

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309834 ((S)-1,3'-bipyrrolidin-1'-yl(4-((7-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309848 ((R)-1,3'-bipyrrolidin-1'-yl(4-((2-methyl-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309847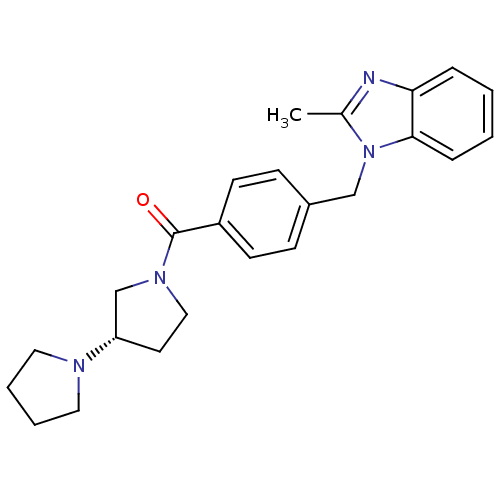 ((S)-1,3'-bipyrrolidin-1'-yl(4-((2-methyl-1H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309845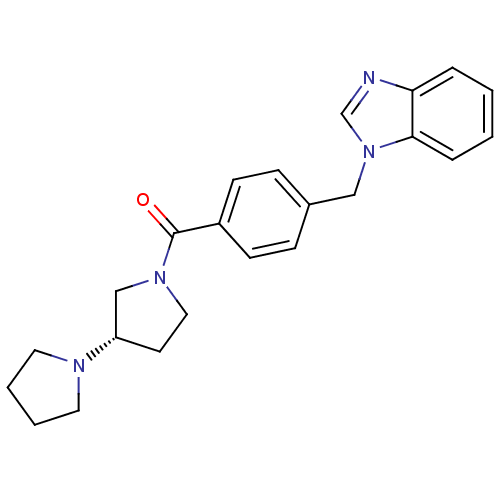 ((S)-1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309837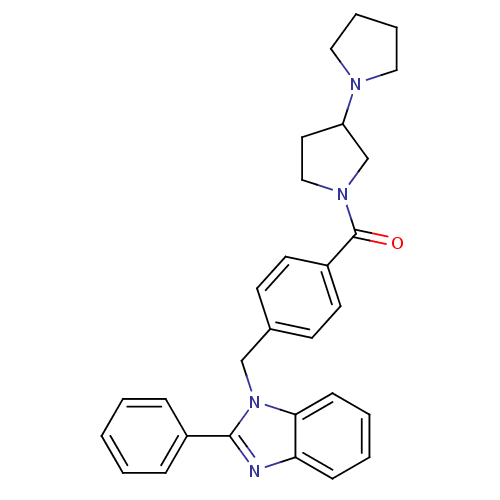 (1,3'-bipyrrolidin-1'-yl(4-((2-phenyl-1H-benzo[d]im...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309851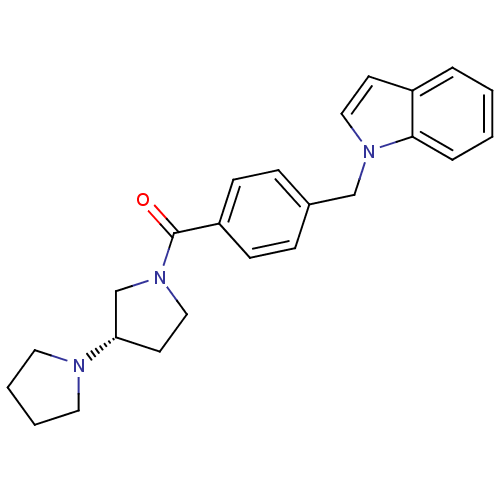 ((S)-1,3'-bipyrrolidin-1'-yl(4-((1H-indol-1-yl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309843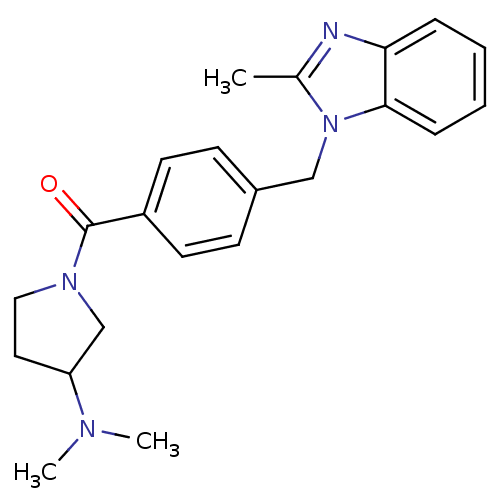 ((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-methyl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50309834 ((S)-1,3'-bipyrrolidin-1'-yl(4-((7-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity to rat histamine H3 receptor | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309844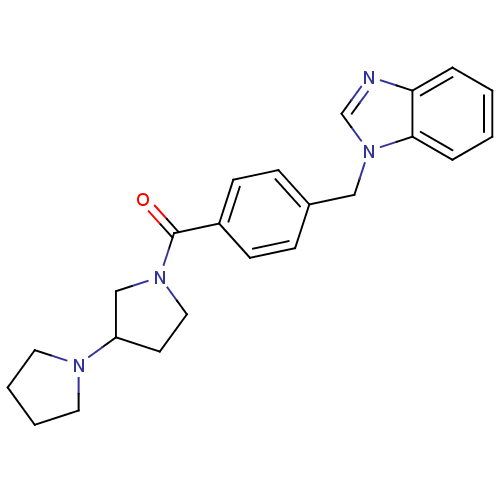 (1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309846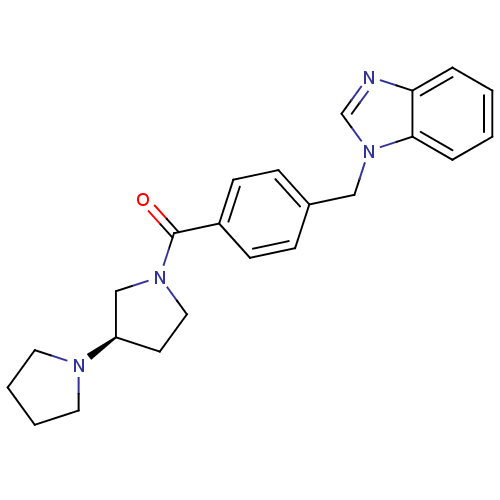 ((R)-1,3'-bipyrrolidin-1'-yl(4-((1H-benzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309839 ((S)-(3-(dimethylamino)pyrrolidin-1-yl)(4-((2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309836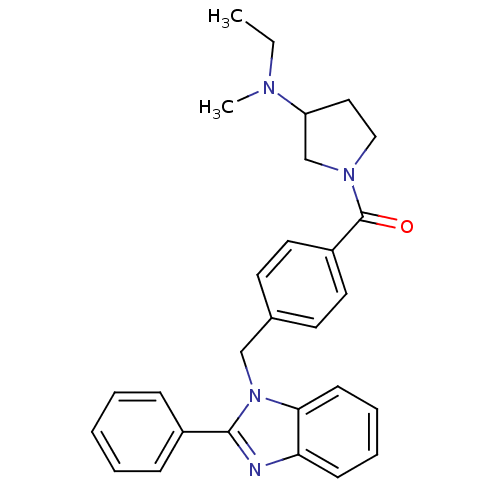 ((3-(ethyl(methyl)amino)pyrrolidin-1-yl)(4-((2-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309835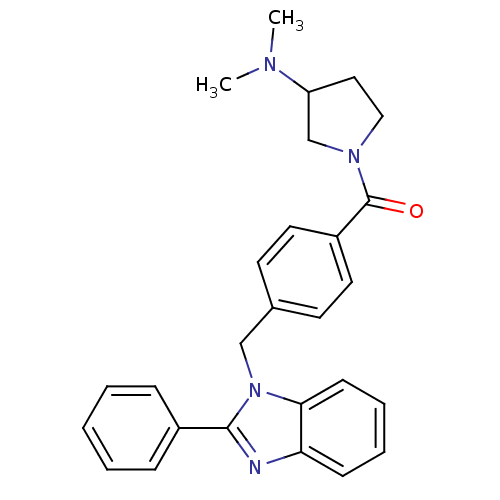 ((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-phenyl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309853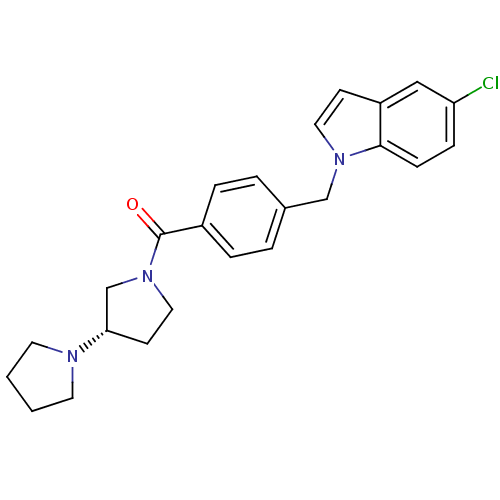 ((S)-1,3'-bipyrrolidin-1'-yl(4-((5-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202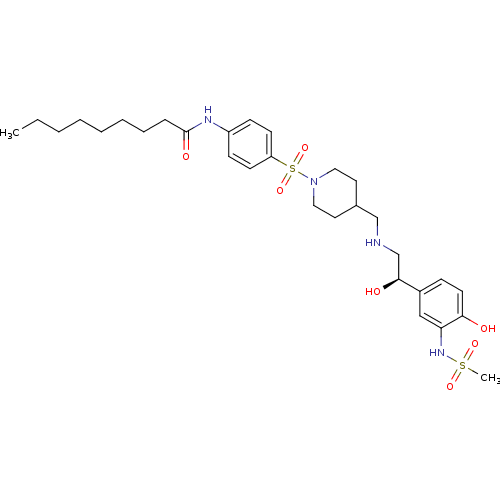 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309852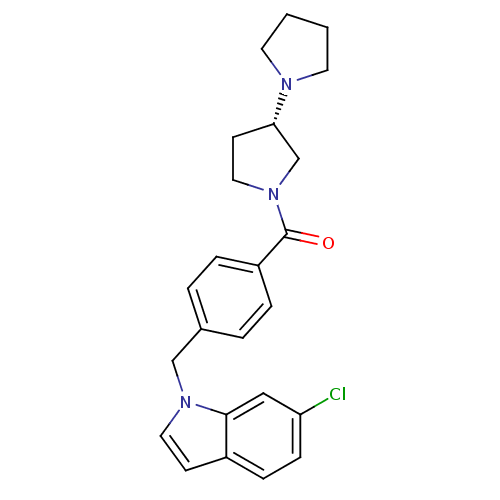 ((S)-1,3'-bipyrrolidin-1'-yl(4-((6-chloro-1H-indol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-2 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309841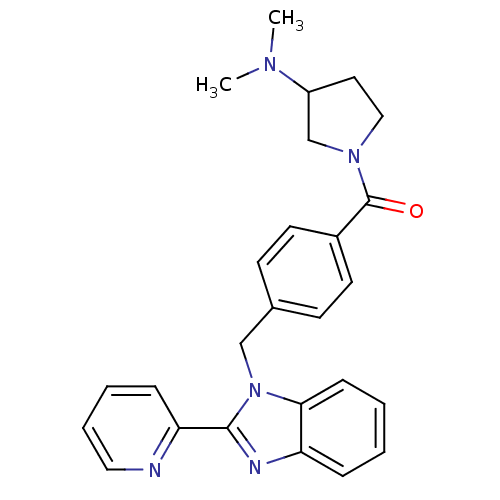 ((3-(dimethylamino)pyrrolidin-1-yl)(4-((2-(pyridin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 235 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119195 (CHEMBL317003 | {1-[4-(4-{[(R)-2-Hydroxy-2-(4-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309842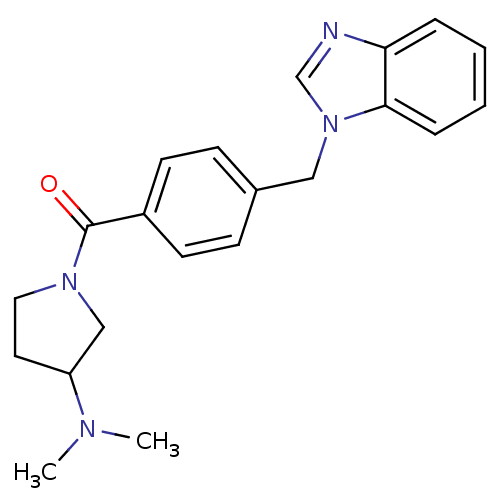 ((4-((1H-benzo[d]imidazol-1-yl)methyl)phenyl)(3-(di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309849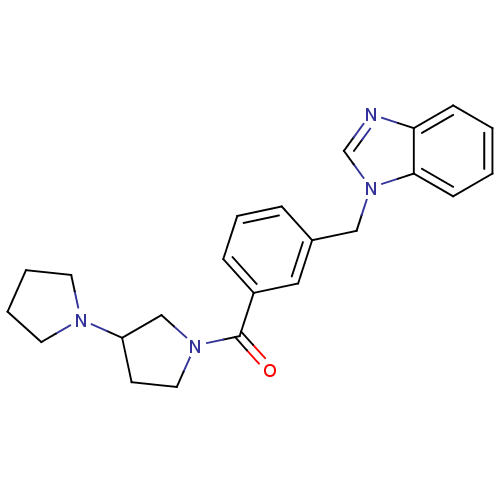 (1,3'-bipyrrolidin-1'-yl(3-((1H-benzo[d]imidazol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309840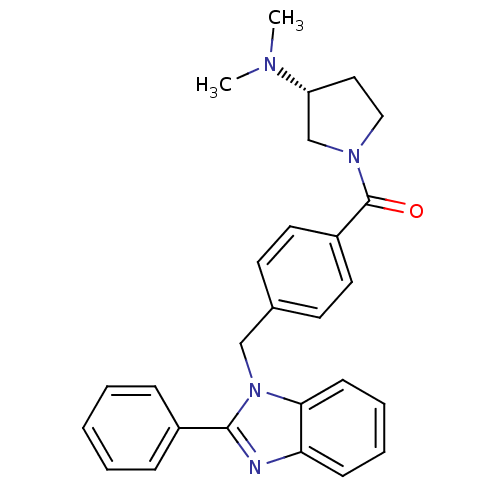 ((R)-(3-(dimethylamino)pyrrolidin-1-yl)(4-((2-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309838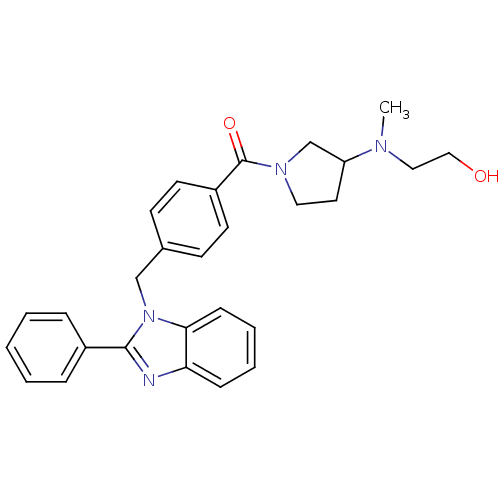 ((3-((2-hydroxyethyl)(methyl)amino)pyrrolidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50309850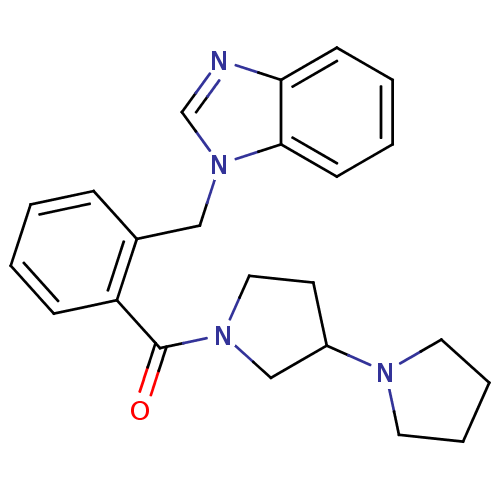 (1,3'-bipyrrolidin-1'-yl(2-((1H-benzo[d]imidazol-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human cloned histamine H3 receptor expressed in HEK293T cells by liquid scintillation spectrometry | Bioorg Med Chem Lett 20: 1237-40 (2010) Article DOI: 10.1016/j.bmcl.2009.11.122 BindingDB Entry DOI: 10.7270/Q2028RNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119202 (CHEMBL99599 | Nonanoic acid [4-(4-{[2-hydroxy-2-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity of compound against Beta-1 adrenergic receptor was determined | Bioorg Med Chem Lett 12: 2963-7 (2002) BindingDB Entry DOI: 10.7270/Q2D21WZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157485 (4-[1-isopropyl-7-(trifluoromethyl)-1H-indazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50119183 (1-[4-(4-{[(S)-2-Hydroxy-3-(4-hydroxy-phenoxy)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-2 