

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379268 (CHEMBL3216556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AChE by Lineweaver-Burk plot analysis | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801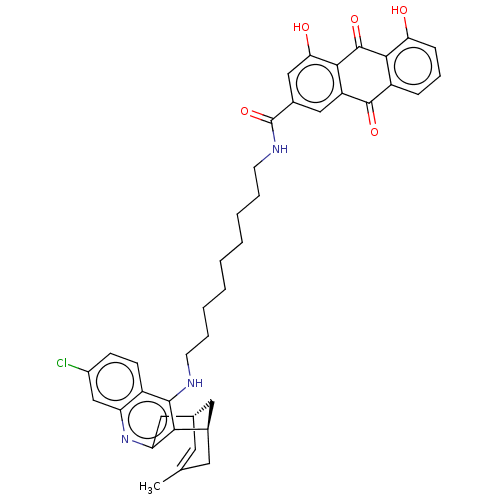 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-inhibitor complex by Li... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate assessed as dissociation constant for enzyme-substrate-inhibitor com... | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292582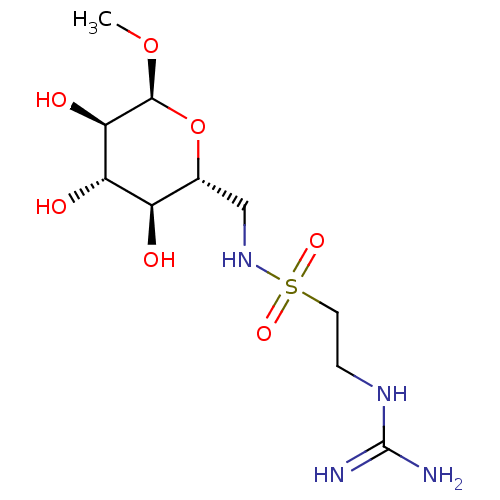 (CHEMBL4162427) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292584 (CHEMBL4172694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292583 (CHEMBL4164794) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292582 (CHEMBL4162427) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292585 (CHEMBL4161382) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292585 (CHEMBL4161382) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of VLA-4 expressed in Jurkat cell line, in a cell-based adhesion assay. | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292584 (CHEMBL4172694) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292590 (CHEMBL4162165) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292586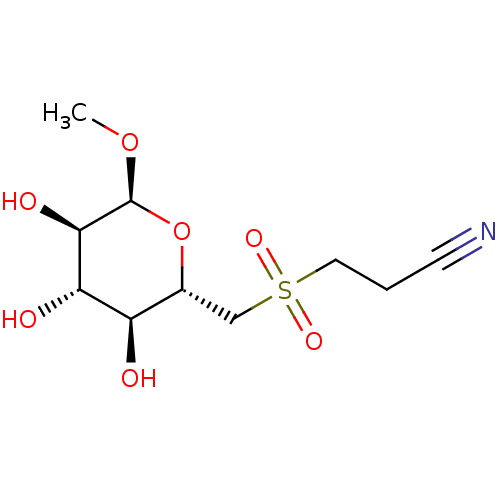 (CHEMBL4169301) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292580 (CHEMBL4172838) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292587 (CHEMBL4167547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292589 (CHEMBL4159624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292586 (CHEMBL4169301) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292590 (CHEMBL4162165) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292589 (CHEMBL4159624) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional glucose-6-phosphate 1-dehydrogenase/6-phosphogluconolactonase (Plasmodium falciparum (isolate 3D7)) | BDBM50292587 (CHEMBL4167547) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum G6PD-6PGL using G6P as substrate in presence of NADP+ by spectrofluorimetric analysis | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292583 (CHEMBL4164794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucose-6-phosphate 1-dehydrogenase (Homo sapiens (Human)) | BDBM50292580 (CHEMBL4172838) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant full length human G6PD expressed in Escherichia coli HB351 using G6P as substrate in presence of NADP+ by spectrofluorimetr... | Eur J