 Found 1219 hits with Last Name = 'song' and Initial = 'l'
Found 1219 hits with Last Name = 'song' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604023
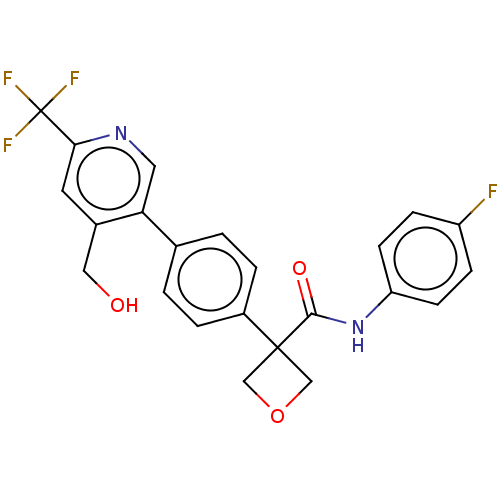
(CHEMBL5192977)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01716
BindingDB Entry DOI: 10.7270/Q2VX0MKR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM513874

(bioRxiv20220126.477782, S-217622 | bioRxiv20220126...)Show SMILES Cn1cnc(Cn2c(=O)[nH]\c(=N/c3cc4cn(C)nc4cc3Cl)n(Cc3cc(F)c(F)cc3F)c2=O)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604021
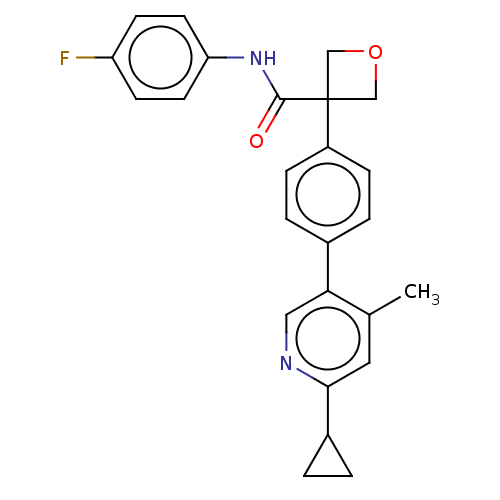
(CHEMBL5207194)Show SMILES Cc1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C1CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50538503
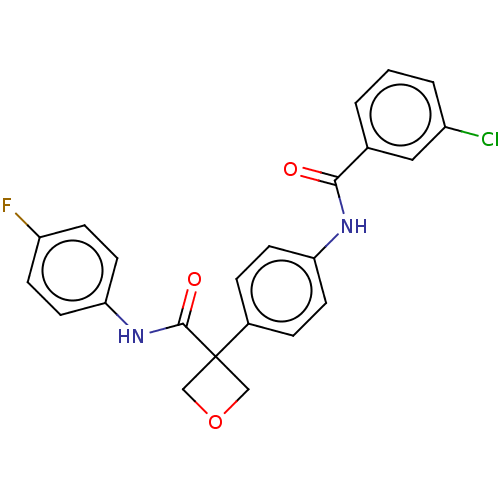
(CHEMBL4645108)Show SMILES Fc1ccc(NC(=O)C2(COC2)c2ccc(NC(=O)c3cccc(Cl)c3)cc2)cc1 Show InChI InChI=1S/C23H18ClFN2O3/c24-17-3-1-2-15(12-17)21(28)26-19-8-4-16(5-9-19)23(13-30-14-23)22(29)27-20-10-6-18(25)7-11-20/h1-12H,13-14H2,(H,26,28)(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM50462572

