 Found 745 hits with Last Name = 'somal' and Initial = 'g'
Found 745 hits with Last Name = 'somal' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598933
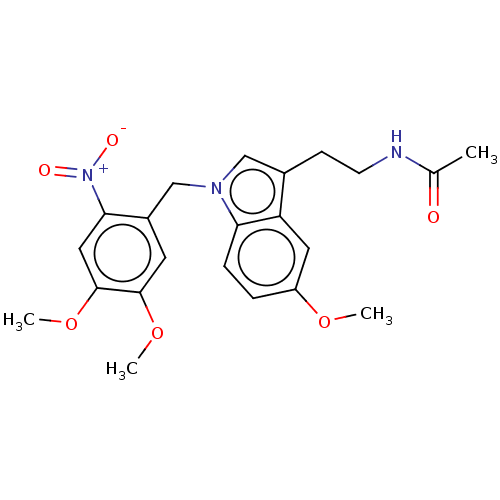
(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598933

(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598931
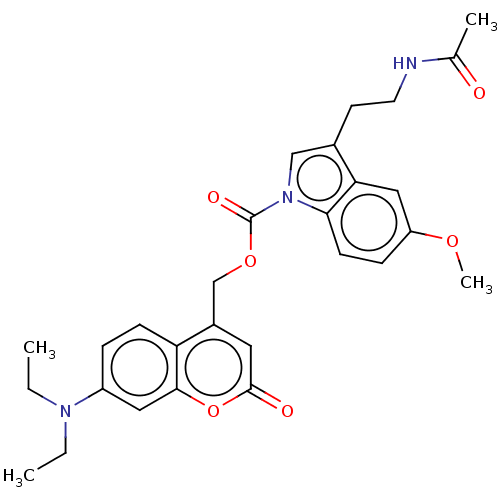
(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598930
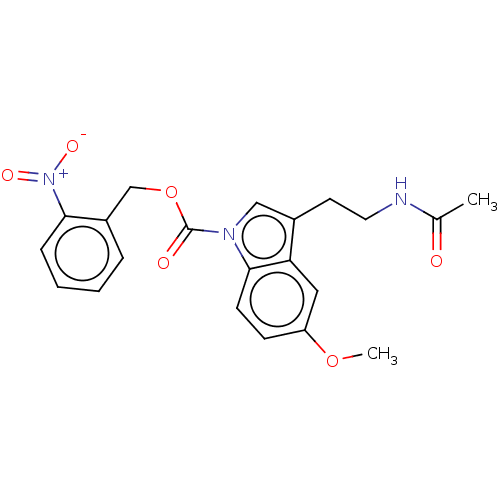
(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598931

(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598930

(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598931

(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598930

(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598931

(CHEMBL5209359)Show SMILES CCN(CC)c1ccc2c(COC(=O)n3cc(CCNC(C)=O)c4cc(OC)ccc34)cc(=O)oc2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598932

(CHEMBL5195595)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1cc(OC)c(OC)cc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598930

(CHEMBL5173623)Show SMILES COc1ccc2n(cc(CCNC(C)=O)c2c1)C(=O)OCc1ccccc1[N+]([O-])=O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50598933

