 Found 6522 hits with Last Name = 'song' and Initial = 'y'
Found 6522 hits with Last Name = 'song' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279

(CHEMBL4436207)Show SMILES [H][C@@]12CCO[C@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(cc1)B(O)O |r| Show InChI InChI=1S/C27H37BN2O9S/c1-18(2)15-30(40(35,36)21-10-8-20(9-11-21)28(33)34)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)39-25-17-38-26-22(25)12-13-37-26/h3-11,18,22-26,31,33-34H,12-17H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM8125

((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...)Show SMILES [H][C@@]1(CO[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H37N3O7S/c1-18(2)15-30(38(33,34)21-10-8-20(28)9-11-21)16-24(31)23(14-19-6-4-3-5-7-19)29-27(32)37-25-17-36-26-22(25)12-13-35-26/h3-11,18,22-26,31H,12-17,28H2,1-2H3,(H,29,32)/t22-,23-,24+,25-,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor Xa (serine protease) was determined |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110577
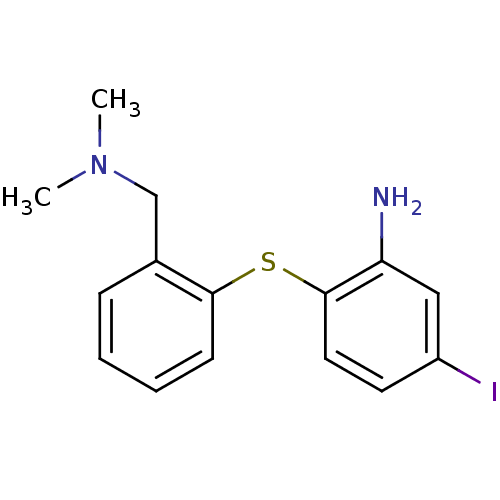
(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110578
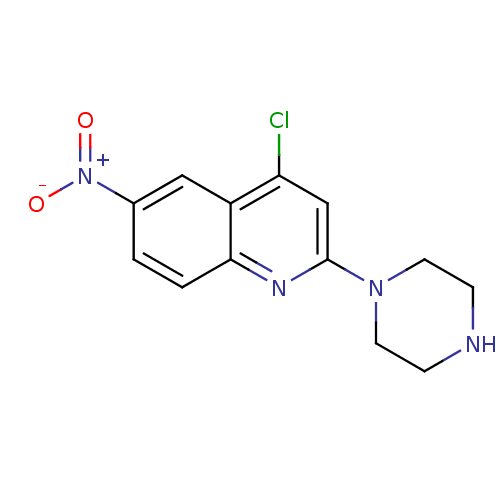
(4-Chloro-6-nitro-2-piperazin-1-yl-quinoline | CHEM...)Show InChI InChI=1S/C13H13ClN4O2/c14-11-8-13(17-5-3-15-4-6-17)16-12-2-1-9(18(19)20)7-10(11)12/h1-2,7-8,15H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193861
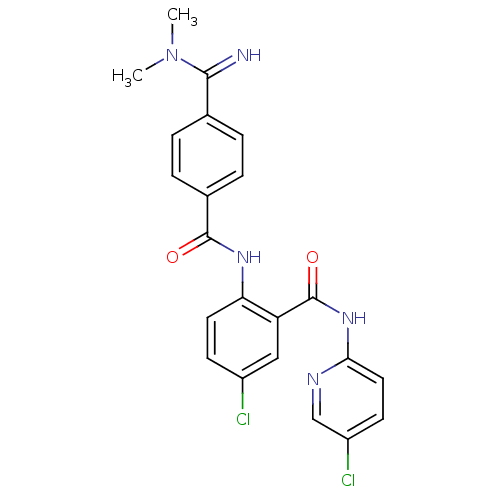