(CHEMBL4249337)Show SMILES Nc1nc2cc(CC[C@H]3C[C@H]([C@H](O)[C@@H]3O)n3ccc4c(N)ncnc34)ccc2cc1Br |r| Show InChI InChI=1S/C22H23BrN6O2/c23-15-8-12-3-1-11(7-16(12)28-21(15)25)2-4-13-9-17(19(31)18(13)30)29-6-5-14-20(24)26-10-27-22(14)29/h1,3,5-8,10,13,17-19,30-31H,2,4,9H2,(H2,25,28)(H2,24,26,27)/t13-,17+,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of full-length human N-terminal FLAG-tagged PRMT5 expressed in Sf9 insect cells using histone H2A as peptide after 120 mins in presence of... |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026
BindingDB Entry DOI: 10.7270/Q2SB490F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01716
BindingDB Entry DOI: 10.7270/Q2VX0MKR |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604026

(CHEMBL5192384)Show SMILES CC(C)(O)c1cc(ncc1-c1ccc(cc1)C1(COC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50220297
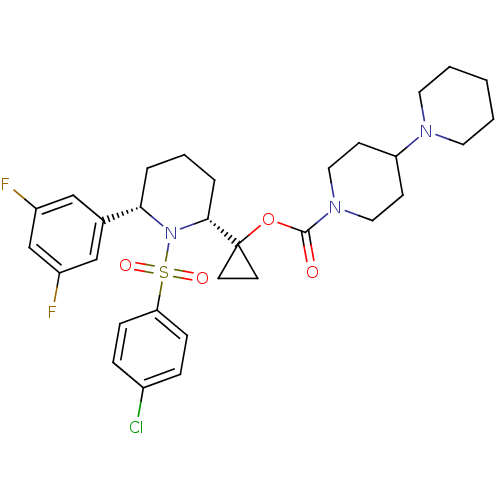
(1-((2R,6S)-1-(4-chlorophenylsulfonyl)-6-(3,5-diflu...)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CCC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C31H38ClF2N3O4S/c32-23-7-9-27(10-8-23)42(39,40)37-28(22-19-24(33)21-25(34)20-22)5-4-6-29(37)31(13-14-31)41-30(38)36-17-11-26(12-18-36)35-15-2-1-3-16-35/h7-10,19-21,26,28-29H,1-6,11-18H2/t28-,29+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase assessed as reduction of membrane Abeta40 level |
Bioorg Med Chem Lett 17: 5330-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.013
BindingDB Entry DOI: 10.7270/Q21G0KZB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330011
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCNCC1 |r| Show InChI InChI=1S/C21H30ClN3O5S/c1-15(2)18-13-29-14-19(25(18)31(27,28)17-5-3-16(22)4-6-17)21(7-8-21)30-20(26)24-11-9-23-10-12-24/h3-6,15,18-19,23H,7-14H2,1-2H3/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50477384

(CHEMBL248276)Show SMILES Fc1cc(F)cc(c1)C1CCCC(N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C31H38ClF2N3O4S/c32-23-7-9-27(10-8-23)42(39,40)37-28(22-19-24(33)21-25(34)20-22)5-4-6-29(37)31(13-14-31)41-30(38)36-17-11-26(12-18-36)35-15-2-1-3-16-35/h7-10,19-21,26,28-29H,1-6,11-18H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes |
Bioorg Med Chem Lett 17: 511-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.011
BindingDB Entry DOI: 10.7270/Q26W9DTK |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589777

(CHEMBL5192350)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1ccco1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589769

(CHEMBL5174111)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1cccs1)C(=O)c1cccnc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589778

(CHEMBL5204987)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1cccs1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589773

(CHEMBL5188908)Show SMILES Clc1ccc(CN2CCN(C[C@H]2C(=O)NCc2cccs2)C(=O)c2cc(=O)[nH]c(=O)[nH]2)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589770