(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM50598933

(CHEMBL5174929)Show SMILES COc1ccc2n(Cc3cc(OC)c(OC)cc3[N+]([O-])=O)cc(CCNC(C)=O)c2c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00717
BindingDB Entry DOI: 10.7270/Q2Z323P9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235829
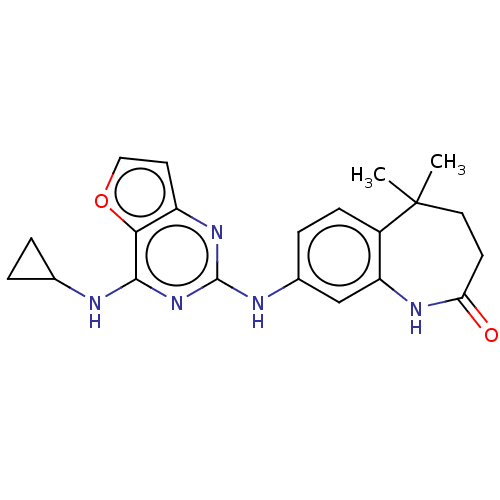
(CHEMBL4098474)Show SMILES CC1(C)CCC(=O)Nc2cc(Nc3nc(NC4CC4)c4occc4n3)ccc12 Show InChI InChI=1S/C21H23N5O2/c1-21(2)9-7-17(27)24-16-11-13(5-6-14(16)21)23-20-25-15-8-10-28-18(15)19(26-20)22-12-3-4-12/h5-6,8,10-12H,3-4,7,9H2,1-2H3,(H,24,27)(H2,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235828
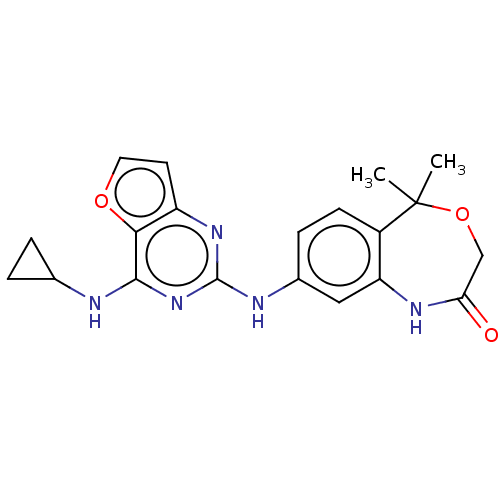
(CHEMBL4090753)Show SMILES CC1(C)OCC(=O)Nc2cc(Nc3nc(NC4CC4)c4occc4n3)ccc12 Show InChI InChI=1S/C20H21N5O3/c1-20(2)13-6-5-12(9-15(13)23-16(26)10-28-20)22-19-24-14-7-8-27-17(14)18(25-19)21-11-3-4-11/h5-9,11H,3-4,10H2,1-2H3,(H,23,26)(H2,21,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235831
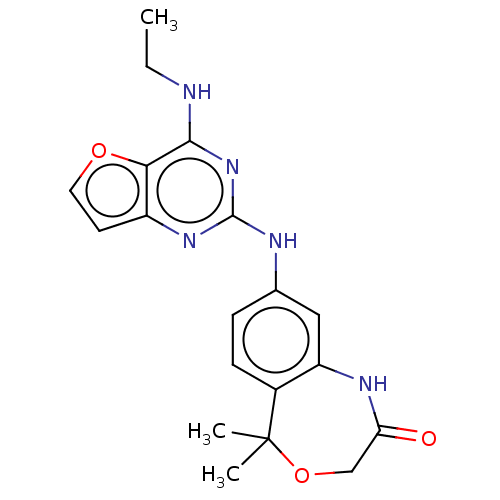
(CHEMBL4093260)Show SMILES CCNc1nc(Nc2ccc3c(NC(=O)COC3(C)C)c2)nc2ccoc12 Show InChI InChI=1S/C19H21N5O3/c1-4-20-17-16-13(7-8-26-16)23-18(24-17)21-11-5-6-12-14(9-11)22-15(25)10-27-19(12,2)3/h5-9H,4,10H2,1-3H3,(H,22,25)(H2,20,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235816

(CHEMBL4069365)Show SMILES CC1(C)OCC(=O)Nc2cc(Nc3nc(NCC(F)(F)F)c4occc4n3)ccc12 Show InChI InChI=1S/C19H18F3N5O3/c1-18(2)11-4-3-10(7-13(11)25-14(28)8-30-18)24-17-26-12-5-6-29-15(12)16(27-17)23-9-19(20,21)22/h3-7H,8-9H2,1-2H3,(H,25,28)(H2,23,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235830
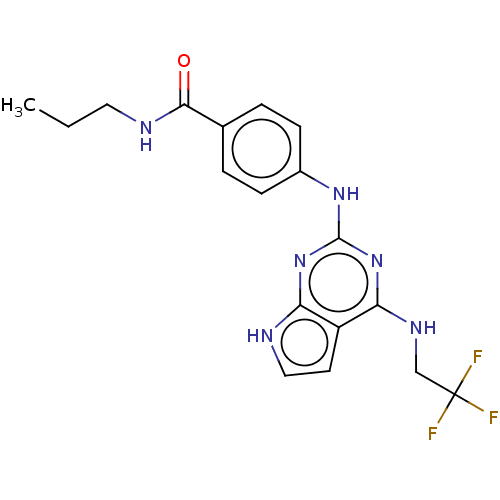
(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235816

