 Found 1740 hits with Last Name = 'soll' and Initial = 'r'
Found 1740 hits with Last Name = 'soll' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221547
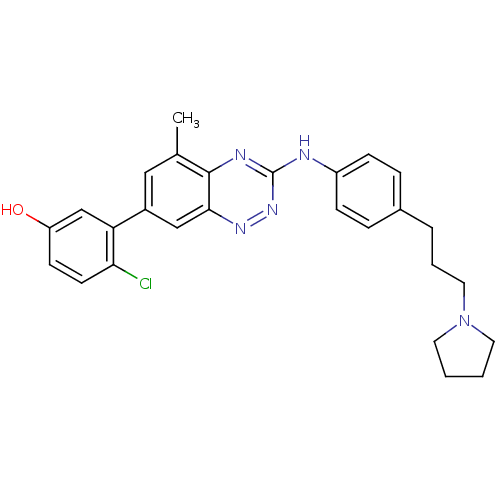
(4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(CCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C27H28ClN5O/c1-18-15-20(23-17-22(34)10-11-24(23)28)16-25-26(18)30-27(32-31-25)29-21-8-6-19(7-9-21)5-4-14-33-12-2-3-13-33/h6-11,15-17,34H,2-5,12-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221559
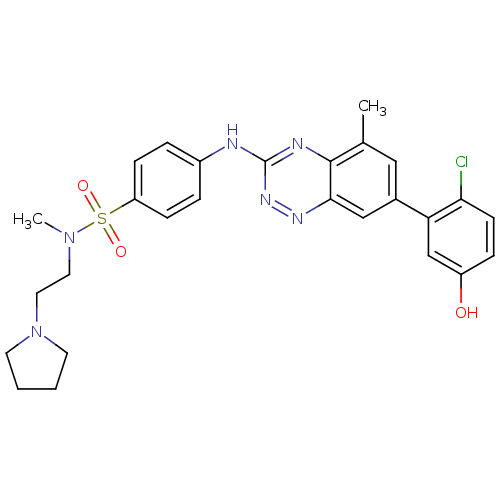
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES CN(CCN1CCCC1)S(=O)(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)cc1 Show InChI InChI=1S/C27H29ClN6O3S/c1-18-15-19(23-17-21(35)7-10-24(23)28)16-25-26(18)30-27(32-31-25)29-20-5-8-22(9-6-20)38(36,37)33(2)13-14-34-11-3-4-12-34/h5-10,15-17,35H,3-4,11-14H2,1-2H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221565
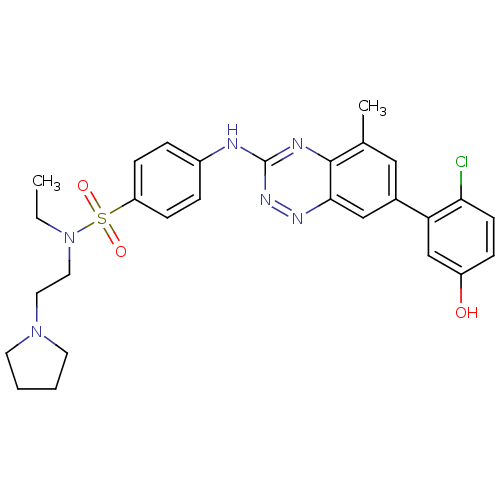
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES CCN(CCN1CCCC1)S(=O)(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)cc1 Show InChI InChI=1S/C28H31ClN6O3S/c1-3-35(15-14-34-12-4-5-13-34)39(37,38)23-9-6-21(7-10-23)30-28-31-27-19(2)16-20(17-26(27)32-33-28)24-18-22(36)8-11-25(24)29/h6-11,16-18,36H,3-5,12-15H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221557
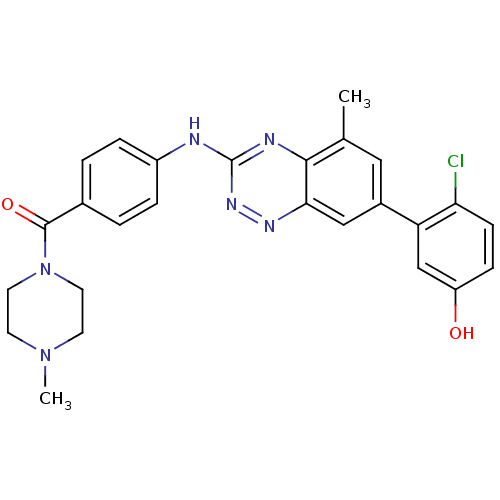
((4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)cc1 Show InChI InChI=1S/C26H25ClN6O2/c1-16-13-18(21-15-20(34)7-8-22(21)27)14-23-24(16)29-26(31-30-23)28-19-5-3-17(4-6-19)25(35)33-11-9-32(2)10-12-33/h3-8,13-15,34H,9-12H2,1-2H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221562
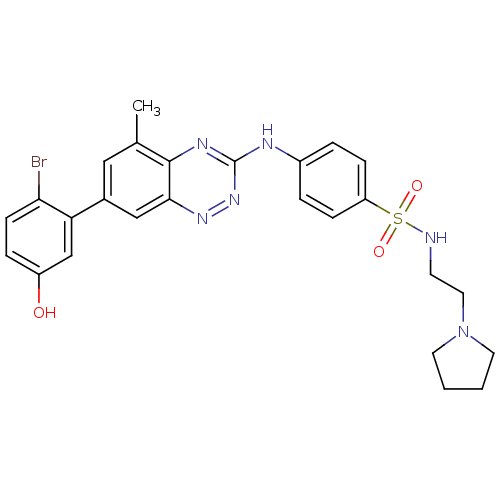
(4-(7-(2-bromo-5-hydroxyphenyl)-5-methylbenzo[e][1,...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1cc(O)ccc1Br Show InChI InChI=1S/C26H27BrN6O3S/c1-17-14-18(22-16-20(34)6-9-23(22)27)15-24-25(17)30-26(32-31-24)29-19-4-7-21(8-5-19)37(35,36)28-10-13-33-11-2-3-12-33/h4-9,14-16,28,34H,2-3,10-13H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50221566
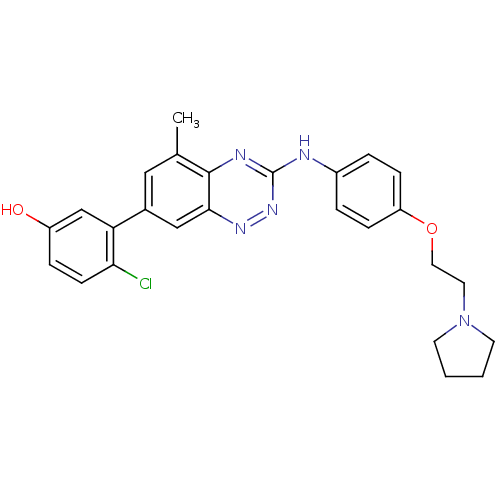
(4-chloro-3-(5-methyl-3-(4-(2-(pyrrolidin-1-yl)etho...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H26ClN5O2/c1-17-14-18(22-16-20(33)6-9-23(22)27)15-24-25(17)29-26(31-30-24)28-19-4-7-21(8-5-19)34-13-12-32-10-2-3-11-32/h4-9,14-16,33H,2-3,10-13H2,1H3,(H,28,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221549
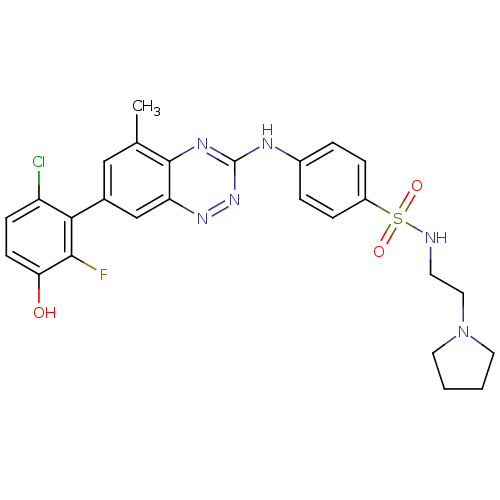
(4-(7-(6-chloro-2-fluoro-3-hydroxyphenyl)-5-methylb...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1c(Cl)ccc(O)c1F Show InChI InChI=1S/C26H26ClFN6O3S/c1-16-14-17(23-20(27)8-9-22(35)24(23)28)15-21-25(16)31-26(33-32-21)30-18-4-6-19(7-5-18)38(36,37)29-10-13-34-11-2-3-12-34/h4-9,14-15,29,35H,2-3,10-13H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070599
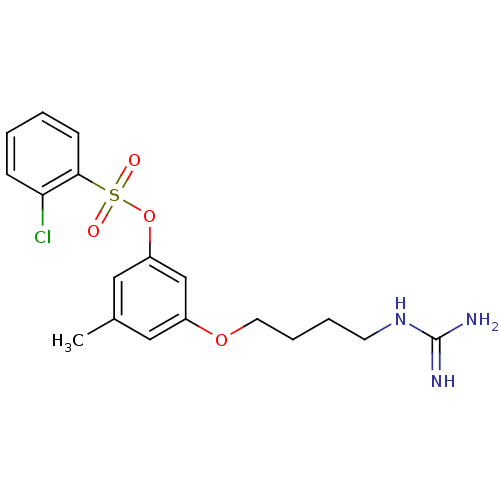
(2-Chloro-benzenesulfonic acid 3-(4-guanidino-butox...)Show SMILES Cc1cc(OCCCCNC(N)=N)cc(OS(=O)(=O)c2ccccc2Cl)c1 Show InChI InChI=1S/C18H22ClN3O4S/c1-13-10-14(25-9-5-4-8-22-18(20)21)12-15(11-13)26-27(23,24)17-7-3-2-6-16(17)19/h2-3,6-7,10-12H,4-5,8-9H2,1H3,(H4,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory constant against human alpha thrombin |
Bioorg Med Chem Lett 10: 1-4 (2000)
BindingDB Entry DOI: 10.7270/Q2HH6J9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221566