(5-chloro-N-(5-chloro-pyridin-2-yl)-2-[4-(N,N-dimet...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H19Cl2N5O2/c1-29(2)20(25)13-3-5-14(6-4-13)21(30)27-18-9-7-15(23)11-17(18)22(31)28-19-10-8-16(24)12-26-19/h3-12,25H,1-2H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249120
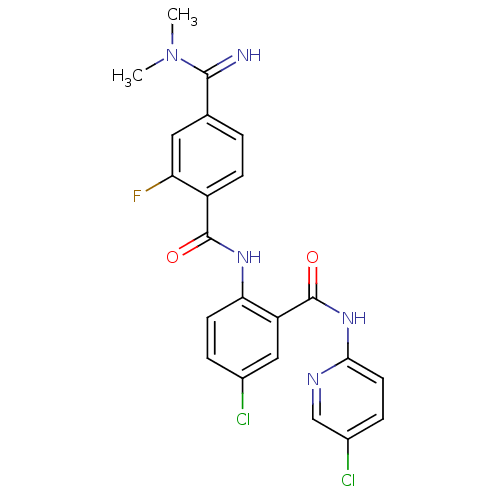
(CHEMBL472967 | N-(4-chloro-2-(5-chloropyridin-2-yl...)Show SMILES CN(C)C(=N)c1ccc(C(=O)Nc2ccc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c(F)c1 Show InChI InChI=1S/C22H18Cl2FN5O2/c1-30(2)20(26)12-3-6-15(17(25)9-12)21(31)28-18-7-4-13(23)10-16(18)22(32)29-19-8-5-14(24)11-27-19/h3-11,26H,1-2H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249423
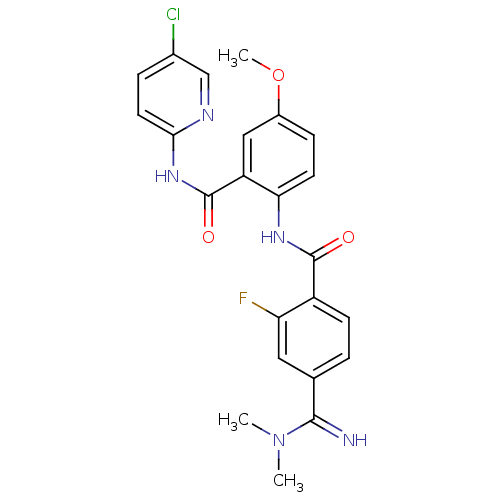
(CHEMBL515919 | N-(2-(5-chloropyridin-2-ylcarbamoyl...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2F)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H21ClFN5O3/c1-30(2)21(26)13-4-7-16(18(25)10-13)22(31)28-19-8-6-15(33-3)11-17(19)23(32)29-20-9-5-14(24)12-27-20/h4-12,26H,1-3H3,(H,28,31)(H,27,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249298
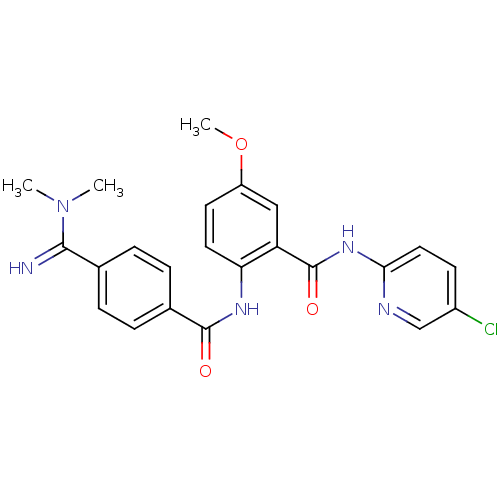
(BEVYXXA | CHEMBL512351 | N-(5-chloropyridin-2-yl)-...)Show SMILES COc1ccc(NC(=O)c2ccc(cc2)C(=N)N(C)C)c(c1)C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C23H22ClN5O3/c1-29(2)21(25)14-4-6-15(7-5-14)22(30)27-19-10-9-17(32-3)12-18(19)23(31)28-20-11-8-16(24)13-26-20/h4-13,25H,1-3H3,(H,27,30)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50063266
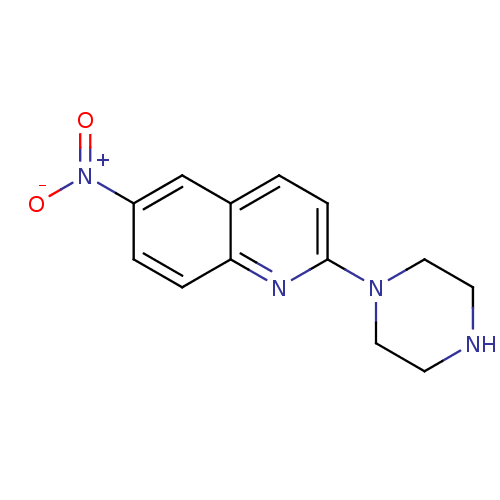