(CHEMBL4069365)Show SMILES CC1(C)OCC(=O)Nc2cc(Nc3nc(NCC(F)(F)F)c4occc4n3)ccc12 Show InChI InChI=1S/C19H18F3N5O3/c1-18(2)11-4-3-10(7-13(11)25-14(28)8-30-18)24-17-26-12-5-6-29-15(12)16(27-17)23-9-19(20,21)22/h3-7H,8-9H2,1-2H3,(H,25,28)(H2,23,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235818
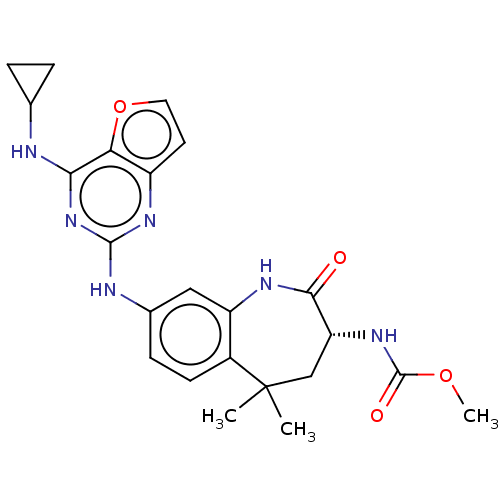
(CHEMBL4071441)Show SMILES COC(=O)N[C@@H]1CC(C)(C)c2ccc(Nc3nc(NC4CC4)c4occc4n3)cc2NC1=O |r| Show InChI InChI=1S/C23H26N6O4/c1-23(2)11-17(28-22(31)32-3)20(30)26-16-10-13(6-7-14(16)23)25-21-27-15-8-9-33-18(15)19(29-21)24-12-4-5-12/h6-10,12,17H,4-5,11H2,1-3H3,(H,26,30)(H,28,31)(H2,24,25,27,29)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235821
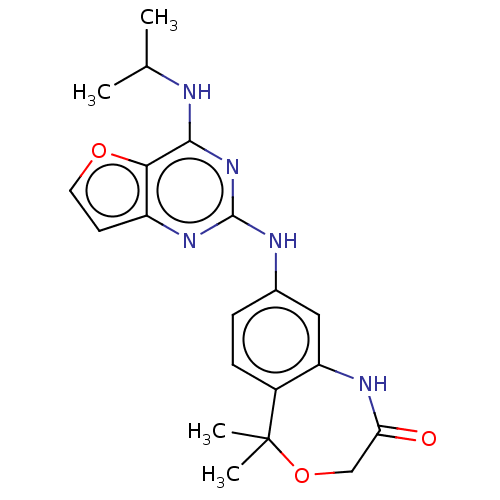
(CHEMBL4100939)Show SMILES CC(C)Nc1nc(Nc2ccc3c(NC(=O)COC3(C)C)c2)nc2ccoc12 Show InChI InChI=1S/C20H23N5O3/c1-11(2)21-18-17-14(7-8-27-17)24-19(25-18)22-12-5-6-13-15(9-12)23-16(26)10-28-20(13,3)4/h5-9,11H,10H2,1-4H3,(H,23,26)(H2,21,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235830

(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235827

(CHEMBL4081974)Show SMILES COC(=O)N[C@@H]1CC(C)(C)c2ccc(Nc3nc(NCC(F)F)c4occc4n3)cc2NC1=O |r| Show InChI InChI=1S/C22H24F2N6O4/c1-22(2)9-15(29-21(32)33-3)19(31)27-14-8-11(4-5-12(14)22)26-20-28-13-6-7-34-17(13)18(30-20)25-10-16(23)24/h4-8,15-16H,9-10H2,1-3H3,(H,27,31)(H,29,32)(H2,25,26,28,30)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration required against 5-lipoxygenase activity in cytosolic fractions of human neutrophils |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235833
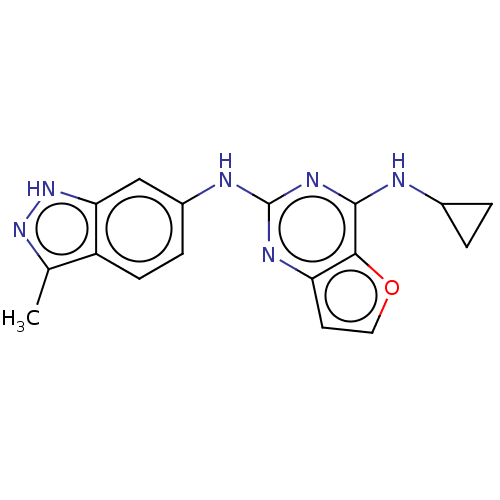
(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235833

