1-33(31,32)15-4-2-3-12(7-15)10-25-19-16(21(22,23)24)11-26-20(29-19)27-14-5-6-17-13(8-14)9-18(30)28-17/h2-8,11H,9-10H2,1H3,(H,28,30)(H2,25,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192889
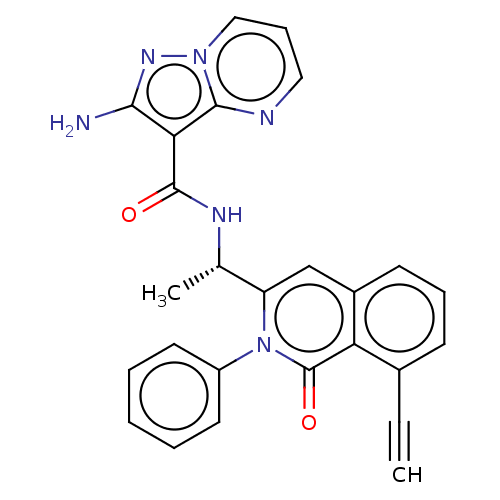
(CHEMBL3975359)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H20N6O2/c1-3-17-9-7-10-18-15-20(32(26(34)21(17)18)19-11-5-4-6-12-19)16(2)29-25(33)22-23(27)30-31-14-8-13-28-24(22)31/h1,4-16H,2H3,(H2,27,30)(H,29,33)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246188

(5-(4-(2-(methylsulfonyl)benzylamino)-5-(trifluorom...)Show SMILES CS(=O)(=O)c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F Show InChI InChI=1S/C21H18F3N5O3S/c1-33(31,32)17-5-3-2-4-12(17)10-25-19-15(21(22,23)24)11-26-20(29-19)27-14-6-7-16-13(8-14)9-18(30)28-16/h2-8,11H,9-10H2,1H3,(H,28,30)(H2,25,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246096
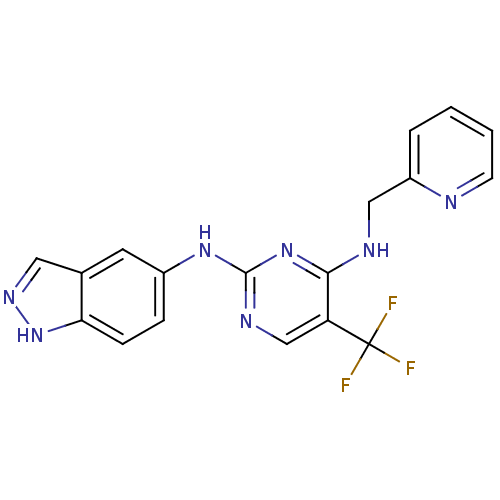
(CHEMBL471526 | N2-(1H-indazol-5-yl)-N4-(pyridin-2-...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3[nH]ncc3c2)nc1NCc1ccccn1 Show InChI InChI=1S/C18H14F3N7/c19-18(20,21)14-10-24-17(26-12-4-5-15-11(7-12)8-25-28-15)27-16(14)23-9-13-3-1-2-6-22-13/h1-8,10H,9H2,(H,25,28)(H2,23,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192880

(CHEMBL3984425 | US10329299, Compound 21 | US106752...)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(C#Cc3cnn(C)c3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H24N8O2/c1-19(34-29(39)26-27(31)35-37-15-7-14-32-28(26)37)24-16-22-9-6-8-21(13-12-20-17-33-36(2)18-20)25(22)30(40)38(24)23-10-4-3-5-11-23/h3-11,14-19H,1-2H3,(H2,31,35)(H,34,39)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246189

(5-(4-(2-(pyrrolidin-1-ylsulfonyl)benzylamino)-5-(t...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3NC(=O)Cc3c2)nc1NCc1ccccc1S(=O)(=O)N1CCCC1 Show InChI InChI=1S/C24H23F3N6O3S/c25-24(26,27)18-14-29-23(30-17-7-8-19-16(11-17)12-21(34)31-19)32-22(18)28-13-15-5-1-2-6-20(15)37(35,36)33-9-3-4-10-33/h1-2,5-8,11,14H,3-4,9-10,12-13H2,(H,31,34)(H2,28,29,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246139