(4-chloro-3-(5-methyl-3-(4-(2-(pyrrolidin-1-yl)etho...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H26ClN5O2/c1-17-14-18(22-16-20(33)6-9-23(22)27)15-24-25(17)29-26(31-30-24)28-19-4-7-21(8-5-19)34-13-12-32-10-2-3-11-32/h4-9,14-16,33H,2-3,10-13H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221563
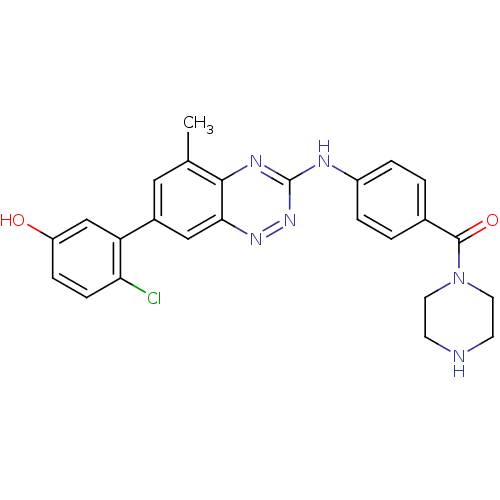
((4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)C(=O)N3CCNCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C25H23ClN6O2/c1-15-12-17(20-14-19(33)6-7-21(20)26)13-22-23(15)29-25(31-30-22)28-18-4-2-16(3-5-18)24(34)32-10-8-27-9-11-32/h2-7,12-14,27,33H,8-11H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221555
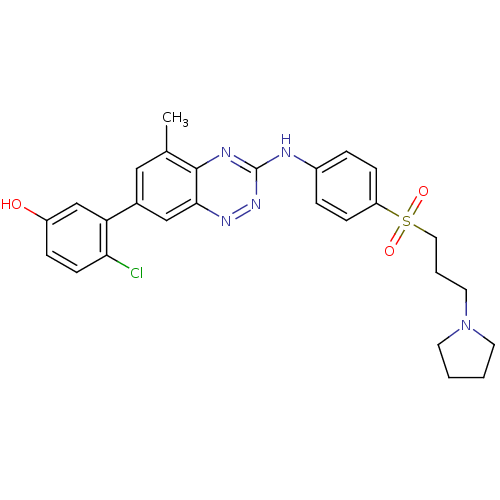
(4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)CCCN3CCCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C27H28ClN5O3S/c1-18-15-19(23-17-21(34)7-10-24(23)28)16-25-26(18)30-27(32-31-25)29-20-5-8-22(9-6-20)37(35,36)14-4-13-33-11-2-3-12-33/h5-10,15-17,34H,2-4,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50376901