(6-Nitro-2-piperazin-1-yl-quinoline | 6-nitroquipaz...)Show InChI InChI=1S/C13H14N4O2/c18-17(19)11-2-3-12-10(9-11)1-4-13(15-12)16-7-5-14-6-8-16/h1-4,9,14H,5-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50113598
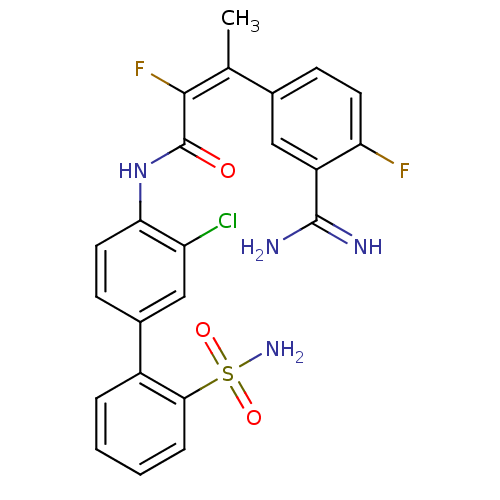
((Z)-3-(3-Carbamimidoyl-4-fluoro-phenyl)-2-fluoro-b...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1ccc(F)c(c1)C(N)=N Show InChI InChI=1S/C23H19ClF2N4O3S/c1-12(13-6-8-18(25)16(10-13)22(27)28)21(26)23(31)30-19-9-7-14(11-17(19)24)15-4-2-3-5-20(15)34(29,32)33/h2-11H,1H3,(H3,27,28)(H,30,31)(H2,29,32,33)/b21-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against factor Xa,activity expressed as Ki nM |
Bioorg Med Chem Lett 12: 1511-5 (2002)
BindingDB Entry DOI: 10.7270/Q2P84B6X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396023
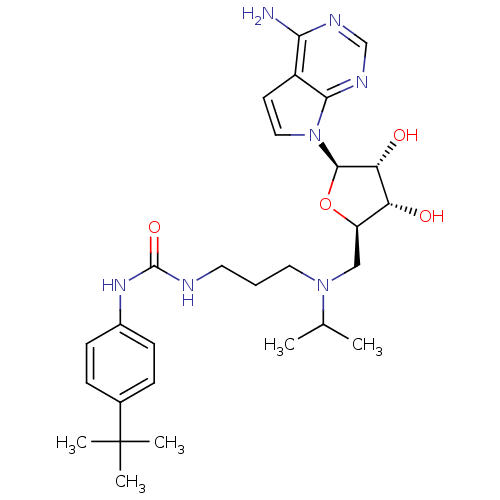
(CHEMBL2169919)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactor |
J Med Chem 56: 8972-83 (2013)
Article DOI: 10.1021/jm4007752
BindingDB Entry DOI: 10.7270/Q2D50PDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396023

(CHEMBL2169919)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110584

(3-(3-Fluoro-propyl)-6-nitro-2-piperazin-1-yl-quino...)Show InChI InChI=1S/C16H19FN4O2/c17-5-1-2-12-10-13-11-14(21(22)23)3-4-15(13)19-16(12)20-8-6-18-7-9-20/h3-4,10-11,18H,1-2,5-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110574
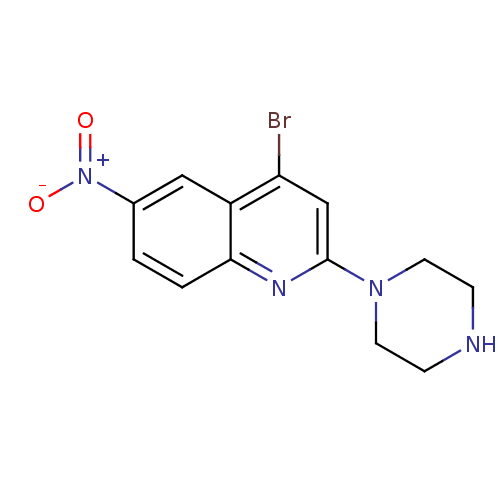
(4-Bromo-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...)Show InChI InChI=1S/C13H13BrN4O2/c14-11-8-13(17-5-3-15-4-6-17)16-12-2-1-9(18(19)20)7-10(11)12/h1-2,7-8,15H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM7840
