

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50434903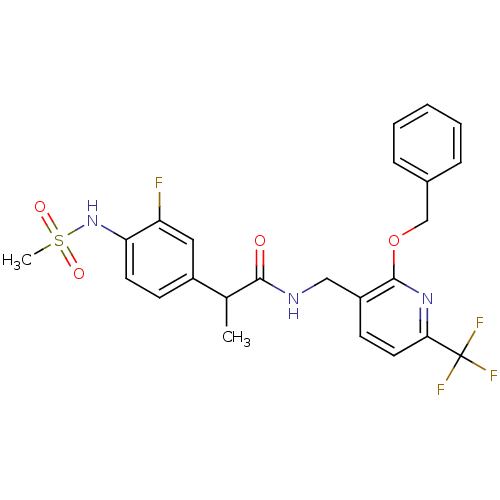 (CHEMBL2385223) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-arachidonoyldopamine-induced activity after 5 mins by FLIPR a... | Eur J Med Chem 64: 589-602 (2013) Article DOI: 10.1016/j.ejmech.2013.04.003 BindingDB Entry DOI: 10.7270/Q2BZ67FC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292202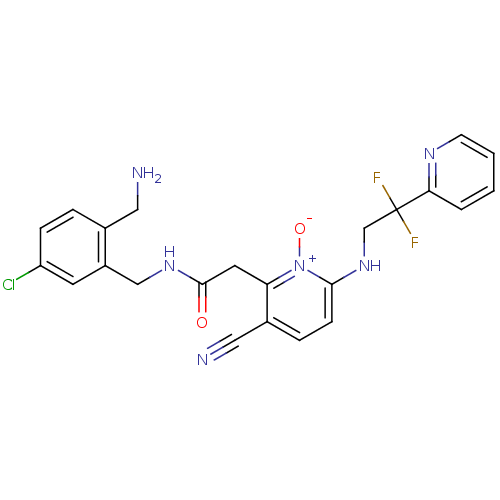 (CHEMBL382542 | N-(2-Aminomethyl-5-chloro-benzyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448761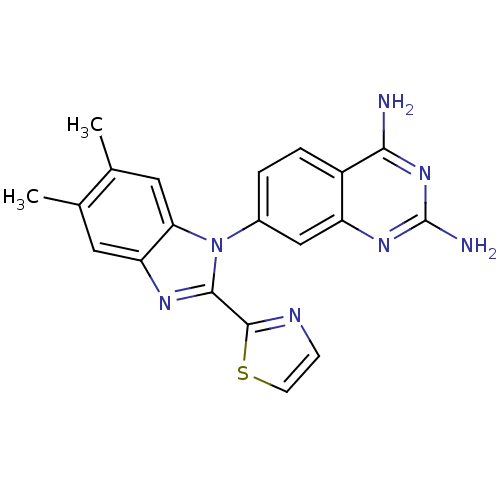 (CHEMBL3128021 | US8835445, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50292203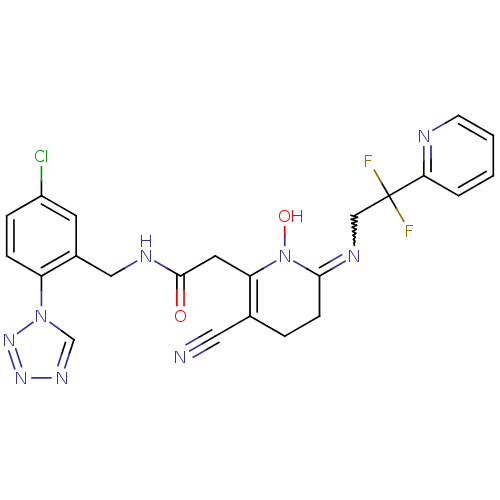 (CHEMBL196030 | N-(5-Chloro-2-tetrazol-1-yl-benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against thrombin in human plasma | Bioorg Med Chem Lett 15: 2771-5 (2005) Article DOI: 10.1016/j.bmcl.2005.03.110 BindingDB Entry DOI: 10.7270/Q2PN96C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50407749 (CHEMBL2112110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards Adenosine deaminase | J Med Chem 39: 277-84 (1996) Article DOI: 10.1021/jm9505674 BindingDB Entry DOI: 10.7270/Q2ZS2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50059414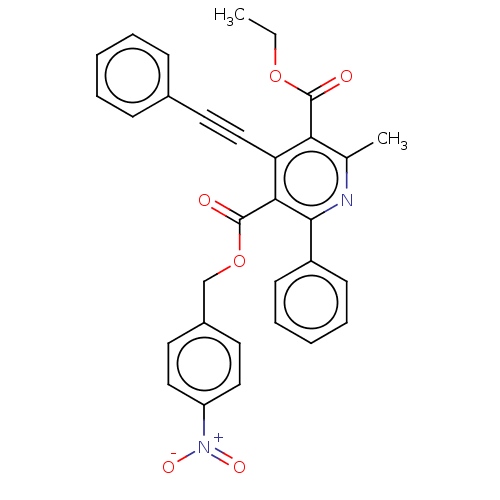 (2-Methyl-6-phenyl-4-phenylethynyl-1,4-dihydro-pyri...