(CHEMBL4062803)Show InChI InChI=1S/C17H16N6O/c1-9-12-5-4-11(8-14(12)23-22-9)19-17-20-13-6-7-24-15(13)16(21-17)18-10-2-3-10/h4-8,10H,2-3H2,1H3,(H,22,23)(H2,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235823

(CHEMBL4096597)Show SMILES CC1(C)CCC(=O)Nc2cc(Nc3nc(NCC(F)(F)F)c4occc4n3)ccc12 Show InChI InChI=1S/C20H20F3N5O2/c1-19(2)7-5-15(29)26-14-9-11(3-4-12(14)19)25-18-27-13-6-8-30-16(13)17(28-18)24-10-20(21,22)23/h3-4,6,8-9H,5,7,10H2,1-2H3,(H,26,29)(H2,24,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235825
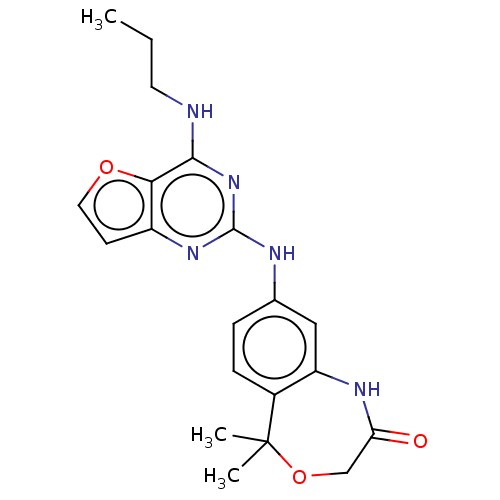
(CHEMBL4065615)Show SMILES CCCNc1nc(Nc2ccc3c(NC(=O)COC3(C)C)c2)nc2ccoc12 Show InChI InChI=1S/C20H23N5O3/c1-4-8-21-18-17-14(7-9-27-17)24-19(25-18)22-12-5-6-13-15(10-12)23-16(26)11-28-20(13,2)3/h5-7,9-10H,4,8,11H2,1-3H3,(H,23,26)(H2,21,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 4
(Homo sapiens (Human)) | BDBM50235830

(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of PAK4 kinase domain (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235826
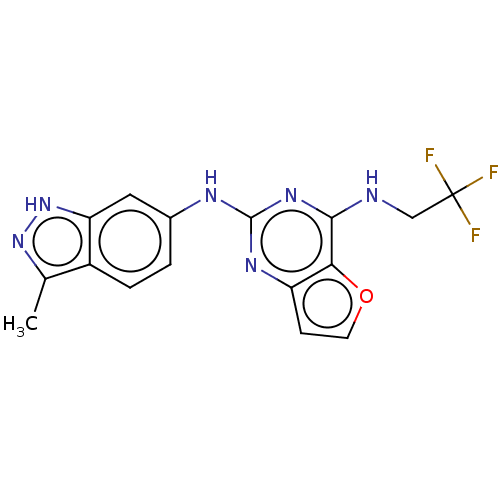
(CHEMBL4087963)Show SMILES Cc1n[nH]c2cc(Nc3nc(NCC(F)(F)F)c4occc4n3)ccc12 Show InChI InChI=1S/C16H13F3N6O/c1-8-10-3-2-9(6-12(10)25-24-8)21-15-22-11-4-5-26-13(11)14(23-15)20-7-16(17,18)19/h2-6H,7H2,1H3,(H,24,25)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50235830