(5-(4-(pyridin-2-ylmethylamino)-5-(trifluoromethyl)...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3NC(=O)Cc3c2)nc1NCc1ccccn1 Show InChI InChI=1S/C19H15F3N6O/c20-19(21,22)14-10-25-18(28-17(14)24-9-13-3-1-2-6-23-13)26-12-4-5-15-11(7-12)8-16(29)27-15/h1-7,10H,8-9H2,(H,27,29)(H2,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192882
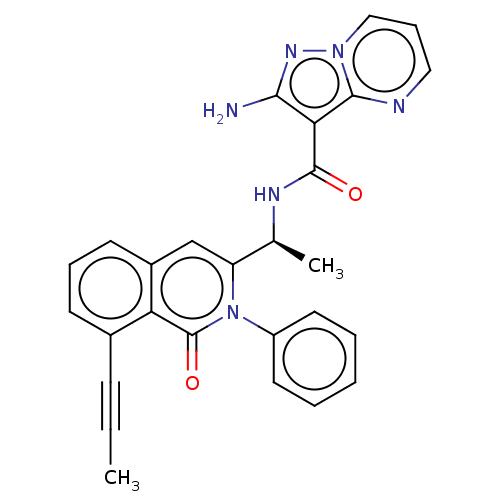
(CHEMBL3937119)Show SMILES CC#Cc1cccc2cc([C@H](C)NC(=O)c3c(N)nn4cccnc34)n(-c3ccccc3)c(=O)c12 |r| Show InChI InChI=1S/C27H22N6O2/c1-3-9-18-10-7-11-19-16-21(33(27(35)22(18)19)20-12-5-4-6-13-20)17(2)30-26(34)23-24(28)31-32-15-8-14-29-25(23)32/h4-8,10-17H,1-2H3,(H2,28,31)(H,30,34)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246284

(CHEMBL487057 | N4-((1R,2R)-2-Dimethylamino-cyclope...)Show SMILES CN(C)[C@@H]1CCC[C@H]1Nc1nc(Nc2ccc3c(CN(C)S3(=O)=O)c2)ncc1C(F)(F)F |r| Show InChI InChI=1S/C20H25F3N6O2S/c1-28(2)16-6-4-5-15(16)26-18-14(20(21,22)23)10-24-19(27-18)25-13-7-8-17-12(9-13)11-29(3)32(17,30)31/h7-10,15-16H,4-6,11H2,1-3H3,(H2,24,25,26,27)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Pyk2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246238
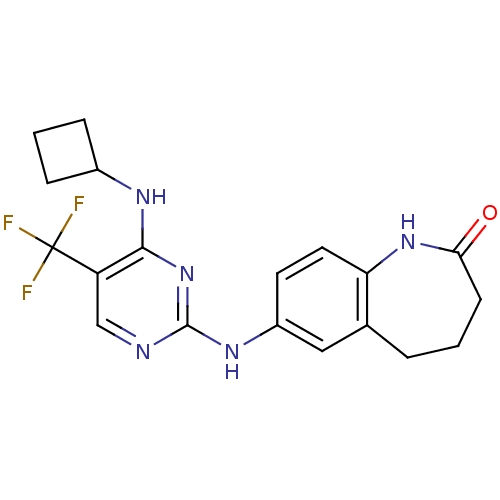
(7-(4-(cyclobutylamino)-5-(trifluoromethyl)pyrimidi...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3NC(=O)CCCc3c2)nc1NC1CCC1 Show InChI InChI=1S/C19H20F3N5O/c20-19(21,22)14-10-23-18(27-17(14)24-12-4-2-5-12)25-13-7-8-15-11(9-13)3-1-6-16(28)26-15/h7-10,12H,1-6H2,(H,26,28)(H2,23,24,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246058
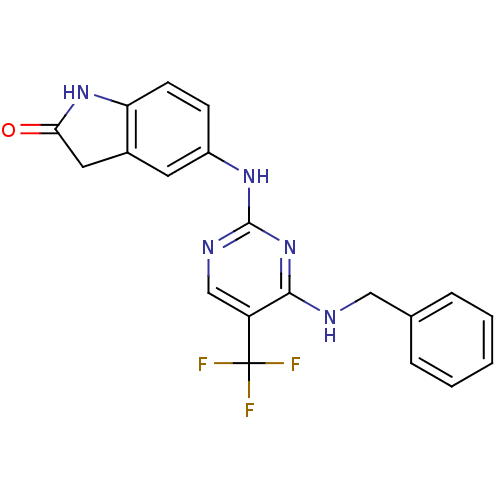
(5-(4-(benzylamino)-5-(trifluoromethyl)pyrimidin-2-...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3NC(=O)Cc3c2)nc1NCc1ccccc1 Show InChI InChI=1S/C20H16F3N5O/c21-20(22,23)15-11-25-19(28-18(15)24-10-12-4-2-1-3-5-12)26-14-6-7-16-13(8-14)9-17(29)27-16/h1-8,11H,9-10H2,(H,27,29)(H2,24,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246286