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at cloned human adenosine A3 receptor expressed in HEK-293 cells was determined using [125I]-AB-MECA as radioligand | J Med Chem 41: 2835-45 (1998) Article DOI: 10.1021/jm980094b BindingDB Entry DOI: 10.7270/Q25Q4V7D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50214974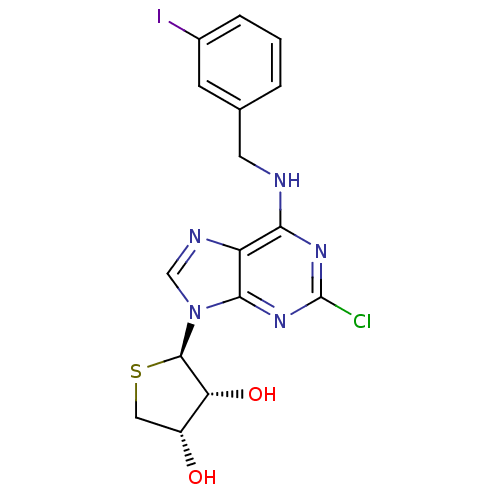 ((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448757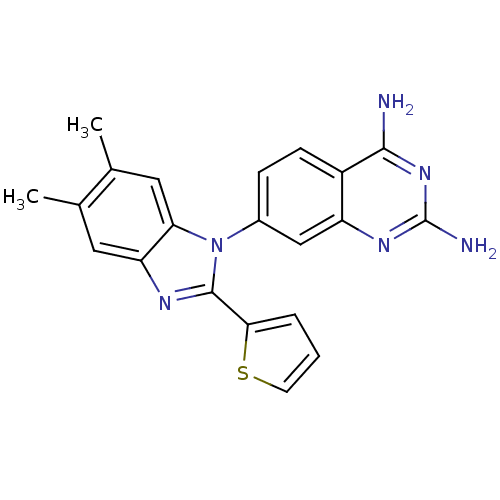 (CHEMBL3128025 | US8835445, 18) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland | Assay Description Inhibition assay using HIV protease and Sulfonamide compounds. | Chem Biol Drug Des 69: 298-313 (2007) Article DOI: 10.1111/j.1747-0285.2007.00514.x BindingDB Entry DOI: 10.7270/Q2TQ6011 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive reversible inhibition of human C-terminal His6-tagged CD73 expressed in HEK293 cells using AMP as substrate preincubated with substrate f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00525 BindingDB Entry DOI: 10.7270/Q29W0K29 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5'-nucleotidase (Homo sapiens (Human)) | BDBM50527134 (CHEMBL4471306 | US20230295213, Compound a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arcus Biosciences, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His-tagged CD73 (27 to 549 residues) expressed in HEK293 cells using AMP as substrate preincubated for 1 h... | J Med Chem 63: 3935-3955 (2020) Article DOI: 10.1021/acs.jmedchem.9b01713 BindingDB Entry DOI: 10.7270/Q2G1648T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12885 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12885 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448759 (CHEMBL3128023 | US8835445, 17) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448758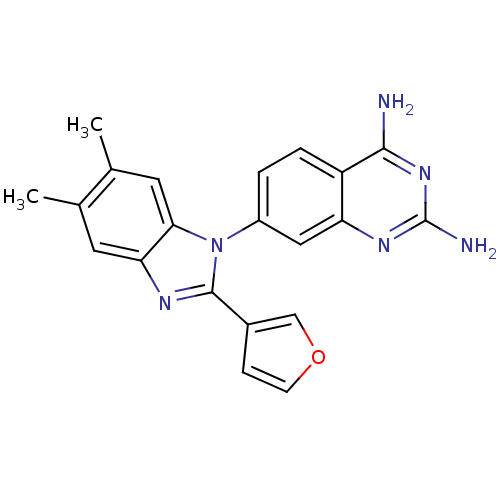 (CHEMBL3128024) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | Bioorg Med Chem 21: 6657-64 (2013) Article DOI: 10.1016/j.bmc.2013.08.015 BindingDB Entry DOI: 10.7270/Q26Q1ZPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030752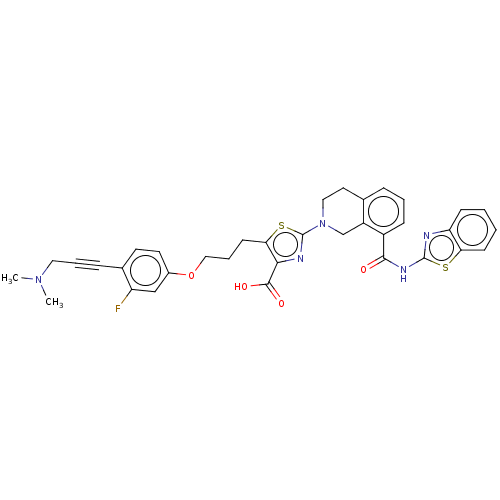 (CHEMBL3342333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus genotype 3a (isolate k3a) (HCV)) | BDBM403653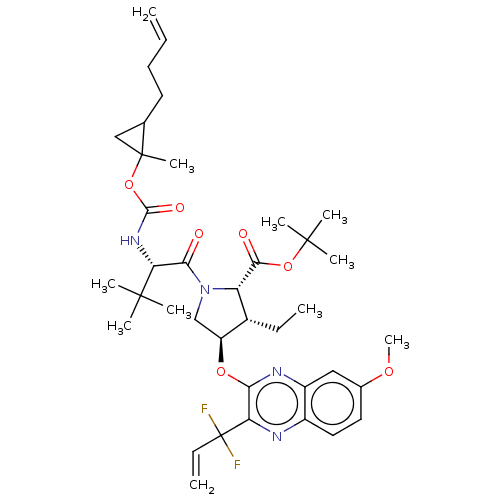 ((1aR,5S,8S,9S,10R,22aR)-5-tert-butyl-N-[(1R,2R)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus (Virus)) | BDBM403592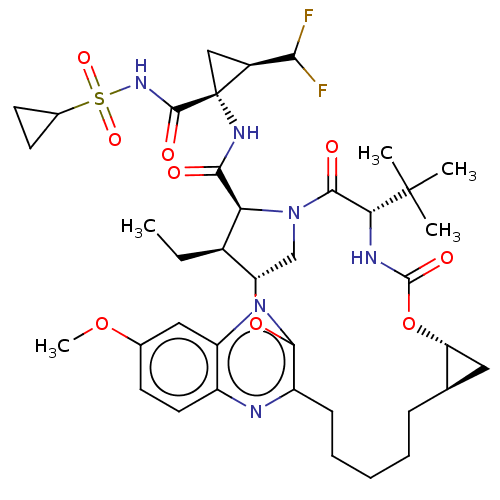 (Preparation of (1aR,5S,8S,9S,10R,22aR)-5-tert-buty...