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157496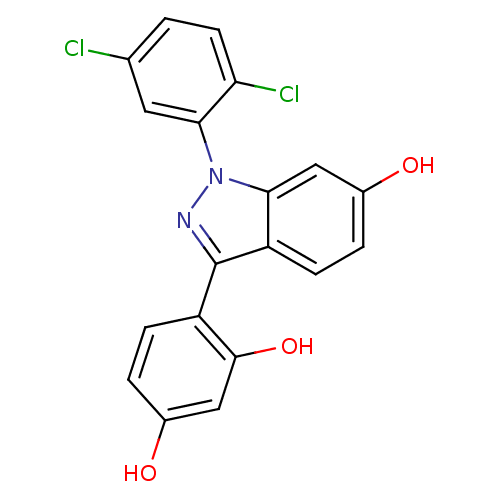 (4-[1-(2,5-dichlorophenyl)-6-hydroxy-1H-indazol-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157488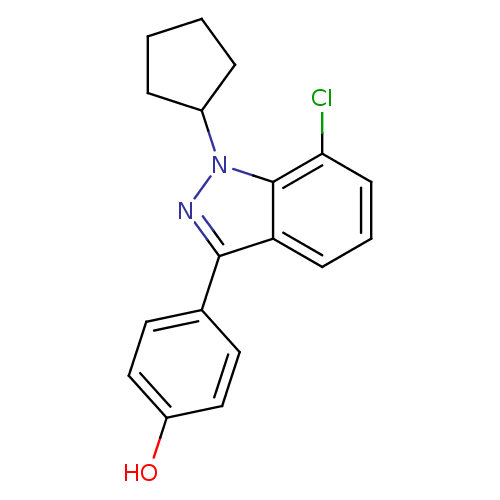 (4-(7-chloro-1-cyclopentyl-1H-indazol-3-yl)phenol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157483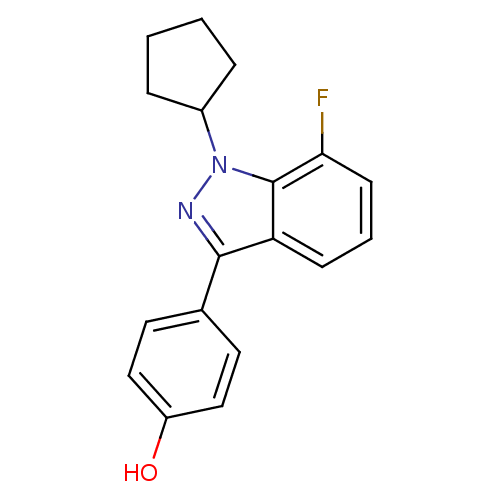 (4-(1-cyclopentyl-7-fluoro-1H-indazol-3-yl)phenol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157501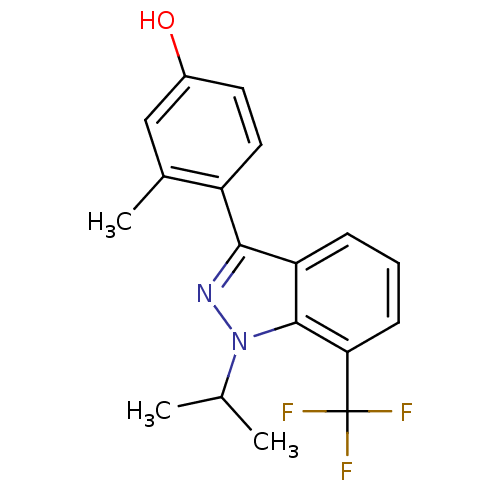 (4-[1-isopropyl-7-(trifluoromethyl)-1H-indazol-3-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157498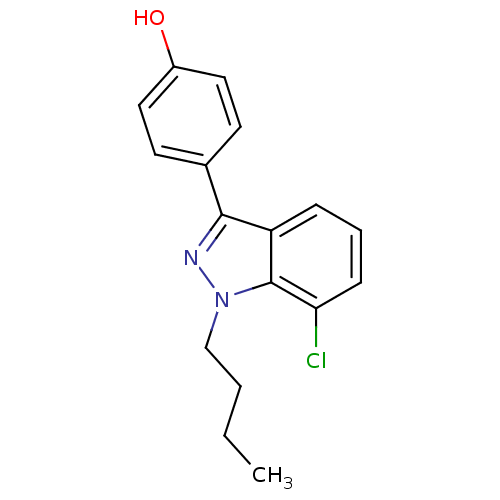 (4-(1-butyl-7-chloro-1H-indazol-3-yl)phenol | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50119183 (1-[4-(4-{[(S)-2-Hydroxy-3-(4-hydroxy-phenoxy)-prop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Compound was tested for the antagonistic activity against Beta-1 adrenergic receptor | Bioorg Med Chem Lett 12: 2957-61 (2002) BindingDB Entry DOI: 