(CHEMBL488247 | N-methyl-N-(2-((2-(4-(methylsulfony...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc(cc2)S(C)(=O)=O)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H22F3N5O4S2/c1-29(35(3,32)33)18-7-5-4-6-14(18)12-25-19-17(21(22,23)24)13-26-20(28-19)27-15-8-10-16(11-9-15)34(2,30)31/h4-11,13H,12H2,1-3H3,(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246237
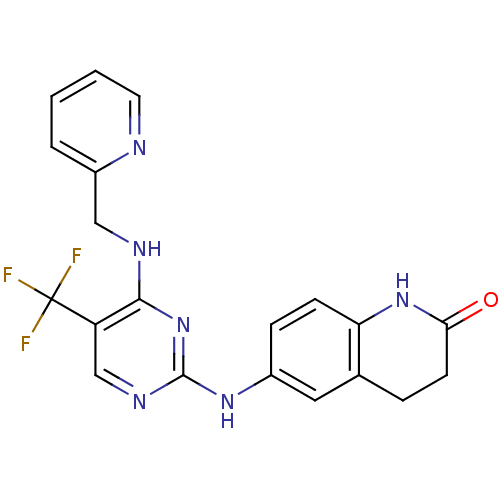
(6-(4-(pyridin-2-ylmethylamino)-5-(trifluoromethyl)...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3NC(=O)CCc3c2)nc1NCc1ccccn1 Show InChI InChI=1S/C20H17F3N6O/c21-20(22,23)15-11-26-19(29-18(15)25-10-14-3-1-2-8-24-14)27-13-5-6-16-12(9-13)4-7-17(30)28-16/h1-3,5-6,8-9,11H,4,7,10H2,(H,28,30)(H2,25,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192899
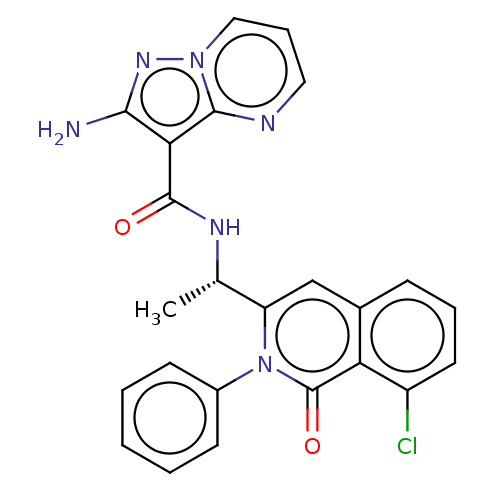
(CHEMBL3963736)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(Cl)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19ClN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246137
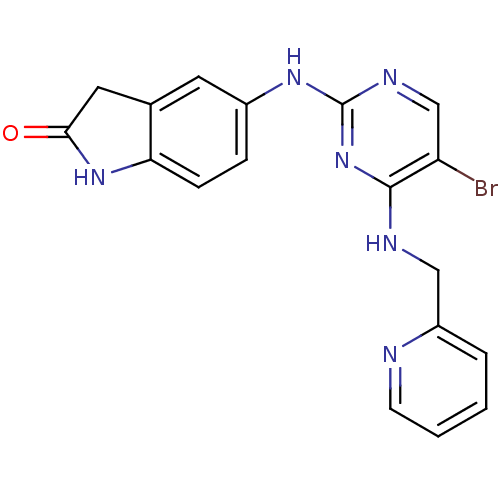
(5-(5-bromo-4-(pyridin-2-ylmethylamino)pyrimidin-2-...)Show InChI InChI=1S/C18H15BrN6O/c19-14-10-22-18(25-17(14)21-9-13-3-1-2-6-20-13)23-12-4-5-15-11(7-12)8-16(26)24-15/h1-7,10H,8-9H2,(H,24,26)(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246190