(CHEMBL5188502)Show SMILES Clc1ccc(cc1Cl)N1CCN(C[C@H]1C(=O)NCc1cccs1)C(=O)c1cncc2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589780
(CHEMBL5188982)Show SMILES Clc1ccc(CN2CCN(C[C@H]2C(=O)NCc2ccco2)C(=O)c2cc(=O)[nH]c(=O)[nH]2)c(Cl)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330016
(((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(1-methylcyc...)Show SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC[C@H]1COC[C@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(C)CC1 |r| Show InChI InChI=1S/C24H36ClN3O6S/c1-23(2,17-29)27-12-10-26(11-13-27)22(30)34-15-19-14-33-16-21(24(3)8-9-24)28(19)35(31,32)20-6-4-18(25)5-7-20/h4-7,19,21,29H,8-17H2,1-3H3/t19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330006
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-isopropylm...)Show SMILES CC(C)[C@@H]1COC[C@@H](N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CC2CCC(C1)N2 |r,TLB:23:25:32:28.29| Show InChI InChI=1S/C23H32ClN3O5S/c1-15(2)20-13-31-14-21(27(20)33(29,30)19-7-3-16(24)4-8-19)23(9-10-23)32-22(28)26-11-17-5-6-18(12-26)25-17/h3-4,7-8,15,17-18,20-21,25H,5-6,9-14H2,1-2H3/t17?,18?,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM419133
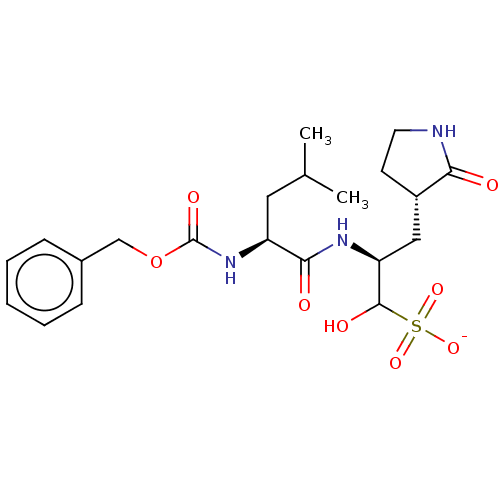
(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589779