(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235817
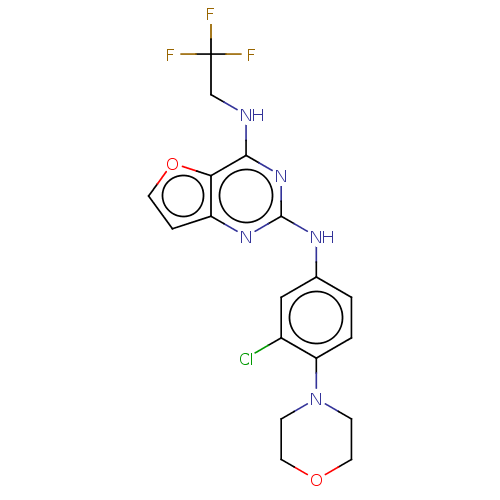
(CHEMBL4078893)Show SMILES FC(F)(F)CNc1nc(Nc2ccc(N3CCOCC3)c(Cl)c2)nc2ccoc12 Show InChI InChI=1S/C18H17ClF3N5O2/c19-12-9-11(1-2-14(12)27-4-7-28-8-5-27)24-17-25-13-3-6-29-15(13)16(26-17)23-10-18(20,21)22/h1-3,6,9H,4-5,7-8,10H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
In vitro antagonist activity at recombinant human Metabotropic glutamate receptor 2 expressed in RGT cells. |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235834
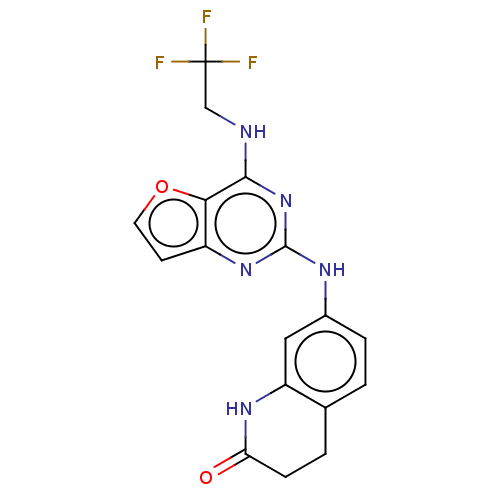
(CHEMBL4088907)Show SMILES FC(F)(F)CNc1nc(Nc2ccc3CCC(=O)Nc3c2)nc2ccoc12 Show InChI InChI=1S/C17H14F3N5O2/c18-17(19,20)8-21-15-14-11(5-6-27-14)24-16(25-15)22-10-3-1-9-2-4-13(26)23-12(9)7-10/h1,3,5-7H,2,4,8H2,(H,23,26)(H2,21,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235824
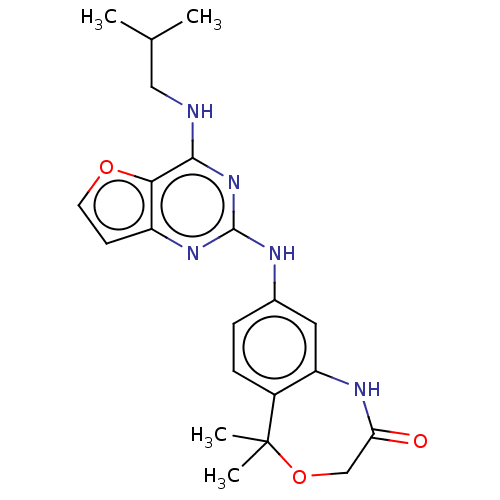
(CHEMBL4082961)Show SMILES CC(C)CNc1nc(Nc2ccc3c(NC(=O)COC3(C)C)c2)nc2ccoc12 Show InChI InChI=1S/C21H25N5O3/c1-12(2)10-22-19-18-15(7-8-28-18)25-20(26-19)23-13-5-6-14-16(9-13)24-17(27)11-29-21(14,3)4/h5-9,12H,10-11H2,1-4H3,(H,24,27)(H2,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235817