10.7270/Q2HT2NP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157491 (4-[1-(2,5-difluorophenyl)-6-hydroxy-1H-indazol-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157504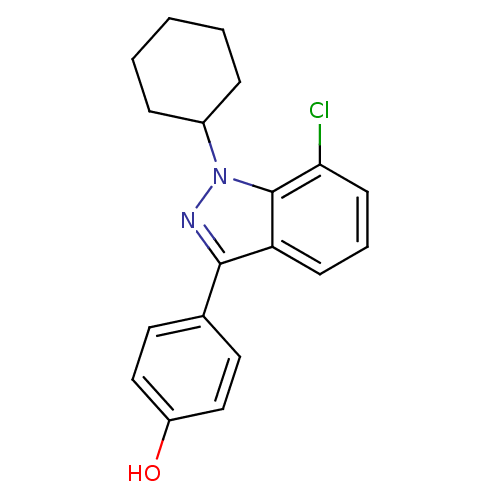 (4-(7-chloro-1-cyclohexyl-1H-indazol-3-yl)phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157489 (4-[1-(3-fluorophenyl)-6-hydroxy-1H-indazol-3-yl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157503 (4-(7-methyl-1-propyl-1H-indazol-3-yl)phenol | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157500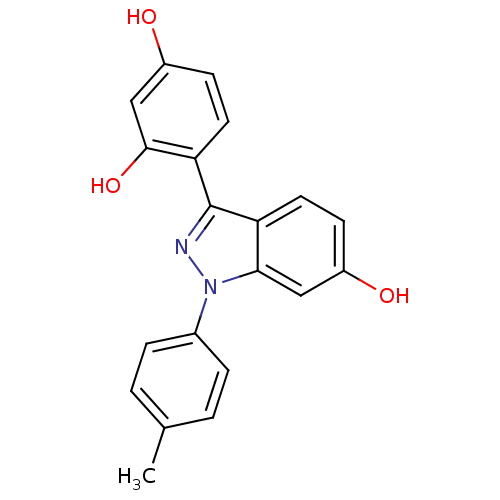 (4-[6-hydroxy-1-(4-methylphenyl)-1H-indazol-3-yl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157486 (4-(6-HYDROXY-1H-INDAZOL-3-YL)BENZENE-1,3-DIOL | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157508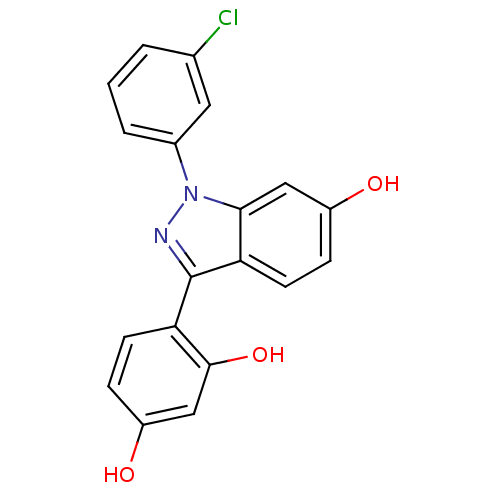 (4-[1-(3-chlorophenyl)-6-hydroxy-1H-indazol-3-yl]be...