(CHEMBL5181886)Show SMILES Clc1ccc(CN2CCN(CC2C(=O)NCc2ccco2)C(=O)c2cc(=O)[nH]c(=O)[nH]2)c(Cl)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50489341
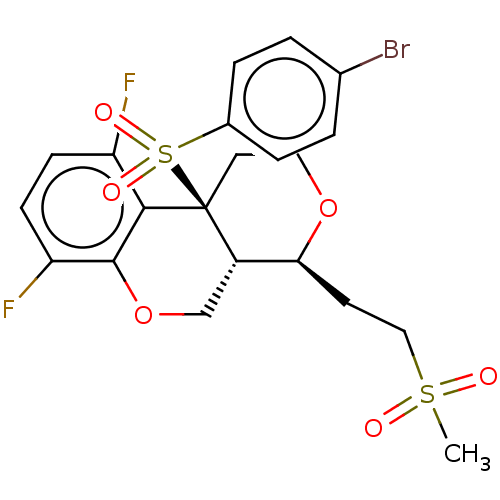
(CHEMBL2314617)Show SMILES [H][C@@]12COc3c(F)ccc(F)c3[C@@]1(CCO[C@H]2CCS(C)(=O)=O)S(=O)(=O)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C21H21BrF2O6S2/c1-31(25,26)11-8-18-15-12-30-20-17(24)7-6-16(23)19(20)21(15,9-10-29-18)32(27,28)14-4-2-13(22)3-5-14/h2-7,15,18H,8-12H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in HEK293 cells transfected with human APPSOW and APPLON after 5 hrs by sandwich imm... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642834
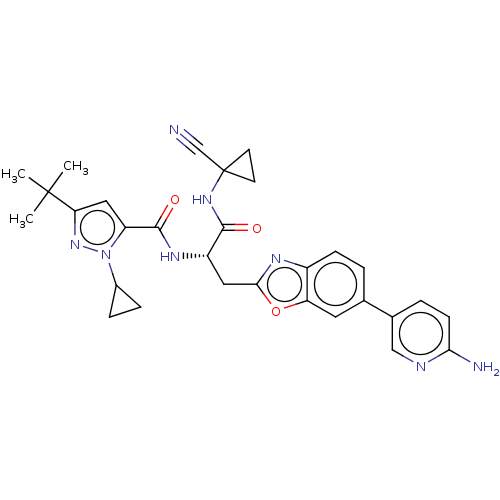
(US11858905, Compound 34)Show SMILES CC(C)(C)c1cc(C(=O)N[C@@H](Cc2nc3ccc(cc3o2)-c2ccc(N)nc2)C(=O)NC2(CC2)C#N)n(n1)C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589775
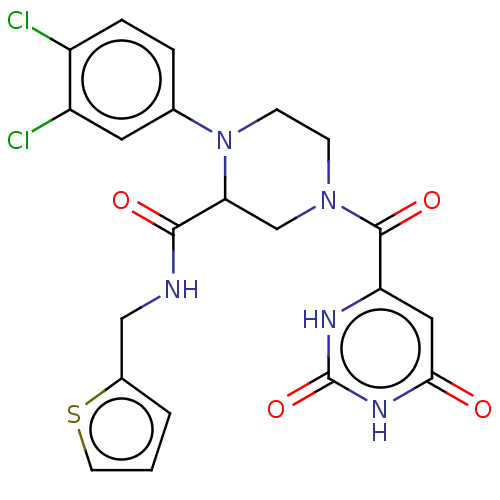
(CHEMBL5172248)Show SMILES Clc1ccc(cc1Cl)N1CCN(CC1C(=O)NCc1cccs1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50477389
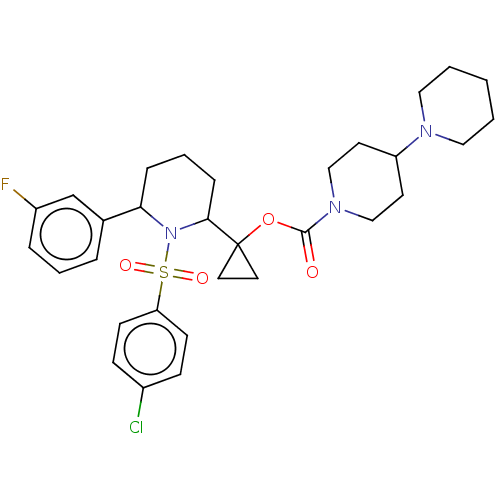
(CHEMBL248285)Show SMILES Fc1cccc(c1)C1CCCC(N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C31H39ClFN3O4S/c32-24-10-12-27(13-11-24)41(38,39)36-28(23-6-4-7-25(33)22-23)8-5-9-29(36)31(16-17-31)40-30(37)35-20-14-26(15-21-35)34-18-2-1-3-19-34/h4,6-7,10-13,22,26,28-29H,1-3,5,8-9,14-21H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes |
Bioorg Med Chem Lett 17: 511-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.011
BindingDB Entry DOI: 10.7270/Q26W9DTK |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50589774