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Purified NS3 protease domain (amino acids 1-181) of the genotype 1b and 3a virus were generated as above. The internally quenched fluorogenic depsipe... | PubChem Bioassay (2006) BindingDB Entry DOI: 10.7270/Q2930WJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448743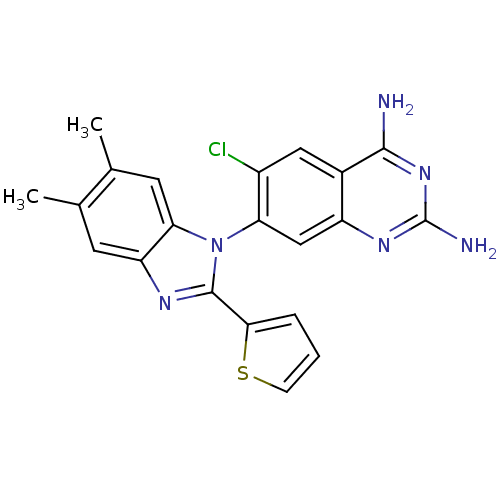 (CHEMBL3128015) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Antagonist activity at human TRPV1 expressed in CHOK1 cells assessed as inhibition of N-acetyldopamine-induced activity after 5 mins by FLIPR assay | J Med Chem 55: 8392-408 (2012) Article DOI: 10.1021/jm300780p BindingDB Entry DOI: 10.7270/Q2TX3GH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50369126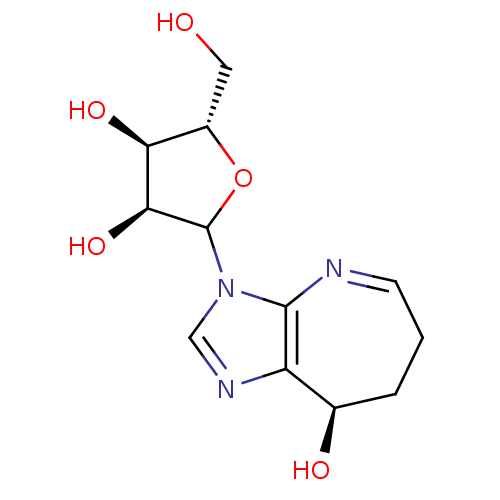 (CONFORMYCIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Binding affinity towards Adenosine deaminase | J Med Chem 39: 277-84 (1996) Article DOI: 10.1021/jm9505674 BindingDB Entry DOI: 10.7270/Q2ZS2X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50117108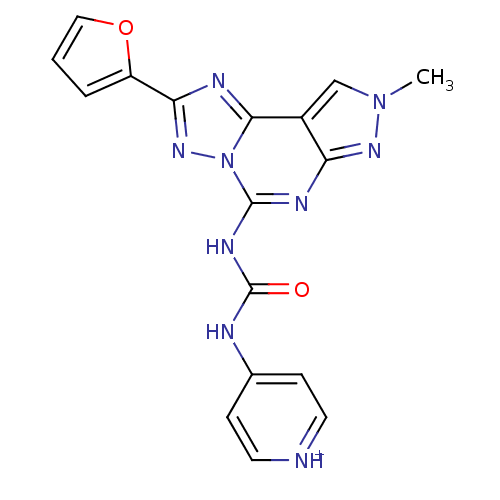 (4-[3-(2-Furan-2-yl-8-methyl-8H-pyrazolo[4,3-e][1,2...