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157502 (4-(7-chloro-1-propyl-1H-indazol-3-yl)phenol | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157507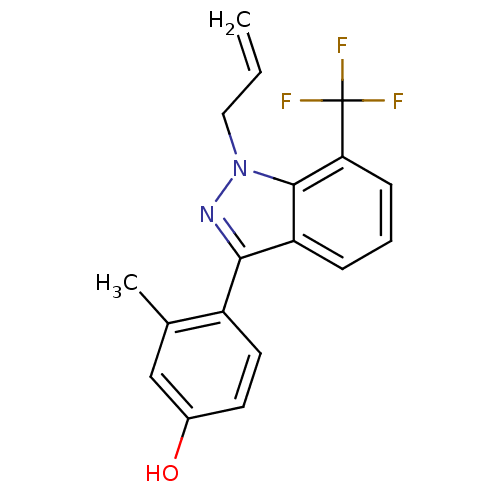 (4-[1-allyl-7-(trifluoromethyl)-1H-indazol-3-yl]-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157487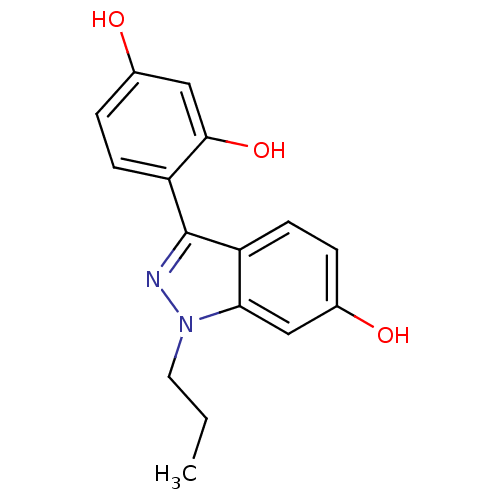 (4-(6-hydroxy-1-propyl-1H-indazol-3-yl)benzene-1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157493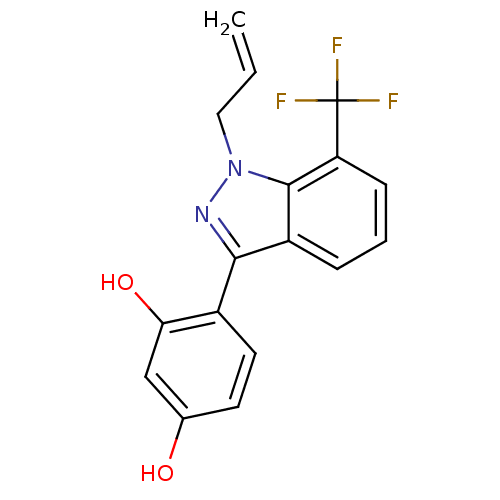 (4-[1-allyl-7-(trifluoromethyl)-1H-indazol-3-yl]ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157505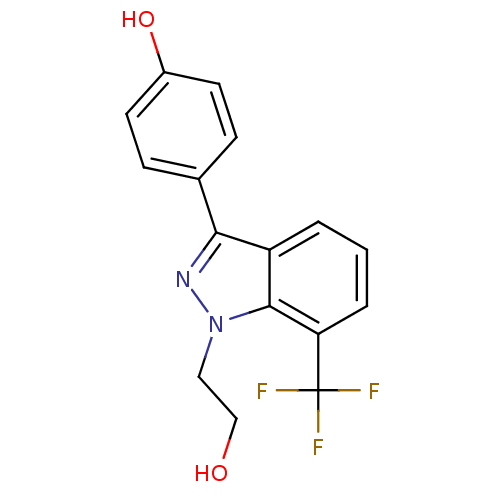 (4-[1-(2-hydroxyethyl)-7-(trifluoromethyl)-1H-indaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157484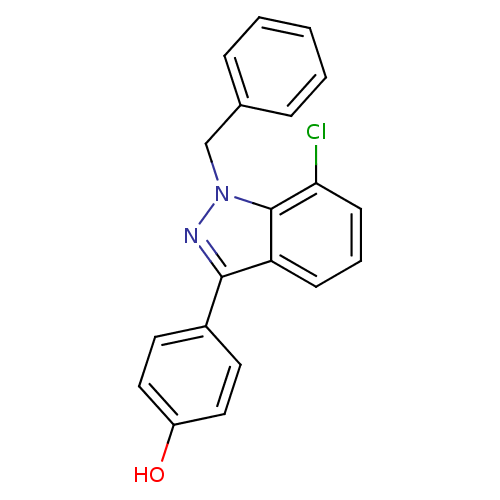 (4-(1-benzyl-7-chloro-1H-indazol-3-yl)phenol | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50157493 (4-[1-allyl-7-(trifluoromethyl)-1H-indazol-3-yl]ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]E2 from ERbeta ligand binding domain | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157494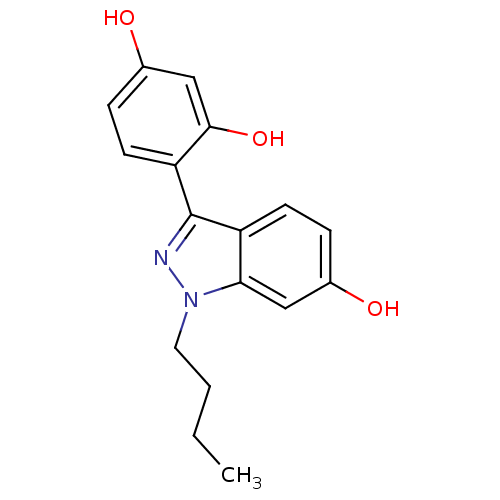 (4-(1-butyl-6-hydroxy-1H-indazol-3-yl)benzene-1,3-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at human ERalpha expressed in HAECT1 cells assessed as inhibition of NFkappaB transcription | J Med Chem 47: 6435-8 (2004) Article DOI: 10.1021/jm049194+ BindingDB Entry DOI: 10.7270/Q2639P7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50220305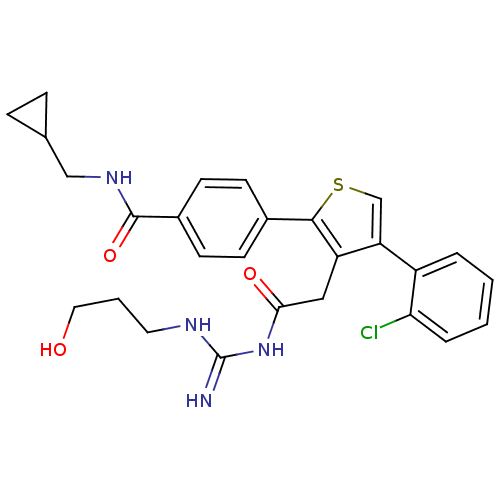 (4-(3-(2-(amino(3-hydroxypropylamino)methyleneamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of BACE1 by FRET assay | Bioorg Med Chem Lett 17: 5353-6 (2007) Article DOI: 10.1016/j.bmcl.2007.08.010 BindingDB Entry DOI: 10.7270/Q2WQ03GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50220314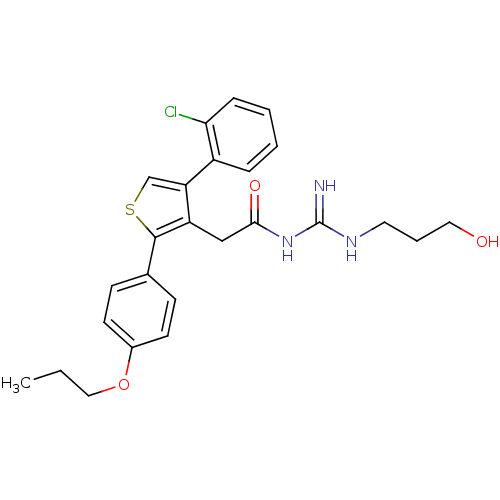 (CHEMBL237063 | N-(amino(3-hydroxypropylamino)methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of BACE1 by FRET assay | Bioorg Med Chem Lett 17: 5353-6 (2007) Article DOI: 10.1016/j.bmcl.2007.08.010 BindingDB Entry DOI: 10.7270/Q2WQ03GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 441 total ) | Next | Last >> |