(CHEMBL5201620)Show SMILES Clc1ccc(cc1Cl)N1CCN(CC1C(=O)NCc1ccco1)C(=O)c1cc(=O)[nH]c(=O)[nH]1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01146
BindingDB Entry DOI: 10.7270/Q2P55SFS |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50477366
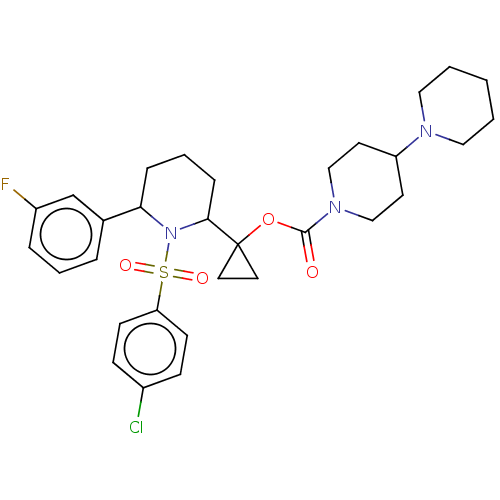
(CHEMBL537890)Show SMILES Cl.Fc1cccc(c1)C1CCCC(N1S(=O)(=O)c1ccc(Cl)cc1)C1(CC1)OC(=O)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C31H39ClFN3O4S.ClH/c32-24-10-12-27(13-11-24)41(38,39)36-28(23-6-4-7-25(33)22-23)8-5-9-29(36)31(16-17-31)40-30(37)35-20-14-26(15-21-35)34-18-2-1-3-19-34;/h4,6-7,10-13,22,26,28-29H,1-3,5,8-9,14-21H2;1H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes |
Bioorg Med Chem Lett 17: 511-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.011
BindingDB Entry DOI: 10.7270/Q26W9DTK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604025
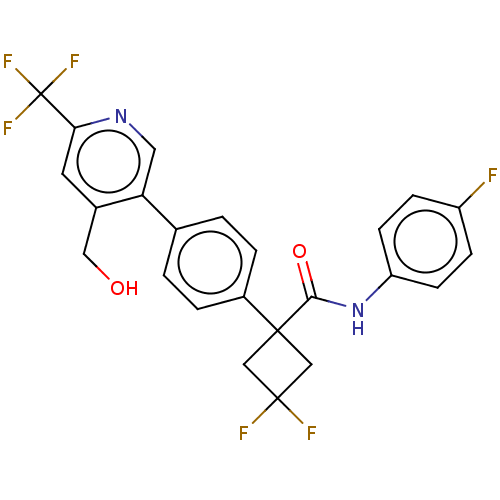
(CHEMBL5173861)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CC(F)(F)C1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642843

(US11858905, Compound 43)Show SMILES CCNc1ccc(cn1)-c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C3(C)CC3)C(=O)NC3(CC3)C#N)oc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330017
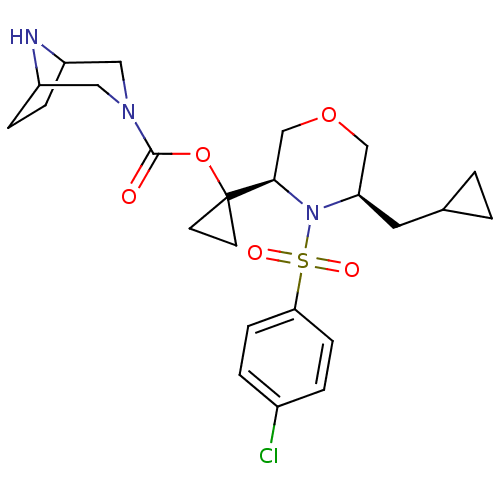
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES Clc1ccc(cc1)S(=O)(=O)N1[C@H](CC2CC2)COC[C@@H]1C1(CC1)OC(=O)N1CC2CCC(C1)N2 |r,TLB:24:26:33:29.30| Show InChI InChI=1S/C24H32ClN3O5S/c25-17-3-7-21(8-4-17)34(30,31)28-20(11-16-1-2-16)14-32-15-22(28)24(9-10-24)33-23(29)27-12-18-5-6-19(13-27)26-18/h3-4,7-8,16,18-20,22,26H,1-2,5-6,9-15H2/t18?,19?,20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50489349

(CHEMBL2314606)Show SMILES [H][C@@]12COc3c(F)ccc(F)c3[C@@]1(CCO[C@H]2CCS(=O)(=O)CCO)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H23ClF2O7S2/c23-14-1-3-15(4-2-14)34(29,30)22-8-10-31-19(7-11-33(27,28)12-9-26)16(22)13-32-21-18(25)6-5-17(24)20(21)22/h1-6,16,19,26H,7-13H2/t16-,19-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in HEK293 cells transfected with human APPSOW and APPLON after 5 hrs by sandwich imm... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50477377