((R)-5-(4-(2-oxoazepan-3-ylamino)-5-(trifluoromethy...)Show SMILES FC(F)(F)c1cnc(Nc2ccc3NC(=O)Cc3c2)nc1N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C19H19F3N6O2/c20-19(21,22)12-9-24-18(25-11-4-5-13-10(7-11)8-15(29)26-13)28-16(12)27-14-3-1-2-6-23-17(14)30/h4-5,7,9,14H,1-3,6,8H2,(H,23,30)(H,26,29)(H2,24,25,27,28)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50192883
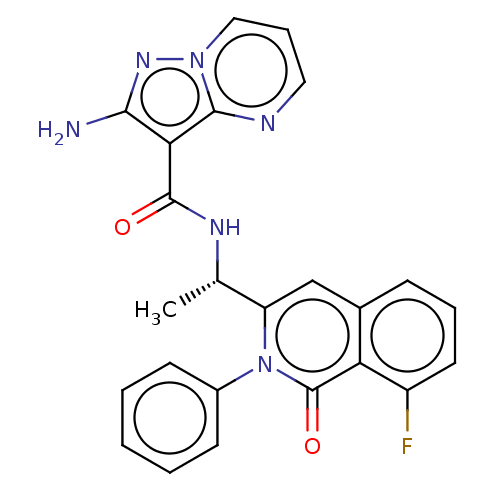
(CHEMBL3976330)Show SMILES C[C@H](NC(=O)c1c(N)nn2cccnc12)c1cc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H19FN6O2/c1-14(28-23(32)20-21(26)29-30-12-6-11-27-22(20)30)18-13-15-7-5-10-17(25)19(15)24(33)31(18)16-8-3-2-4-9-16/h2-14H,1H3,(H2,26,29)(H,28,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant full length N-terminal His-tagged PI3Kgamma expressed in Sf9 insect cells using diC8PIP2 as substrate incubated for 1... |
ACS Med Chem Lett 7: 862-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00238
BindingDB Entry DOI: 10.7270/Q2N018HX |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246379
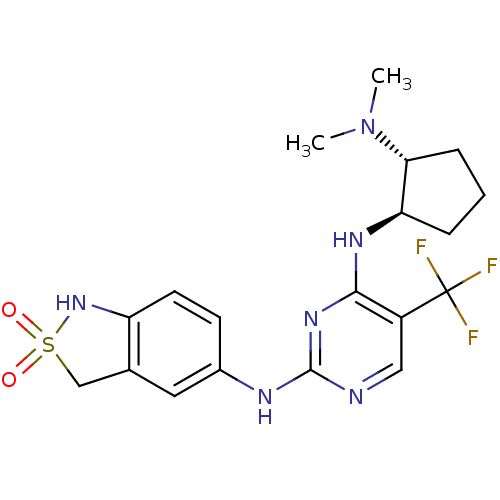
(CHEMBL452342 | N4-((1R,2R)-2-Dimethylamino-cyclope...)Show SMILES CN(C)[C@@H]1CCC[C@H]1Nc1nc(Nc2ccc3NS(=O)(=O)Cc3c2)ncc1C(F)(F)F |r| Show InChI InChI=1S/C19H23F3N6O2S/c1-28(2)16-5-3-4-15(16)25-17-13(19(20,21)22)9-23-18(26-17)24-12-6-7-14-11(8-12)10-31(29,30)27-14/h6-9,15-16,27H,3-5,10H2,1-2H3,(H2,23,24,25,26)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246236
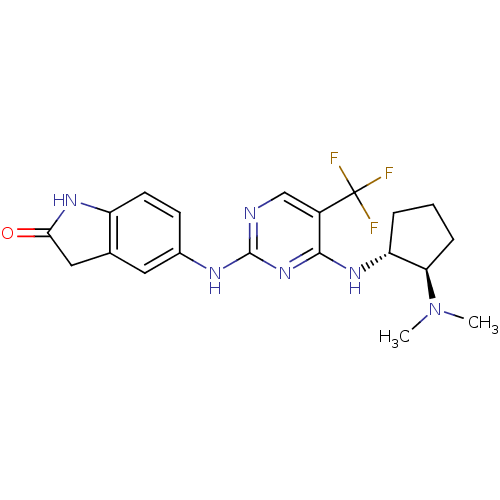
(5-(4-((1R,2R)-2-(dimethylamino)cyclopentylamino)-5...)Show SMILES CN(C)[C@@H]1CCC[C@H]1Nc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O/c1-29(2)16-5-3-4-15(16)27-18-13(20(21,22)23)10-24-19(28-18)25-12-6-7-14-11(8-12)9-17(30)26-14/h6-8,10,15-16H,3-5,9H2,1-2H3,(H,26,30)(H2,24,25,27,28)/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation |
Bioorg Med Chem Lett 18: 6071-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.030
BindingDB Entry DOI: 10.7270/Q2C8295H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data