(CHEMBL4078893)Show SMILES FC(F)(F)CNc1nc(Nc2ccc(N3CCOCC3)c(Cl)c2)nc2ccoc12 Show InChI InChI=1S/C18H17ClF3N5O2/c19-12-9-11(1-2-14(12)27-4-7-28-8-5-27)24-17-25-13-3-6-29-15(13)16(26-17)23-10-18(20,21)22/h1-3,6,9H,4-5,7-8,10H2,(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235820
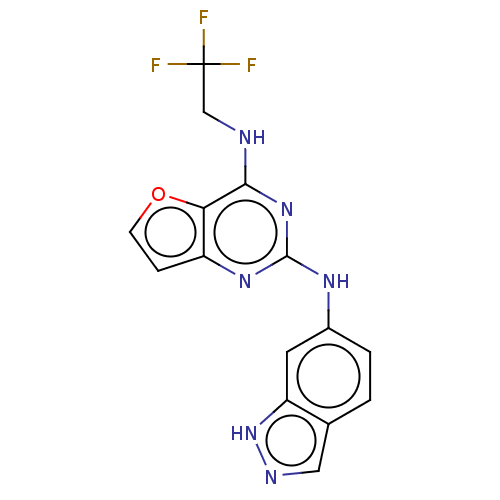
(CHEMBL4066664)Show InChI InChI=1S/C15H11F3N6O/c16-15(17,18)7-19-13-12-10(3-4-25-12)22-14(23-13)21-9-2-1-8-6-20-24-11(8)5-9/h1-6H,7H2,(H,20,24)(H2,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged Syk catalytic domain (356 to 635 residues) (unknown origin) using biotin-TYR1 as substrate after 60 mins by TR-FR... |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 8
(Homo sapiens (Human)) | BDBM50235830

(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM50235830

(CHEMBL2006765)Show SMILES CCCNC(=O)c1ccc(Nc2nc(NCC(F)(F)F)c3cc[nH]c3n2)cc1 Show InChI InChI=1S/C18H19F3N6O/c1-2-8-23-16(28)11-3-5-12(6-4-11)25-17-26-14-13(7-9-22-14)15(27-17)24-10-18(19,20)21/h3-7,9H,2,8,10H2,1H3,(H,23,28)(H3,22,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50235820

(CHEMBL4066664)Show InChI InChI=1S/C15H11F3N6O/c16-15(17,18)7-19-13-12-10(3-4-25-12)22-14(23-13)21-9-2-1-8-6-20-24-11(8)5-9/h1-6H,7H2,(H,20,24)(H2,19,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of Syk (unknown origin) after 60 mins by TR-FRET assay |
Bioorg Med Chem Lett 26: 5562-5567 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.077
BindingDB Entry DOI: 10.7270/Q2WQ061V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287215

(US9567339, Example A.4.11)Show SMILES Cc1c(NC2COC2)cccc1-c1ccc(C(N)=O)c2[nH]c(cc12)C1=CCN(CC1)S(C)(=O)=O |t:28| Show InChI InChI=1S/C25H28N4O4S/c1-15-18(4-3-5-22(15)27-17-13-33-14-17)19-6-7-20(25(26)30)24-21(19)12-23(28-24)16-8-10-29(11-9-16)34(2,31)32/h3-8,12,17,27-28H,9-11,13-14H2,1-2H3,(H2,26,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287233

(US9567339, Example A.7.2)Show SMILES Cc1c(cccc1-n1cnc2ccccc2c1=O)-c1ccc(C(N)=O)c2[nH]c(cc12)-c1cccnc1 Show InChI InChI=1S/C29H21N5O2/c1-17-19(8-4-10-26(17)34-16-32-24-9-3-2-7-21(24)29(34)36)20-11-12-22(28(30)35)27-23(20)14-25(33-27)18-6-5-13-31-15-18/h2-16,33H,1H3,(H2,30,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287259
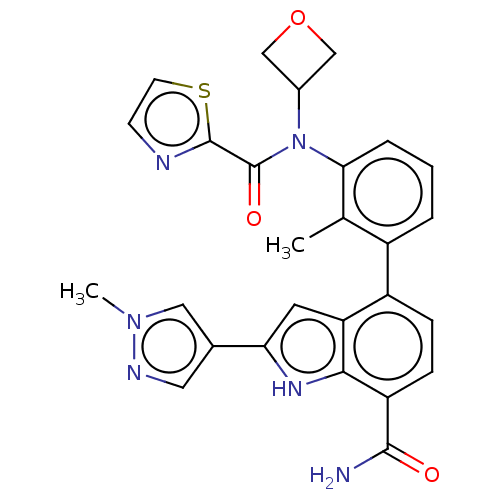
(US9567339, Example A.10.3)Show SMILES Cc1c(cccc1-c1ccc(C(N)=O)c2[nH]c(cc12)-c1cnn(C)c1)N(C1COC1)C(=O)c1nccs1 Show InChI InChI=1S/C27H24N6O3S/c1-15-18(4-3-5-23(15)33(17-13-36-14-17)27(35)26-29-8-9-37-26)19-6-7-20(25(28)34)24-21(19)10-22(31-24)16-11-30-32(2)12-16/h3-12,17,31H,13-14H2,1-2H3,(H2,28,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287358