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]-MRE 3008-F20 from Human Adenosine A3 receptor expressed in HEK-293 cells | J Med Chem 45: 3579-82 (2002) BindingDB Entry DOI: 10.7270/Q2SN08B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030759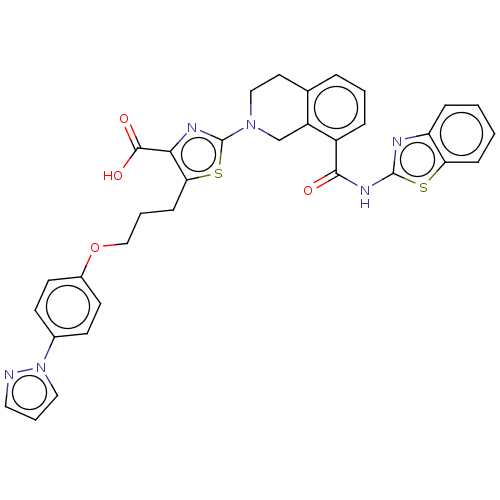 (CHEMBL3342194) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030758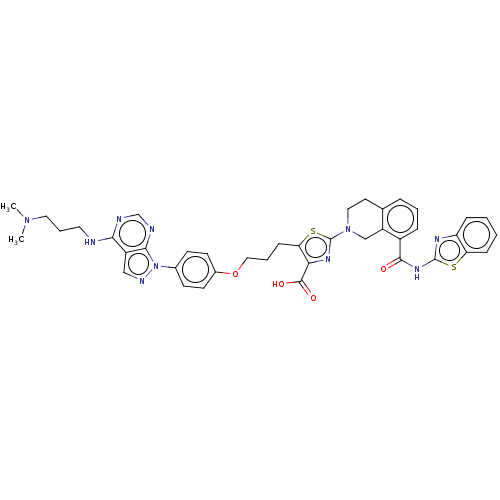 (CHEMBL3342195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030757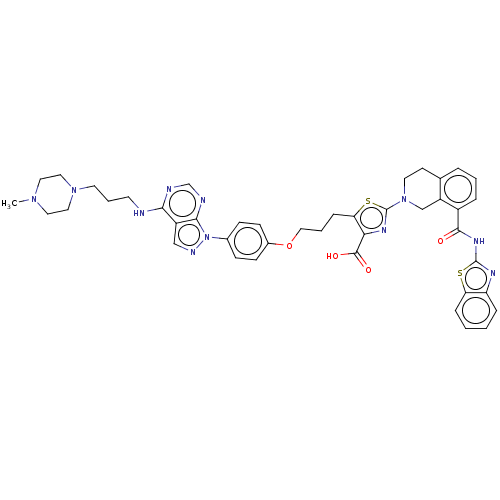 (CHEMBL3342196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030754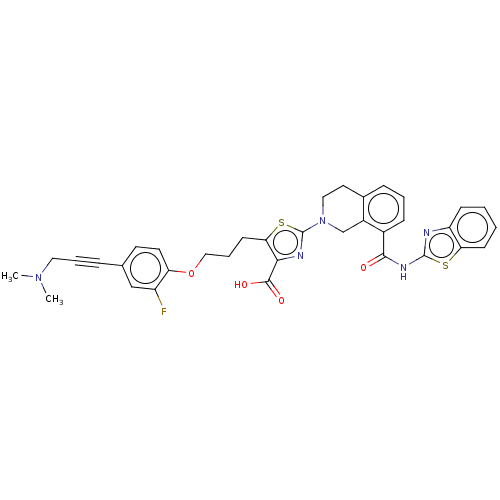 (CHEMBL3342332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50214981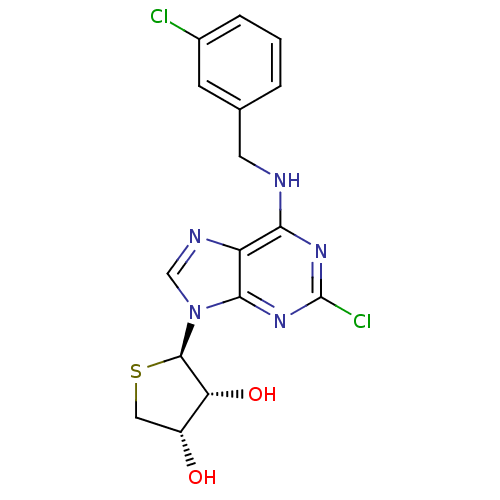 ((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University Curated by ChEMBL | Assay Description Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay | J Med Chem 60: 7459-7475 (2017) Article DOI: 10.1021/acs.jmedchem.7b00805 BindingDB Entry DOI: 10.7270/Q2XK8HQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448744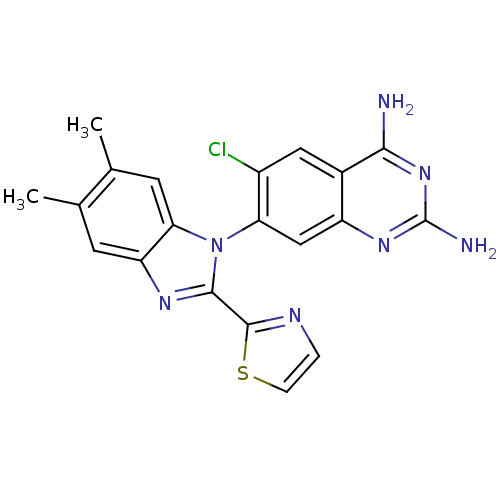 (CHEMBL3128014 | US8835445, 25 | US8835445, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448740 (CHEMBL3128018) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM50448756 (CHEMBL3128026 | US8835445, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics Inc. Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DHFR using dihydrofolate as substrate preincubated for 10 mins followed by substrate addition by spectrophotometr... | J Med Chem 57: 651-68 (2014) Article DOI: 10.1021/jm401204g BindingDB Entry DOI: 10.7270/Q2PN974G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50479738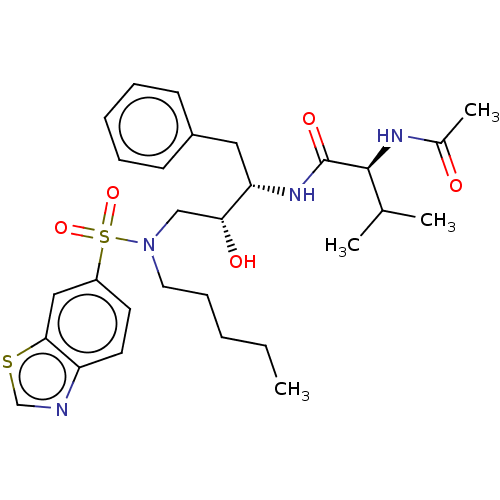 (CHEMBL514810) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50479738 (CHEMBL514810) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12893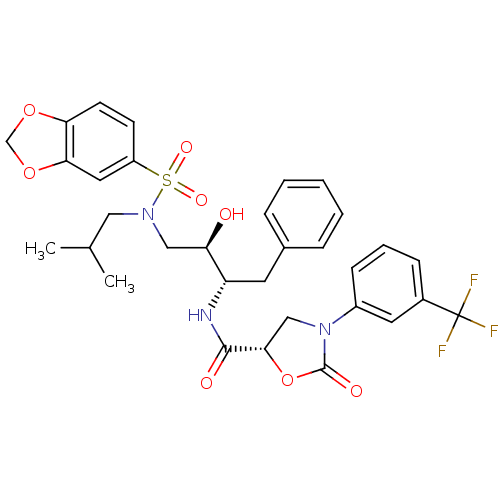 ((5S)-N-[(1S,2R)-3-[[(Benzo[1,3]dioxole-5-sulfonyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12894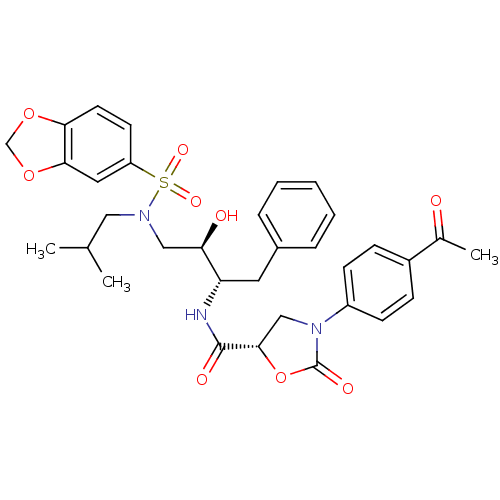 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM570555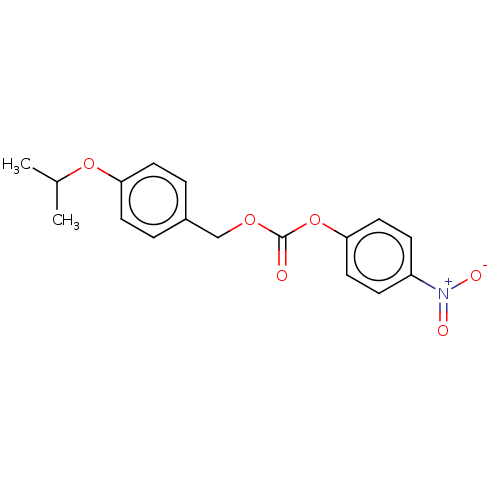 (US11440884, Example 18 | [4-(propan-2-yloxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZC864D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12893 ((5S)-N-[(1S,2R)-3-[[(Benzo[1,3]dioxole-5-sulfonyl)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12894 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55995 total ) | Next | Last >> |