(CHEMBL248070)Show SMILES C[C@H]1CN(CCN1C(=O)OC1(CC1)C1CCCC(N1S(=O)(=O)c1ccc(Cl)cc1)c1cccc(F)c1)C(C)(CO)CO Show InChI InChI=1S/C30H39ClFN3O6S/c1-21-18-33(29(2,19-36)20-37)15-16-34(21)28(38)41-30(13-14-30)27-8-4-7-26(22-5-3-6-24(32)17-22)35(27)42(39,40)25-11-9-23(31)10-12-25/h3,5-6,9-12,17,21,26-27,36-37H,4,7-8,13-16,18-20H2,1-2H3/t21-,26?,27?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes |
Bioorg Med Chem Lett 17: 511-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.011
BindingDB Entry DOI: 10.7270/Q26W9DTK |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50393452

(CHEMBL2159511)Show SMILES CS(=O)(=O)CC[C@@H]1OCC[C@@]2([C@H]1COc1c(F)ccc(F)c21)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H21ClF2O6S2/c1-31(25,26)11-8-18-15-12-30-20-17(24)7-6-16(23)19(20)21(15,9-10-29-18)32(27,28)14-4-2-13(22)3-5-14/h2-7,15,18H,8-12H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in HEK293 cells transfected with human APPSOW and APPLON after 5 hrs by sandwich imm... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 5
(Homo sapiens (Human)) | BDBM415557

(US10428104, Example 88)Show SMILES Nc1ncnc2n(ccc12)[C@@H]1O[C@H]([C@H](O)c2ccc(Cl)c(F)c2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H16ClFN4O4/c18-9-2-1-7(5-10(9)19)11(24)14-12(25)13(26)17(27-14)23-4-3-8-15(20)21-6-22-16(8)23/h1-6,11-14,17,24-26H,(H2,20,21,22)/t11-,12+,13-,14-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Jinan
Curated by ChEMBL
| Assay Description
Inhibition of PRMT5 (unknown origin) |
Bioorg Med Chem Lett 28: 3693-3699 (2018)
Article DOI: 10.1016/j.bmcl.2018.10.026
BindingDB Entry DOI: 10.7270/Q2SB490F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Presenilin-2
(Homo sapiens (Human)) | BDBM50393452

(CHEMBL2159511)Show SMILES CS(=O)(=O)CC[C@@H]1OCC[C@@]2([C@H]1COc1c(F)ccc(F)c21)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H21ClF2O6S2/c1-31(25,26)11-8-18-15-12-30-20-17(24)7-6-16(23)19(20)21(15,9-10-29-18)32(27,28)14-4-2-13(22)3-5-14/h2-7,15,18H,8-12H2,1H3/t15-,18-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PS2 gamma-secretase expressed in HEK cells harboring Awe-Lon gene assessed as reduction of amyloid beta-42 production |
ACS Med Chem Lett 3: 892-896 (2012)
Article DOI: 10.1021/ml300044f
BindingDB Entry DOI: 10.7270/Q2WD41PQ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642846

(US11858905, Compound 46)Show SMILES COCCN1CCC(=CC1)c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C3(C)CC3)C(=O)NC3(CC3)C#N)oc2c1 |r,c:7| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50489349