(US9567339, Example E.1.4)Show SMILES NC(=O)c1ccc(-c2cncc(NC(=O)C=C)c2)c2cc[nH]c12 Show InChI InChI=1S/C17H14N4O2/c1-2-15(22)21-11-7-10(8-19-9-11)12-3-4-14(17(18)23)16-13(12)5-6-20-16/h2-9,20H,1H2,(H2,18,23)(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287375
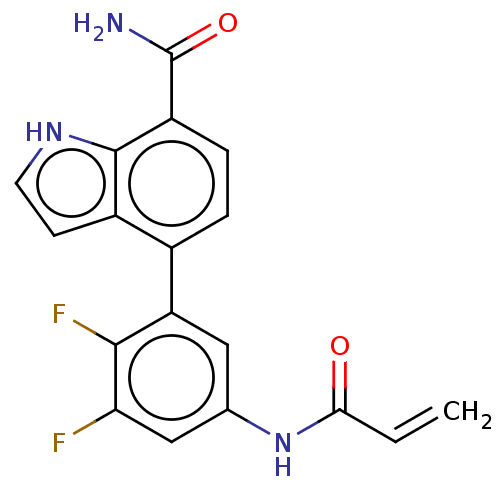
(US9567339, Example E.1.21)Show SMILES NC(=O)c1ccc(-c2cc(NC(=O)C=C)cc(F)c2F)c2cc[nH]c12 Show InChI InChI=1S/C18H13F2N3O2/c1-2-15(24)23-9-7-13(16(20)14(19)8-9)10-3-4-12(18(21)25)17-11(10)5-6-22-17/h2-8,22H,1H2,(H2,21,25)(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287270

(US9567339, Example A.15.1)Show SMILES Cn1cc(cn1)-c1cc2c(ccc(C(N)=O)c2[nH]1)-c1cccc(c1CO)-n1ccc2cc(cc(F)c2c1=O)C1CC1 Show InChI InChI=1S/C32H26FN5O3/c1-37-15-20(14-35-37)27-13-24-22(7-8-23(31(34)40)30(24)36-27)21-3-2-4-28(25(21)16-39)38-10-9-18-11-19(17-5-6-17)12-26(33)29(18)32(38)41/h2-4,7-15,17,36,39H,5-6,16H2,1H3,(H2,34,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK [393-659]
(Homo sapiens (Human)) | BDBM287296
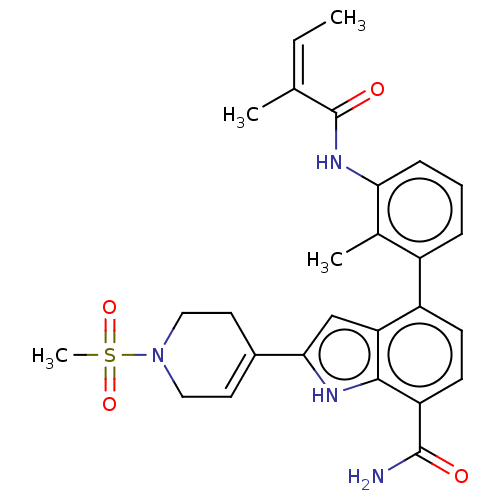
(US9567339, Example D.7.1)Show SMILES C\C=C(\C)C(=O)Nc1cccc(c1C)-c1ccc(C(N)=O)c2[nH]c(cc12)C1=CCN(CC1)S(C)(=O)=O |t:29| Show InChI InChI=1S/C27H30N4O4S/c1-5-16(2)27(33)30-23-8-6-7-19(17(23)3)20-9-10-21(26(28)32)25-22(20)15-24(29-25)18-11-13-31(14-12-18)36(4,34)35/h5-11,15,29H,12-14H2,1-4H3,(H2,28,32)(H,30,33)/b16-5- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | 6.5 | 25 |
AbbVie Inc.
US Patent
| Assay Description
The in-house BTK corresponds to recombinant human catalytic domain (aa 393-659), which was expressed in SF9 cells with an N-terminal his tag and puri... |
US Patent US9567339 (2017)
BindingDB Entry DOI: 10.7270/Q2P2715T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data