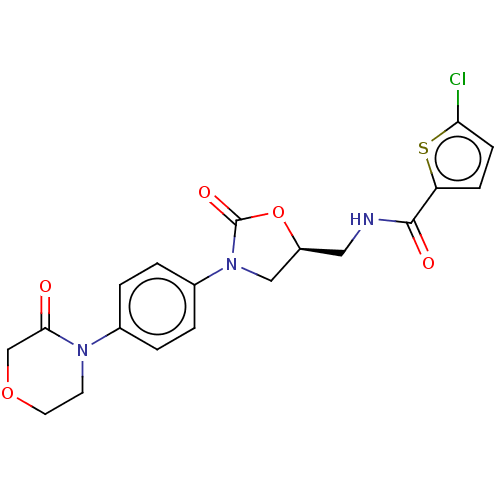
(RIVAROXABAN | US8822458, 44 | US8822458, 97)Show SMILES Clc1ccc(s1)C(=O)NC[C@H]1CN(C(=O)O1)c1ccc(cc1)N1CCOCC1=O |r| Show InChI InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980
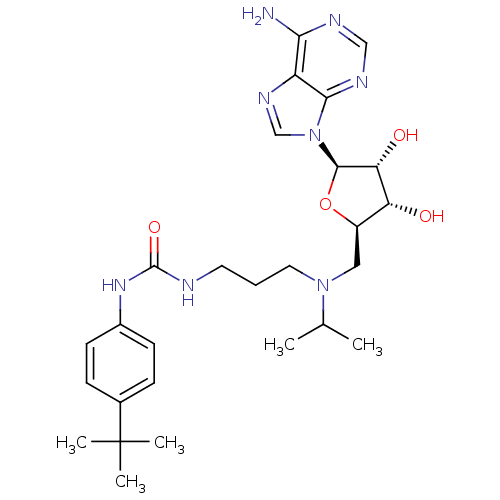
(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50292847
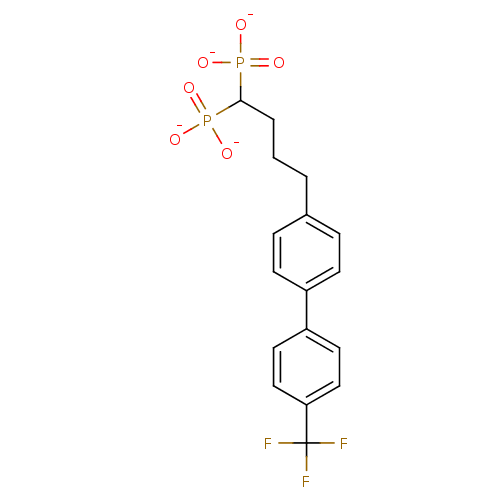
(4-[4-(4-Trifluoromethylphenyl)phenyl)]butyldiphosp...)Show SMILES [O-]P([O-])(=O)C(CCCc1ccc(cc1)-c1ccc(cc1)C(F)(F)F)P([O-])([O-])=O Show InChI InChI=1S/C17H19F3O6P2/c18-17(19,20)15-10-8-14(9-11-15)13-6-4-12(5-7-13)2-1-3-16(27(21,22)23)28(24,25)26/h4-11,16H,1-3H2,(H2,21,22,23)(H2,24,25,26)/p-4 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay |
J Med Chem 52: 976-88 (2009)
Article DOI: 10.1021/jm801023u
BindingDB Entry DOI: 10.7270/Q2VM4C87 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980

(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactor |
J Med Chem 56: 8972-83 (2013)
Article DOI: 10.1021/jm4007752
BindingDB Entry DOI: 10.7270/Q2D50PDQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM22416

((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...)Show SMILES Fc1ccc(cc1)[C@@H]1CCNC[C@H]1COc1ccc2OCOc2c1 Show InChI InChI=1S/C19H20FNO3/c20-15-3-1-13(2-4-15)17-7-8-21-10-14(17)11-22-16-5-6-18-19(9-16)24-12-23-18/h1-6,9,14,17,21H,7-8,10-12H2/t14-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140413
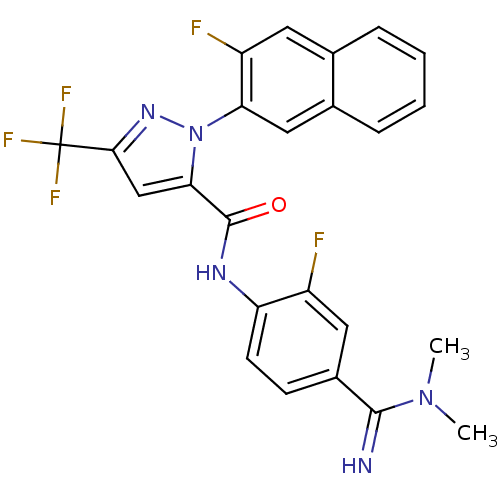