(CHEMBL2314606)Show SMILES [H][C@@]12COc3c(F)ccc(F)c3[C@@]1(CCO[C@H]2CCS(=O)(=O)CCO)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H23ClF2O7S2/c23-14-1-3-15(4-2-14)34(29,30)22-8-10-31-19(7-11-33(27,28)12-9-26)16(22)13-32-21-18(25)6-5-17(24)20(21)22/h1-6,16,19,26H,7-13H2/t16-,19-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta40 production in HEK293 cell membranes transfected with human APPSOW and APPLON mutant after 5 hrs... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50477372
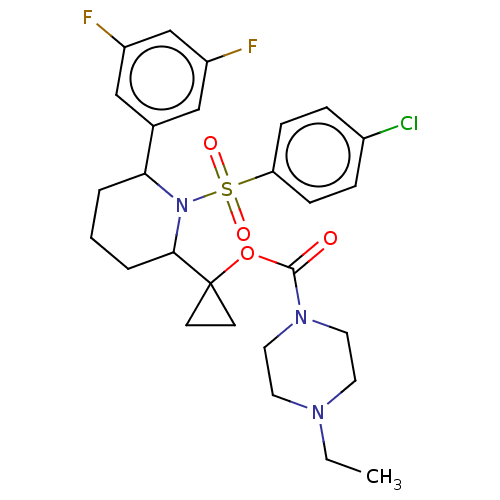
(CHEMBL245413)Show SMILES CCN1CCN(CC1)C(=O)OC1(CC1)C1CCCC(N1S(=O)(=O)c1ccc(Cl)cc1)c1cc(F)cc(F)c1 Show InChI InChI=1S/C27H32ClF2N3O4S/c1-2-31-12-14-32(15-13-31)26(34)37-27(10-11-27)25-5-3-4-24(19-16-21(29)18-22(30)17-19)33(25)38(35,36)23-8-6-20(28)7-9-23/h6-9,16-18,24-25H,2-5,10-15H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of gamma-secretase mediated amyloid beta-40 production in HEK293 cell membranes |
Bioorg Med Chem Lett 17: 511-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.011
BindingDB Entry DOI: 10.7270/Q26W9DTK |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642835

(US11858905, Compound 35)Show SMILES Cc1cc(ccn1)-c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C3(C)CC3)C(=O)NCC#N)oc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50489349

(CHEMBL2314606)Show SMILES [H][C@@]12COc3c(F)ccc(F)c3[C@@]1(CCO[C@H]2CCS(=O)(=O)CCO)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H23ClF2O7S2/c23-14-1-3-15(4-2-14)34(29,30)22-8-10-31-19(7-11-33(27,28)12-9-26)16(22)13-32-21-18(25)6-5-17(24)20(21)22/h1-6,16,19,26H,7-13H2/t16-,19-,22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta40 production in HEK293 cells transfected with human APPSOW and APPLON after 5 hrs by sandwich imm... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50604024

(CHEMBL5193283)Show SMILES OCc1cc(ncc1-c1ccc(cc1)C1(CCC1)C(=O)Nc1ccc(F)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01670
BindingDB Entry DOI: 10.7270/Q2VH5SX2 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642829
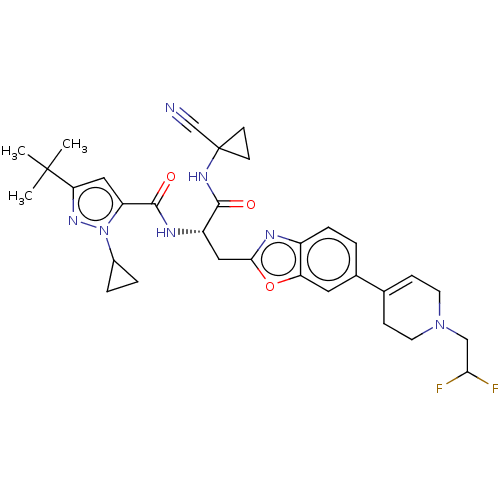
(US11858905, Compound 29)Show SMILES CC(C)(C)c1cc(C(=O)N[C@@H](Cc2nc3ccc(cc3o2)C2=CCN(CC(F)F)CC2)C(=O)NC2(CC2)C#N)n(n1)C1CC1 |r,t:23| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50393452

(CHEMBL2159511)Show SMILES CS(=O)(=O)CC[C@@H]1OCC[C@@]2([C@H]1COc1c(F)ccc(F)c21)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H21ClF2O6S2/c1-31(25,26)11-8-18-15-12-30-20-17(24)7-6-16(23)19(20)21(15,9-10-29-18)32(27,28)14-4-2-13(22)3-5-14/h2-7,15,18H,8-12H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PS1 gamma-secretase assessed as reduction of amyloid beta-40 production by membrane based assay |
ACS Med Chem Lett 3: 892-896 (2012)
Article DOI: 10.1021/ml300044f
BindingDB Entry DOI: 10.7270/Q2WD41PQ |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50393452