(CHEMBL256101 | TG-100855)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCC[N+]4([O-])CCCC4)cc3)nc12)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H25Cl2N5O2/c1-17-15-18(24-21(27)5-4-6-22(24)28)16-23-25(17)30-26(32-31-23)29-19-7-9-20(10-8-19)35-14-13-33(34)11-2-3-12-33/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl by luminescence based kinase assay |
Drug Metab Dispos 35: 929-36 (2007)
Article DOI: 10.1124/dmd.106.014290
BindingDB Entry DOI: 10.7270/Q2CV4JMV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126647
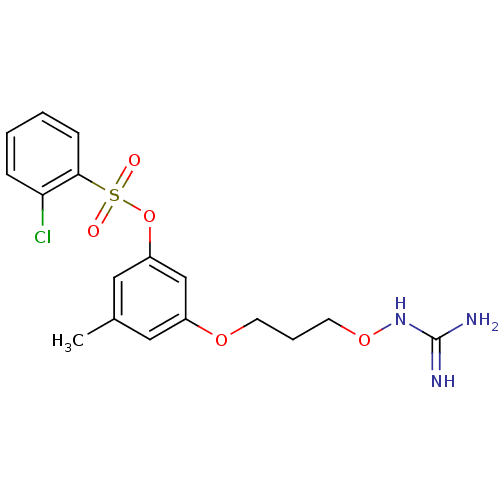
(3-[3-({[(Z)-amino(imino)methyl]amino}oxy)propoxy]-...)Show SMILES Cc1cc(OCCCONC(N)=N)cc(OS(=O)(=O)c2ccccc2Cl)c1 Show InChI InChI=1S/C17H20ClN3O5S/c1-12-9-13(24-7-4-8-25-21-17(19)20)11-14(10-12)26-27(22,23)16-6-3-2-5-15(16)18/h2-3,5-6,9-11H,4,7-8H2,1H3,(H4,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was tested against alpha-human thrombin |
Bioorg Med Chem Lett 13: 1495-8 (2003)
BindingDB Entry DOI: 10.7270/Q2668CJ9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221558
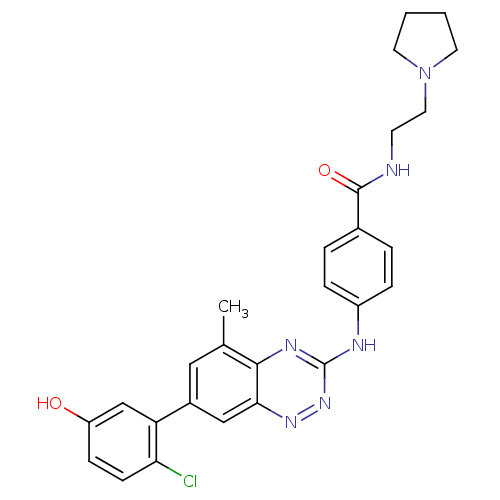
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)C(=O)NCCN3CCCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C27H27ClN6O2/c1-17-14-19(22-16-21(35)8-9-23(22)28)15-24-25(17)31-27(33-32-24)30-20-6-4-18(5-7-20)26(36)29-10-13-34-11-2-3-12-34/h4-9,14-16,35H,2-3,10-13H2,1H3,(H,29,36)(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221560
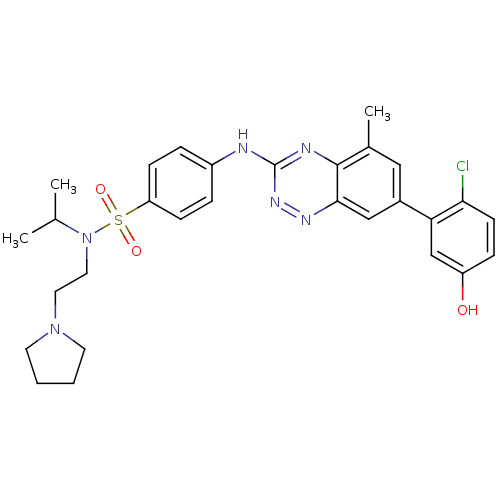
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES CC(C)N(CCN1CCCC1)S(=O)(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)cc1 Show InChI InChI=1S/C29H33ClN6O3S/c1-19(2)36(15-14-35-12-4-5-13-35)40(38,39)24-9-6-22(7-10-24)31-29-32-28-20(3)16-21(17-27(28)33-34-29)25-18-23(37)8-11-26(25)30/h6-11,16-19,37H,4-5,12-15H2,1-3H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221553
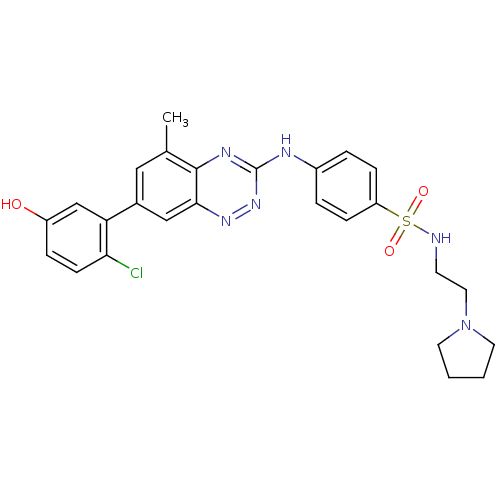
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C26H27ClN6O3S/c1-17-14-18(22-16-20(34)6-9-23(22)27)15-24-25(17)30-26(32-31-24)29-19-4-7-21(8-5-19)37(35,36)28-10-13-33-11-2-3-12-33/h4-9,14-16,28,34H,2-3,10-13H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221550
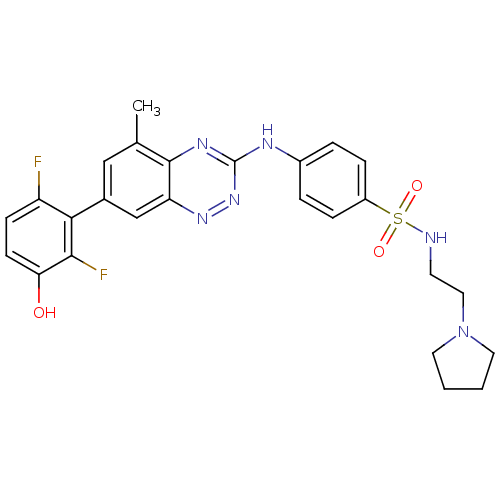
(4-(7-(2,6-difluoro-3-hydroxyphenyl)-5-methylbenzo[...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1c(F)ccc(O)c1F Show InChI InChI=1S/C26H26F2N6O3S/c1-16-14-17(23-20(27)8-9-22(35)24(23)28)15-21-25(16)31-26(33-32-21)30-18-4-6-19(7-5-18)38(36,37)29-10-13-34-11-2-3-12-34/h4-9,14-15,29,35H,2-3,10-13H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221556
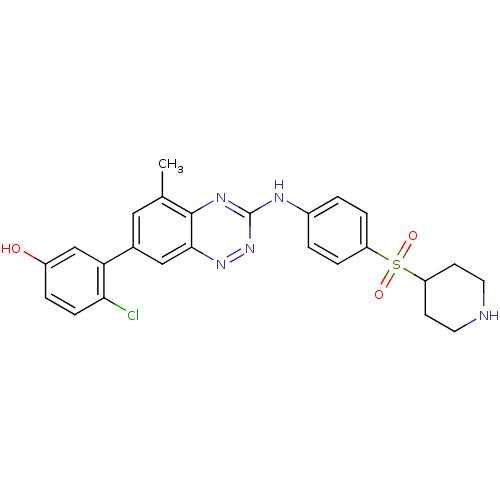
(4-chloro-3-(5-methyl-3-(4-(piperidin-4-ylsulfonyl)...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)C3CCNCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C25H24ClN5O3S/c1-15-12-16(21-14-18(32)4-7-22(21)26)13-23-24(15)29-25(31-30-23)28-17-2-5-19(6-3-17)35(33,34)20-8-10-27-11-9-20/h2-7,12-14,20,27,32H,8-11H2,1H3,(H,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221561