(2-(3-Fluoro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2cc3ccccc3cc2F)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18F5N5O/c1-33(2)22(30)15-7-8-18(16(25)10-15)31-23(35)20-12-21(24(27,28)29)32-34(20)19-11-14-6-4-3-5-13(14)9-17(19)26/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140424
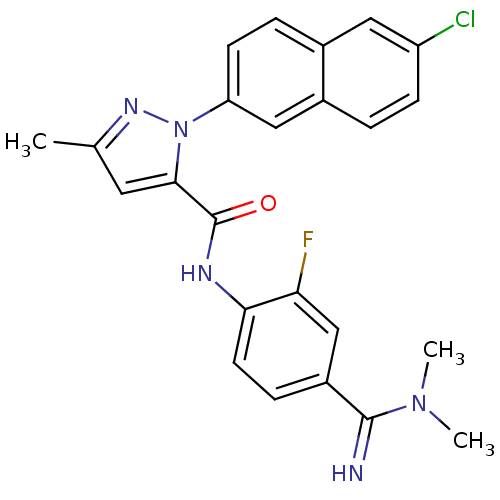
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C24H21ClFN5O/c1-14-10-22(24(32)28-21-9-6-17(13-20(21)26)23(27)30(2)3)31(29-14)19-8-5-15-11-18(25)7-4-16(15)12-19/h4-13,27H,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140443
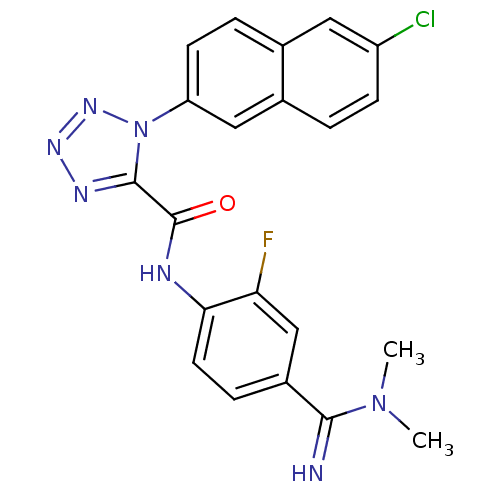
(1-(6-Chloro-naphthalen-2-yl)-1H-tetrazole-5-carbox...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2nnnn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 Show InChI InChI=1S/C21H17ClFN7O/c1-29(2)19(24)14-5-8-18(17(23)11-14)25-21(31)20-26-27-28-30(20)16-7-4-12-9-15(22)6-3-13(12)10-16/h3-11,24H,1-2H3,(H,25,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396980

(CHEMBL2171169)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C27H40N8O4/c1-16(2)34(12-6-11-29-26(38)33-18-9-7-17(8-10-18)27(3,4)5)13-19-21(36)22(37)25(39-19)35-15-32-20-23(28)30-14-31-24(20)35/h7-10,14-16,19,21-22,25,36-37H,6,11-13H2,1-5H3,(H2,28,30,31)(H2,29,33,38)/t19-,21-,22-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396979
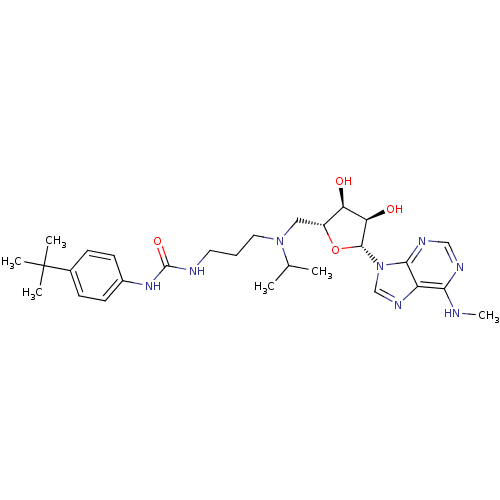