(CHEMBL2159511)Show SMILES CS(=O)(=O)CC[C@@H]1OCC[C@@]2([C@H]1COc1c(F)ccc(F)c21)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C21H21ClF2O6S2/c1-31(25,26)11-8-18-15-12-30-20-17(24)7-6-16(23)19(20)21(15,9-10-29-18)32(27,28)14-4-2-13(22)3-5-14/h2-7,15,18H,8-12H2,1H3/t15-,18-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta40 production in HEK293 cell membranes transfected with human APPSOW and APPLON mutant after 5 hrs... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50489338

(CHEMBL2314613)Show SMILES [H][C@@]12COc3c(F)ccc(F)c3[C@@]1(CCO[C@H]2CCS(C)(=O)=O)S(=O)(=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C21H20F4O6S2/c1-32(26,27)9-6-18-13-11-31-20-16(24)5-4-15(23)19(20)21(13,7-8-30-18)33(28,29)12-2-3-14(22)17(25)10-12/h2-5,10,13,18H,6-9,11H2,1H3/t13-,18-,21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta42 production in HEK293 cells transfected with human APPSOW and APPLON after 5 hrs by sandwich imm... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Presenilin-1
(Homo sapiens (Human)) | BDBM50489342

(CHEMBL2314603)Show SMILES [H][C@@]12COc3c(F)ccc(F)c3[C@@]1(CCO[C@H]2CCS(=O)(=O)c1ncc[nH]1)S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H21ClF2N2O6S2/c24-14-1-3-15(4-2-14)36(31,32)23-8-11-33-19(7-12-35(29,30)22-27-9-10-28-22)16(23)13-34-21-18(26)6-5-17(25)20(21)23/h1-6,9-10,16,19H,7-8,11-13H2,(H,27,28)/t16-,19-,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase-mediated amyloid beta40 production in HEK293 cell membranes transfected with human APPSOW and APPLON mutant after 5 hrs... |
Bioorg Med Chem Lett 23: 844-9 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.047
BindingDB Entry DOI: 10.7270/Q2J96988 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50330023
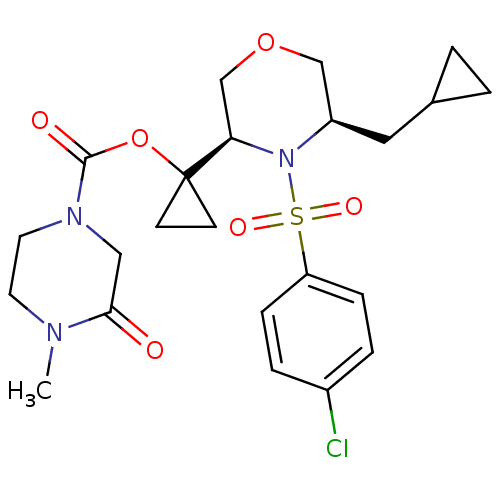
(1-((3R,5R)-4-(4-chlorophenylsulfonyl)-5-(cycloprop...)Show SMILES CN1CCN(CC1=O)C(=O)OC1(CC1)[C@H]1COC[C@@H](CC2CC2)N1S(=O)(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C23H30ClN3O6S/c1-25-10-11-26(13-21(25)28)22(29)33-23(8-9-23)20-15-32-14-18(12-16-2-3-16)27(20)34(30,31)19-6-4-17(24)5-7-19/h4-7,16,18,20H,2-3,8-15H2,1H3/t18-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Lab.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 after 30 mins |
Bioorg Med Chem Lett 20: 6606-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.028
BindingDB Entry DOI: 10.7270/Q2RF5V79 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM642826

(US11858905, Compound 26)Show SMILES CN1CCN(CC1)c1ccc2nc(C[C@H](NC(=O)c3cc(nn3C3CC3)C(C)(C)C)C(=O)NC3(CC3)C#N)oc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data