(4-chloro-3-(3-(4-(1-(2-(diethylamino)ethyl)piperid...)Show SMILES CCN(CC)CCN1CCC(CC1)S(=O)(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)cc1 Show InChI InChI=1S/C31H37ClN6O3S/c1-4-37(5-2)16-17-38-14-12-26(13-15-38)42(40,41)25-9-6-23(7-10-25)33-31-34-30-21(3)18-22(19-29(30)35-36-31)27-20-24(39)8-11-28(27)32/h6-11,18-20,26,39H,4-5,12-17H2,1-3H3,(H,33,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149022
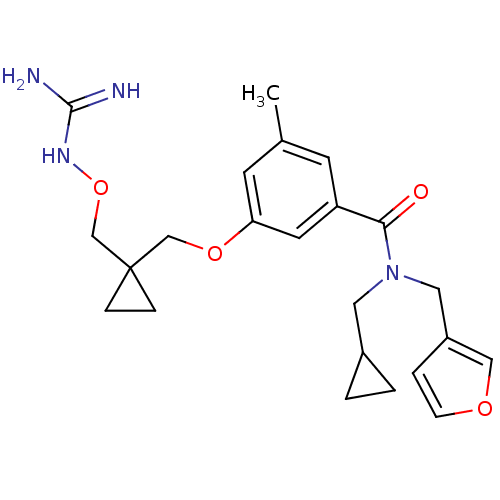
(CHEMBL421081 | Oxyguanidine derivative)Show SMILES Cc1cc(OCC2(CONC(N)=N)CC2)cc(c1)C(=O)N(CC1CC1)Cc1ccoc1 Show InChI InChI=1S/C23H30N4O4/c1-16-8-19(21(28)27(11-17-2-3-17)12-18-4-7-29-13-18)10-20(9-16)30-14-23(5-6-23)15-31-26-22(24)25/h4,7-10,13,17H,2-3,5-6,11-12,14-15H2,1H3,(H4,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023
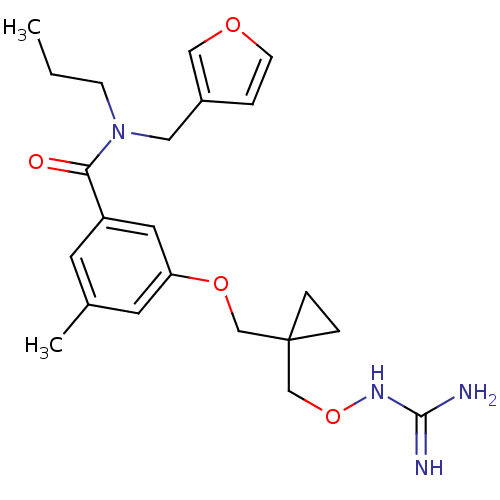
(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50376901

(CHEMBL256101 | TG-100855)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCC[N+]4([O-])CCCC4)cc3)nc12)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H25Cl2N5O2/c1-17-15-18(24-21(27)5-4-6-22(24)28)16-23-25(17)30-26(32-31-23)29-19-7-9-20(10-8-19)35-14-13-33(34)11-2-3-12-33/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Lyn by luminescence based kinase assay |
Drug Metab Dispos 35: 929-36 (2007)
Article DOI: 10.1124/dmd.106.014290
BindingDB Entry DOI: 10.7270/Q2CV4JMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221552

(CHEMBL249819 | US8481536, 545 | {4-[7-(2-chloro-5-...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)C(=O)N3CCS(=O)(=O)CC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C25H22ClN5O4S/c1-15-12-17(20-14-19(32)6-7-21(20)26)13-22-23(15)28-25(30-29-22)27-18-4-2-16(3-5-18)24(33)31-8-10-36(34,35)11-9-31/h2-7,12-14,32H,8-11H2,1H3,(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221551
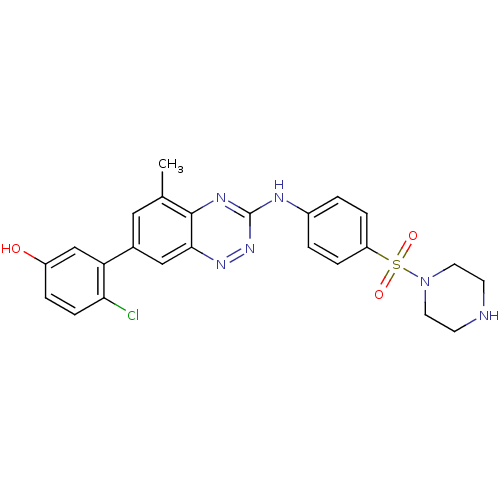
(4-chloro-3-(5-methyl-3-(4-(piperazin-1-ylsulfonyl)...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)N3CCNCC3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C24H23ClN6O3S/c1-15-12-16(20-14-18(32)4-7-21(20)25)13-22-23(15)28-24(30-29-22)27-17-2-5-19(6-3-17)35(33,34)31-10-8-26-9-11-31/h2-7,12-14,26,32H,8-11H2,1H3,(H,27,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083968