Med Chem 146: 108-122 (2018) Article DOI: 10.1016/j.ejmech.2018.01.044 BindingDB Entry DOI: 10.7270/Q2CN76FJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10590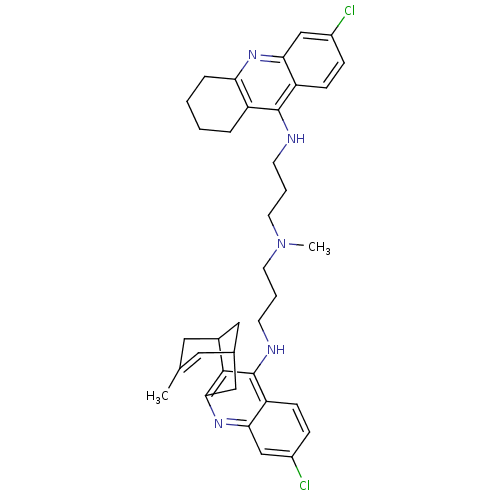 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10586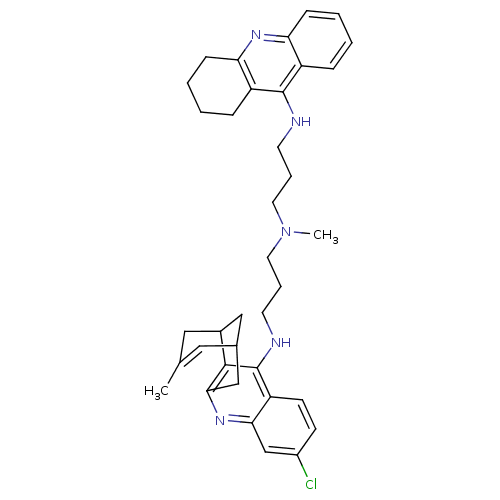 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman method | Bioorg Med Chem Lett 24: 5435-8 (2015) Article DOI: 10.1016/j.bmcl.2014.10.025 BindingDB Entry DOI: 10.7270/Q2G162DS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340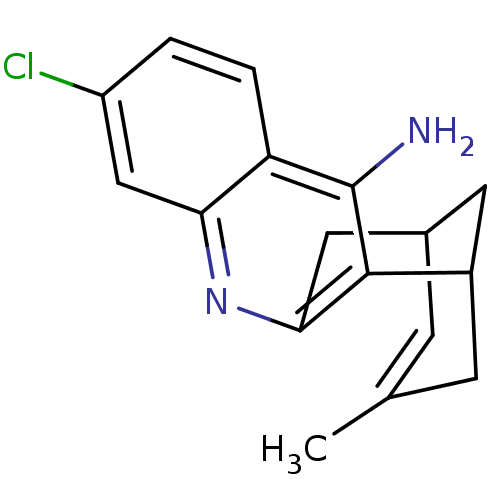 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10587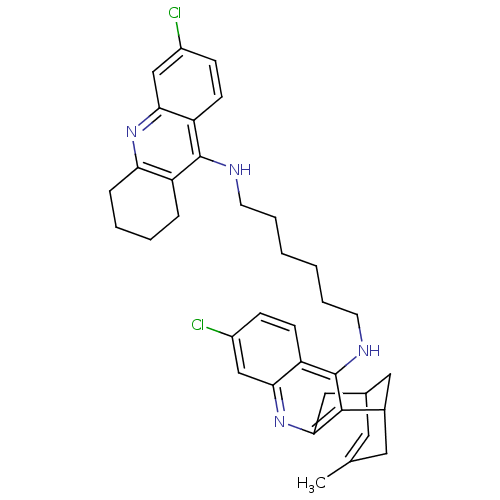 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10583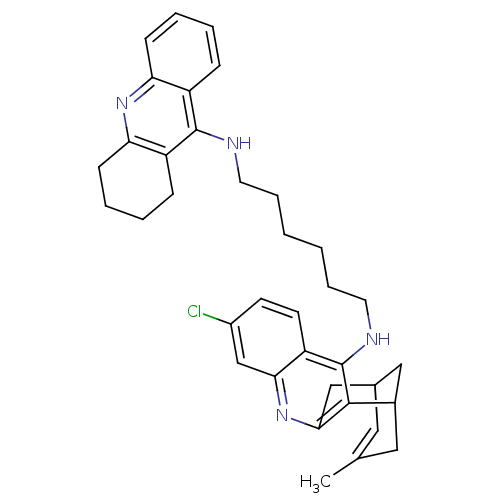 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007795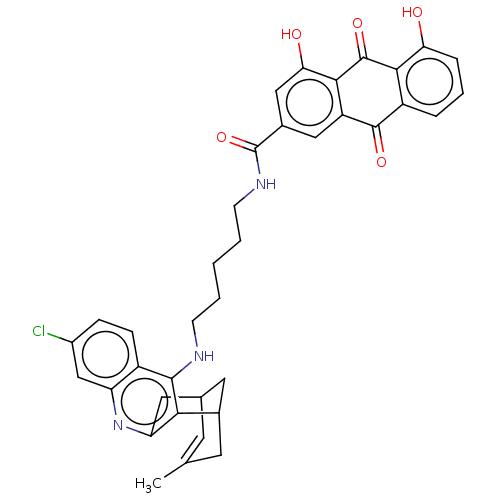 (CHEMBL3233826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10589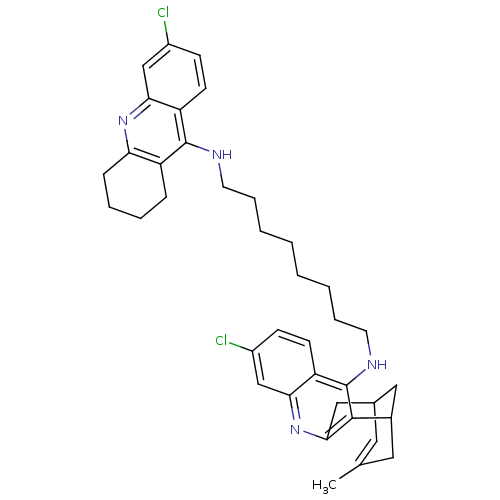 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10585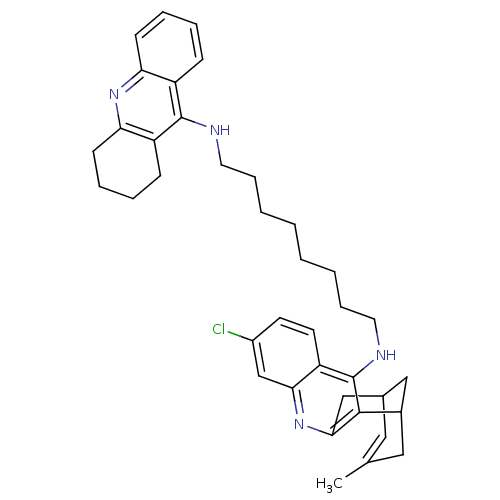 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108994 (CHEMBL3600555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007796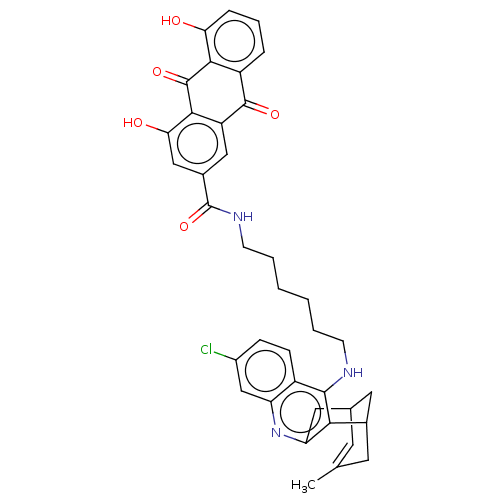 (CHEMBL3233827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108990 (CHEMBL3600551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10584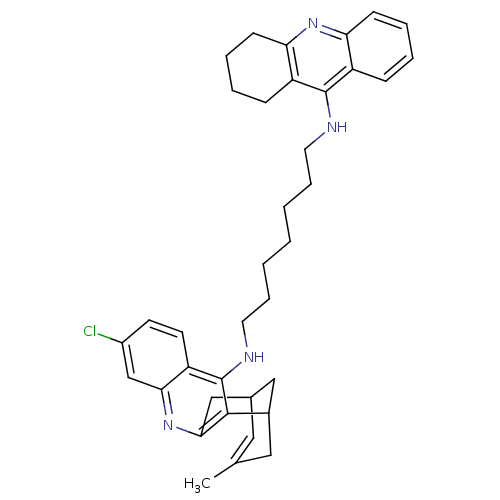 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202364 (US9238626, (-)-(Ib) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007801 (CHEMBL3233832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007797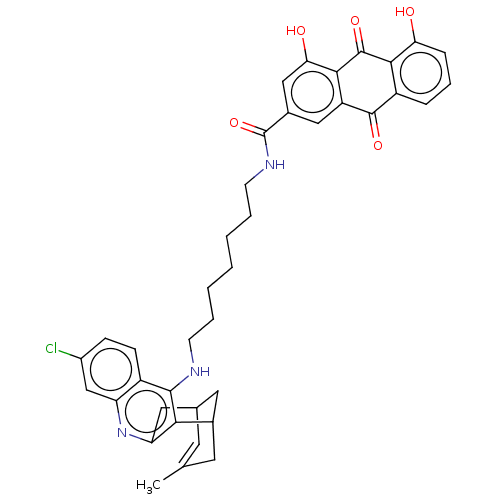 (CHEMBL3233828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50027470 (CHEMBL3216778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108991 (CHEMBL3600552) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM202363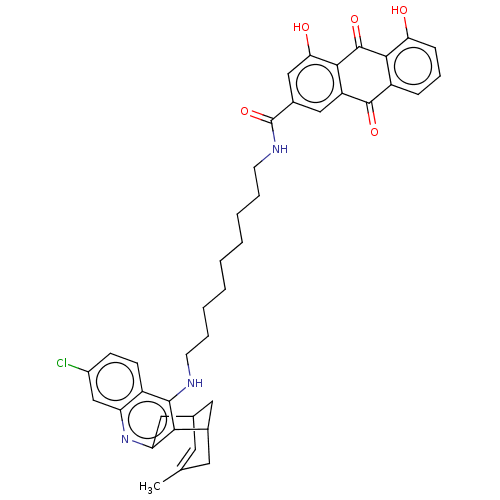 (US9238626, (+/-)-(Ib) HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description AChE inhibitory activity of compounds (Ia)-(Ie) and (-)-(Ib) and (+)-(Ib) was evaluated spectrophotometrically at 25° C. by the method of Ellma... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50007799 (CHEMBL3233830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108995 (CHEMBL3600556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10588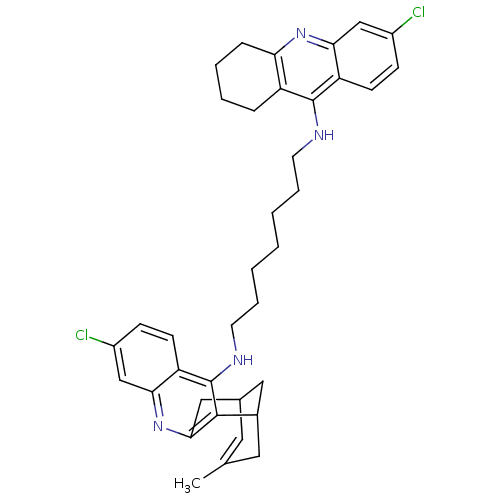 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 239 total ) | Next | Last >> |