(3-[((3E)-3-{[(E)-amino(imino)methyl]hydrazono}prop...)Show SMILES Cc1cc(OCCC=NNC(N)=N)cc(OS(=O)(=O)c2ccccc2C(F)(F)F)c1 |w:8.8| Show InChI InChI=1S/C18H19F3N4O4S/c1-12-9-13(28-8-4-7-24-25-17(22)23)11-14(10-12)29-30(26,27)16-6-3-2-5-15(16)18(19,20)21/h2-3,5-7,9-11H,4,8H2,1H3,(H4,22,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory constant against human alpha thrombin |
Bioorg Med Chem Lett 10: 1-4 (2000)
BindingDB Entry DOI: 10.7270/Q2HH6J9C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070597
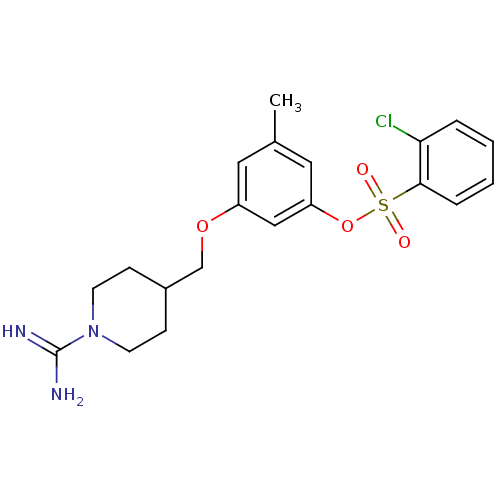
(2-Chloro-benzenesulfonic acid 3-(1-carbamimidoyl-p...)Show SMILES Cc1cc(OCC2CCN(CC2)C(N)=N)cc(OS(=O)(=O)c2ccccc2Cl)c1 Show InChI InChI=1S/C20H24ClN3O4S/c1-14-10-16(27-13-15-6-8-24(9-7-15)20(22)23)12-17(11-14)28-29(25,26)19-5-3-2-4-18(19)21/h2-5,10-12,15H,6-9,13H2,1H3,(H3,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for its ability to inhibit serine protease thrombin using succinyl-Ala-p-nitroanilide as substrate |
Bioorg Med Chem Lett 8: 1595-600 (1999)
BindingDB Entry DOI: 10.7270/Q20864GK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070597

(2-Chloro-benzenesulfonic acid 3-(1-carbamimidoyl-p...)Show SMILES Cc1cc(OCC2CCN(CC2)C(N)=N)cc(OS(=O)(=O)c2ccccc2Cl)c1 Show InChI InChI=1S/C20H24ClN3O4S/c1-14-10-16(27-13-15-6-8-24(9-7-15)20(22)23)12-17(11-14)28-29(25,26)19-5-3-2-4-18(19)21/h2-5,10-12,15H,6-9,13H2,1H3,(H3,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceutical, Inc.
Curated by ChEMBL
| Assay Description
Anti- Thrombin activity using standard chromogenic assay |
Bioorg Med Chem Lett 10: 79-82 (2000)
BindingDB Entry DOI: 10.7270/Q2GH9H6F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50070597

(2-Chloro-benzenesulfonic acid 3-(1-carbamimidoyl-p...)Show SMILES Cc1cc(OCC2CCN(CC2)C(N)=N)cc(OS(=O)(=O)c2ccccc2Cl)c1 Show InChI InChI=1S/C20H24ClN3O4S/c1-14-10-16(27-13-15-6-8-24(9-7-15)20(22)23)12-17(11-14)28-29(25,26)19-5-3-2-4-18(19)21/h2-5,10-12,15H,6-9,13H2,1H3,(H3,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of Thrombin |
Bioorg Med Chem Lett 10: 83-5 (2000)
BindingDB Entry DOI: 10.7270/Q2BR8RDT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083963

(3-[((3E)-3-{[(E)-amino(imino)methyl]hydrazono}prop...)Show SMILES Cc1cc(OCCC=NNC(N)=N)cc(OS(=O)(=O)c2cccc3cccnc23)c1 |w:8.8| Show InChI InChI=1S/C20H21N5O4S/c1-14-11-16(28-10-4-9-24-25-20(21)22)13-17(12-14)29-30(26,27)18-7-2-5-15-6-3-8-23-19(15)18/h2-3,5-9,11-13H,4,10H2,1H3,(H4,21,22,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory constant against human alpha thrombin |
Bioorg Med Chem Lett 10: 1-4 (2000)
BindingDB Entry DOI: 10.7270/Q2HH6J9C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126640

(3-[3-({[(Z)-amino(imino)methyl]amino}oxy)propoxy]-...)Show SMILES Cc1cc(OCCCONC(N)=N)cc(OS(=O)(=O)c2ccccc2S(=O)(=O)c2ccccc2)c1 Show InChI InChI=1S/C23H25N3O7S2/c1-17-14-18(31-12-7-13-32-26-23(24)25)16-19(15-17)33-35(29,30)22-11-6-5-10-21(22)34(27,28)20-8-3-2-4-9-20/h2-6,8-11,14-16H,7,12-13H2,1H3,(H4,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was tested against alpha-human thrombin |
Bioorg Med Chem Lett 13: 1495-8 (2003)
BindingDB Entry DOI: 10.7270/Q2668CJ9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50198826

(4-(7-(2-chloro-6-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(=O)(=O)NCCN3CCCC3)nc12)-c1c(O)cccc1Cl Show InChI InChI=1S/C26H27ClN6O3S/c1-17-15-18(24-21(27)5-4-6-23(24)34)16-22-25(17)30-26(32-31-22)29-19-7-9-20(10-8-19)37(35,36)28-11-14-33-12-2-3-13-33/h4-10,15-16,28,34H,2-3,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221564

(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES CN(CCN1CCOCC1)S(=O)(=O)c1ccc(Nc2nnc3cc(cc(C)c3n2)-c2cc(O)ccc2Cl)cc1 Show InChI InChI=1S/C27H29ClN6O4S/c1-18-15-19(23-17-21(35)5-8-24(23)28)16-25-26(18)30-27(32-31-25)29-20-3-6-22(7-4-20)39(36,37)33(2)9-10-34-11-13-38-14-12-34/h3-8,15-17,35H,9-14H2,1-2H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50376901

(CHEMBL256101 | TG-100855)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCC[N+]4([O-])CCCC4)cc3)nc12)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H25Cl2N5O2/c1-17-15-18(24-21(27)5-4-6-22(24)28)16-23-25(17)30-26(32-31-23)29-19-7-9-20(10-8-19)35-14-13-33(34)11-2-3-12-33/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Yes by luminescence based kinase assay |
Drug Metab Dispos 35: 929-36 (2007)
Article DOI: 10.1124/dmd.106.014290
BindingDB Entry DOI: 10.7270/Q2CV4JMV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083971
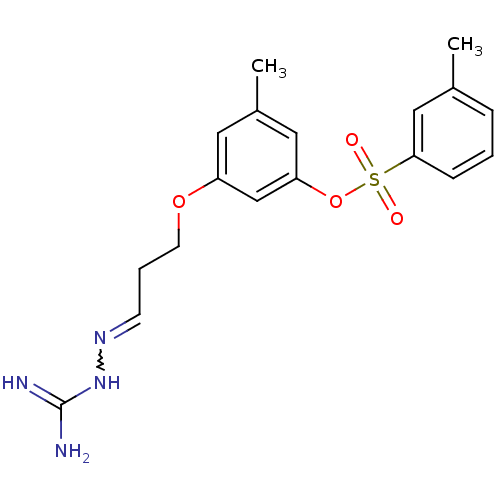
(3-[((3E)-3-{[(E)-amino(imino)methyl]hydrazono}prop...)Show SMILES Cc1cccc(c1)S(=O)(=O)Oc1cc(C)cc(OCCC=NNC(N)=N)c1 |w:21.22| Show InChI InChI=1S/C18H22N4O4S/c1-13-5-3-6-17(11-13)27(23,24)26-16-10-14(2)9-15(12-16)25-8-4-7-21-22-18(19)20/h3,5-7,9-12H,4,8H2,1-2H3,(H4,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory constant against human alpha thrombin |
Bioorg Med Chem Lett 10: 1-4 (2000)
BindingDB Entry DOI: 10.7270/Q2HH6J9C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149015
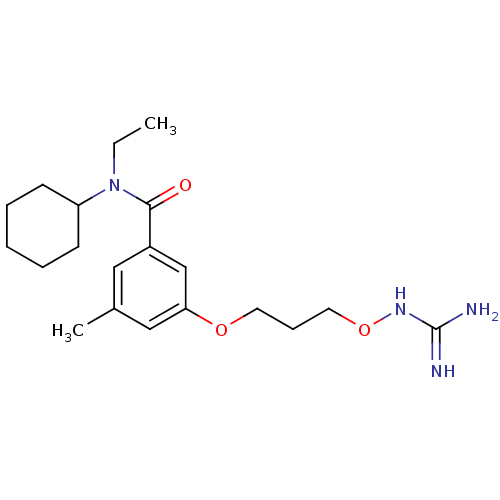
(CHEMBL122144 | Oxyguanidine derivative)Show SMILES CCN(C1CCCCC1)C(=O)c1cc(C)cc(OCCCONC(N)=N)c1 Show InChI InChI=1S/C20H32N4O3/c1-3-24(17-8-5-4-6-9-17)19(25)16-12-15(2)13-18(14-16)26-10-7-11-27-23-20(21)22/h12-14,17H,3-11H2,1-2H3,(H4,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149020
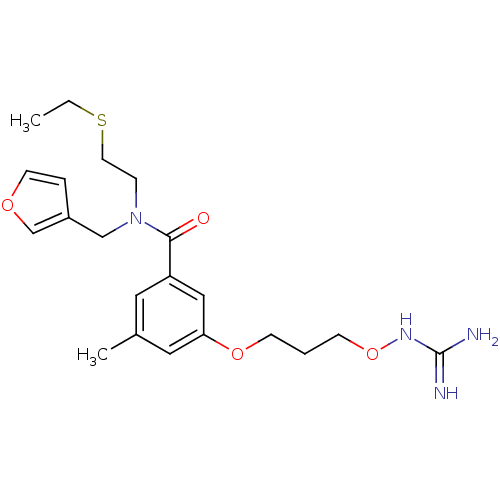
(CHEMBL122696 | Oxyguanidine derivative)Show SMILES CCSCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCCCONC(N)=N)c1 Show InChI InChI=1S/C21H30N4O4S/c1-3-30-10-6-25(14-17-5-9-27-15-17)20(26)18-11-16(2)12-19(13-18)28-7-4-8-29-24-21(22)23/h5,9,11-13,15H,3-4,6-8,10,14H2,1-2H3,(H4,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126634

(3-[3-({[(Z)-amino(imino)methyl]amino}oxy)propoxy]-...)Show SMILES Cc1cc(OCCCONC(N)=N)cc(OS(=O)(=O)c2cccc3cccnc23)c1 Show InChI InChI=1S/C20H22N4O5S/c1-14-11-16(27-9-4-10-28-24-20(21)22)13-17(12-14)29-30(25,26)18-7-2-5-15-6-3-8-23-19(15)18/h2-3,5-8,11-13H,4,9-10H2,1H3,(H4,21,22,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was tested against alpha-human thrombin |
Bioorg Med Chem Lett 13: 1495-8 (2003)
BindingDB Entry DOI: 10.7270/Q2668CJ9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126642

(3-[3-({[(Z)-amino(imino)methyl]amino}oxy)propoxy]-...)Show SMILES COc1ccccc1S(=O)(=O)Oc1cc(C)cc(OCCCONC(N)=N)c1 Show InChI InChI=1S/C18H23N3O6S/c1-13-10-14(25-8-5-9-26-21-18(19)20)12-15(11-13)27-28(22,23)17-7-4-3-6-16(17)24-2/h3-4,6-7,10-12H,5,8-9H2,1-2H3,(H4,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was tested against alpha-human thrombin |
Bioorg Med Chem Lett 13: 1495-8 (2003)
BindingDB Entry DOI: 10.7270/Q2668CJ9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50148990
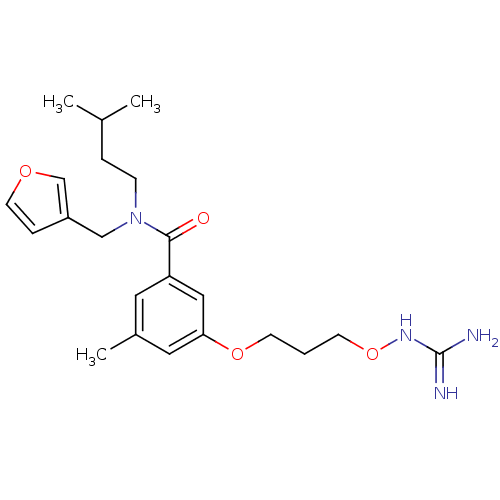
(CHEMBL421243 | Oxyguanidine derivative)Show SMILES CC(C)CCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCCCONC(N)=N)c1 Show InChI InChI=1S/C22H32N4O4/c1-16(2)5-7-26(14-18-6-10-28-15-18)21(27)19-11-17(3)12-20(13-19)29-8-4-9-30-25-22(23)24/h6,10-13,15-16H,4-5,7-9,14H2,1-3H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50193874
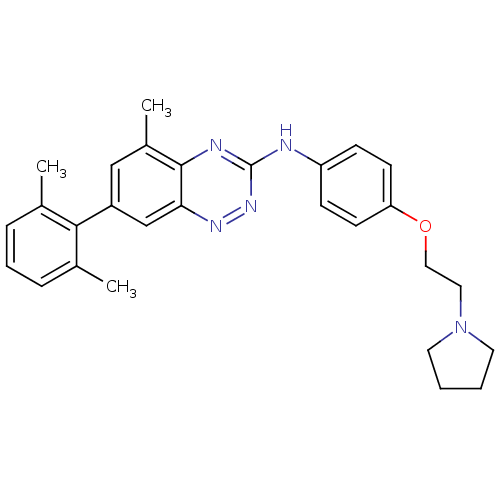
(7-(2,6-dimethylphenyl)-5-methyl-N-(4-(2-(pyrrolidi...)Show SMILES Cc1cccc(C)c1-c1cc(C)c2nc(Nc3ccc(OCCN4CCCC4)cc3)nnc2c1 Show InChI InChI=1S/C28H31N5O/c1-19-7-6-8-20(2)26(19)22-17-21(3)27-25(18-22)31-32-28(30-27)29-23-9-11-24(12-10-23)34-16-15-33-13-4-5-14-33/h6-12,17-18H,4-5,13-16H2,1-3H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50376901

(CHEMBL256101 | TG-100855)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCC[N+]4([O-])CCCC4)cc3)nc12)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H25Cl2N5O2/c1-17-15-18(24-21(27)5-4-6-22(24)28)16-23-25(17)30-26(32-31-23)29-19-7-9-20(10-8-19)35-14-13-33(34)11-2-3-12-33/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Src by luminescence based kinase assay |
Drug Metab Dispos 35: 929-36 (2007)
Article DOI: 10.1124/dmd.106.014290
BindingDB Entry DOI: 10.7270/Q2CV4JMV |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50376901

(CHEMBL256101 | TG-100855)Show SMILES Cc1cc(cc2nnc(Nc3ccc(OCC[N+]4([O-])CCCC4)cc3)nc12)-c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H25Cl2N5O2/c1-17-15-18(24-21(27)5-4-6-22(24)28)16-23-25(17)30-26(32-31-23)29-19-7-9-20(10-8-19)35-14-13-33(34)11-2-3-12-33/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by luminescence based kinase assay |
Drug Metab Dispos 35: 929-36 (2007)
Article DOI: 10.1124/dmd.106.014290
BindingDB Entry DOI: 10.7270/Q2CV4JMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221554
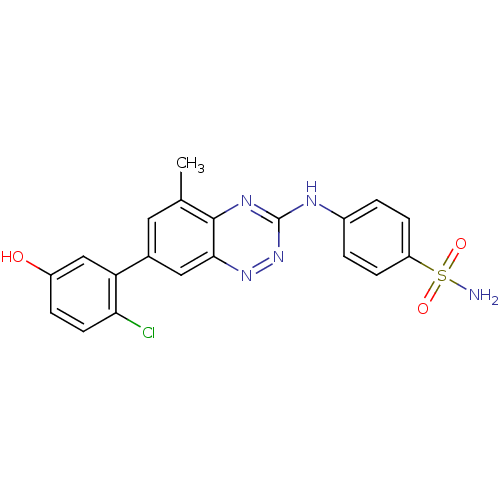
(4-(7-(2-chloro-5-hydroxyphenyl)-5-methylbenzo[e][1...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(cc3)S(N)(=O)=O)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C20H16ClN5O3S/c1-11-8-12(16-10-14(27)4-7-17(16)21)9-18-19(11)24-20(26-25-18)23-13-2-5-15(6-3-13)30(22,28)29/h2-10,27H,1H3,(H2,22,28,29)(H,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50126652

(2-METHANESULFONYL-BENZENESULFONIC ACID 3-METHYL-5-...)Show SMILES Cc1cc(OCC2(CONC(N)=N)CC2)cc(OS(=O)(=O)c2ccccc2S(C)(=O)=O)c1 Show InChI InChI=1S/C20H25N3O7S2/c1-14-9-15(28-12-20(7-8-20)13-29-23-19(21)22)11-16(10-14)30-32(26,27)18-6-4-3-5-17(18)31(2,24)25/h3-6,9-11H,7-8,12-13H2,1-2H3,(H4,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound was tested against alpha-human thrombin |
Bioorg Med Chem Lett 13: 1495-8 (2003)
BindingDB Entry DOI: 10.7270/Q2668CJ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50148994
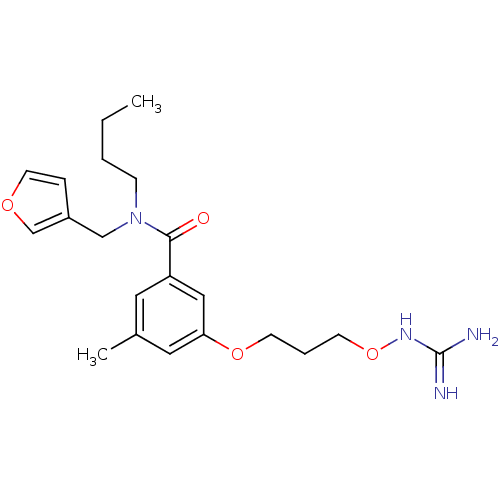
(CHEMBL421096 | Oxyguanidine derivative)Show SMILES CCCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCCCONC(N)=N)c1 Show InChI InChI=1S/C21H30N4O4/c1-3-4-7-25(14-17-6-10-27-15-17)20(26)18-11-16(2)12-19(13-18)28-8-5-9-29-24-21(22)23/h6,10-13,15H,3-5,7-9,14H2,1-2H3,(H4,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149008
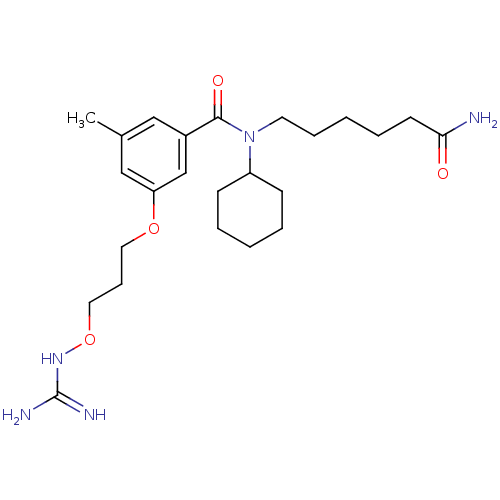
(CHEMBL122205 | Oxyguanidine derivative)Show SMILES Cc1cc(OCCCONC(N)=N)cc(c1)C(=O)N(CCCCCC(N)=O)C1CCCCC1 Show InChI InChI=1S/C24H39N5O4/c1-18-15-19(17-21(16-18)32-13-8-14-33-28-24(26)27)23(31)29(20-9-4-2-5-10-20)12-7-3-6-11-22(25)30/h15-17,20H,2-14H2,1H3,(H2,25,30)(H4,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083969
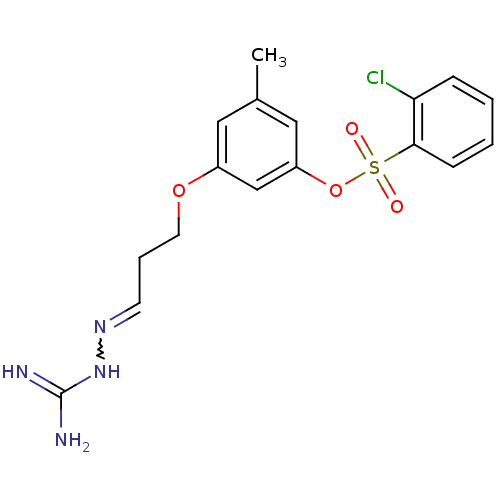
(3-[((3E)-3-{[(E)-amino(imino)methyl]hydrazono}prop...)Show SMILES Cc1cc(OCCC=NNC(N)=N)cc(OS(=O)(=O)c2ccccc2Cl)c1 |w:8.8| Show InChI InChI=1S/C17H19ClN4O4S/c1-12-9-13(25-8-4-7-21-22-17(19)20)11-14(10-12)26-27(23,24)16-6-3-2-5-15(16)18/h2-3,5-7,9-11H,4,8H2,1H3,(H4,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory constant against human alpha thrombin |
Bioorg Med Chem Lett 10: 1-4 (2000)
BindingDB Entry DOI: 10.7270/Q2HH6J9C |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50148988
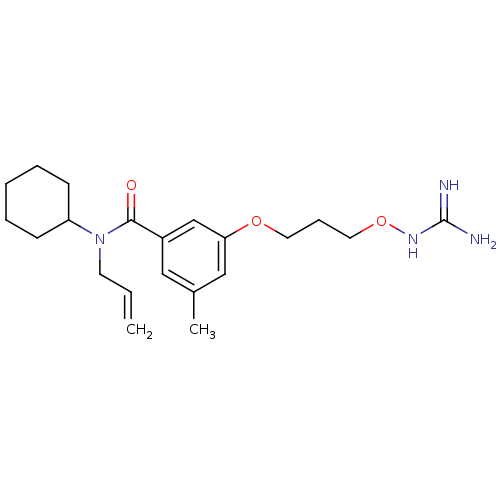
(CHEMBL123028 | Oxyguanidine derivative)Show SMILES Cc1cc(OCCCONC(N)=N)cc(c1)C(=O)N(CC=C)C1CCCCC1 Show InChI InChI=1S/C21H32N4O3/c1-3-10-25(18-8-5-4-6-9-18)20(26)17-13-16(2)14-19(15-17)27-11-7-12-28-24-21(22)23/h3,13-15,18H,1,4-12H2,2H3,(H4,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149017
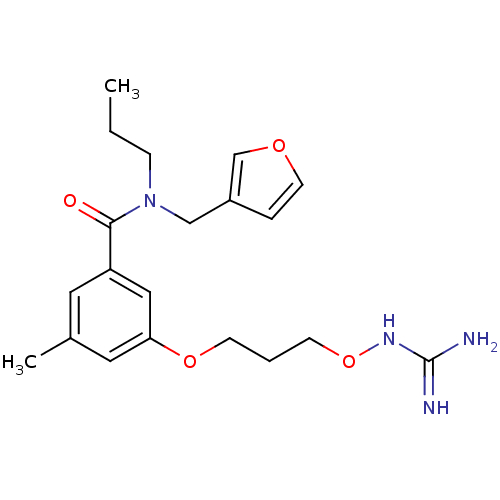
(CHEMBL341539 | Oxyguanidine derivative)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCCCONC(N)=N)c1 Show InChI InChI=1S/C20H28N4O4/c1-3-6-24(13-16-5-9-26-14-16)19(25)17-10-15(2)11-18(12-17)27-7-4-8-28-23-20(21)22/h5,9-12,14H,3-4,6-8,13H2,1-2H3,(H4,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 14: 3727-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.002
BindingDB Entry DOI: 10.7270/Q2D79C0Q |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50083967

(3-[((3E)-3-{[(E)-amino(imino)methyl]hydrazono}prop...)Show SMILES Cc1cc(OCCC=NNC(N)=N)cc(OS(=O)(=O)c2ccccc2C#N)c1 |w:8.8| Show InChI InChI=1S/C18H19N5O4S/c1-13-9-15(26-8-4-7-22-23-18(20)21)11-16(10-13)27-28(24,25)17-6-3-2-5-14(17)12-19/h2-3,5-7,9-11H,4,8H2,1H3,(H4,20,21,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
3-Dimensional Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory constant against human alpha thrombin |
Bioorg Med Chem Lett 10: 1-4 (2000)
BindingDB Entry DOI: 10.7270/Q2HH6J9C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data