(CHEMBL2171170)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCCNC(=O)Nc2ccc(cc2)C(C)(C)C)C(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H42N8O4/c1-17(2)35(13-7-12-30-27(39)34-19-10-8-18(9-11-19)28(3,4)5)14-20-22(37)23(38)26(40-20)36-16-33-21-24(29-6)31-15-32-25(21)36/h8-11,15-17,20,22-23,26,37-38H,7,12-14H2,1-6H3,(H,29,31,32)(H2,30,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L catalytic domain amino acid (1 to 472) using [3H]-SAM after 30 mins by scintillation counter |
J Med Chem 55: 8066-74 (2012)
Article DOI: 10.1021/jm300917h
BindingDB Entry DOI: 10.7270/Q2TD9ZGG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123431
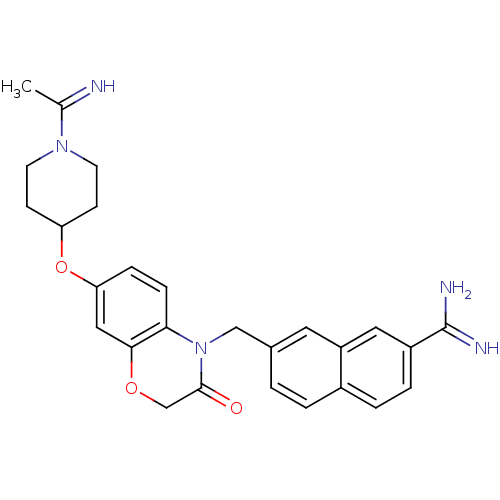
(7-{7-[1-(1-Imino-ethyl)-piperidin-4-yloxy]-3-oxo-2...)Show SMILES CC(=N)N1CCC(CC1)Oc1ccc2N(Cc3ccc4ccc(cc4c3)C(N)=N)C(=O)COc2c1 Show InChI InChI=1S/C27H29N5O3/c1-17(28)31-10-8-22(9-11-31)35-23-6-7-24-25(14-23)34-16-26(33)32(24)15-18-2-3-19-4-5-20(27(29)30)13-21(19)12-18/h2-7,12-14,22,28H,8-11,15-16H2,1H3,(H3,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards factor Xa |
Bioorg Med Chem Lett 13: 561-6 (2003)
BindingDB Entry DOI: 10.7270/Q2DB816X |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50396979

(CHEMBL2171170)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCCNC(=O)Nc2ccc(cc2)C(C)(C)C)C(C)C)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C28H42N8O4/c1-17(2)35(13-7-12-30-27(39)34-19-10-8-18(9-11-19)28(3,4)5)14-20-22(37)23(38)26(40-20)36-16-33-21-24(29-6)31-15-32-25(21)36/h8-11,15-17,20,22-23,26,37-38H,7,12-14H2,1-6H3,(H,29,31,32)(H2,30,34,39)/t20-,22-,23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactor |
J Med Chem 56: 8972-83 (2013)
Article DOI: 10.1021/jm4007752
BindingDB Entry DOI: 10.7270/Q2D50PDQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140410
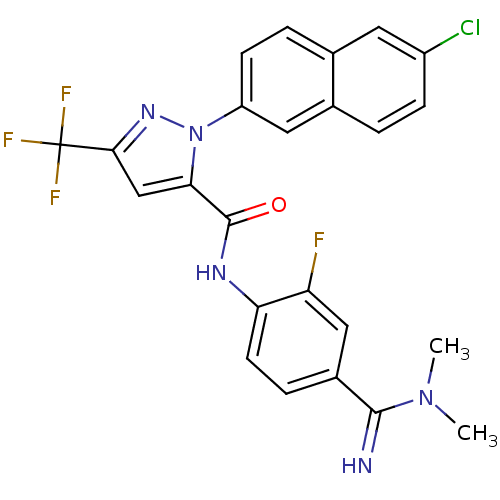
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN(C)C(=N)c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H18ClF4N5O/c1-33(2)22(30)15-5-8-19(18(26)11-15)31-23(35)20-12-21(24(27,28)29)32-34(20)17-7-4-13-9-16(25)6-3-14(13)10-17/h3-12,30H,1-2H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of 5-FAM-Bid peptide binding to Bcl-2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 26: 5207-5211 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.061
BindingDB Entry DOI: 10.7270/Q2PN97K9 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM21447

(4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...)Show SMILES CN(C)CC[C@H](CSc1ccccc1)Nc1ccc(cc1[N+]([O-])=O)S(=O)(=O)NC(=O)c1ccc(cc1)N1CCN(Cc2ccccc2-c2ccc(Cl)cc2)CC1 |r| Show InChI InChI=1S/C42H45ClN6O5S2/c1-46(2)23-22-35(30-55-37-9-4-3-5-10-37)44-40-21-20-38(28-41(40)49(51)52)56(53,54)45-42(50)32-14-18-36(19-15-32)48-26-24-47(25-27-48)29-33-8-6-7-11-39(33)31-12-16-34(43)17-13-31/h3-21,28,35,44H,22-27,29-30H2,1-2H3,(H,45,50)/t35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of 5-FAM-Bid peptide binding to Bcl-XL (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 26: 5207-5211 (2016)
Article DOI: 10.1016/j.bmcl.2016.09.061
BindingDB Entry DOI: 10.7270/Q2PN97K9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140422
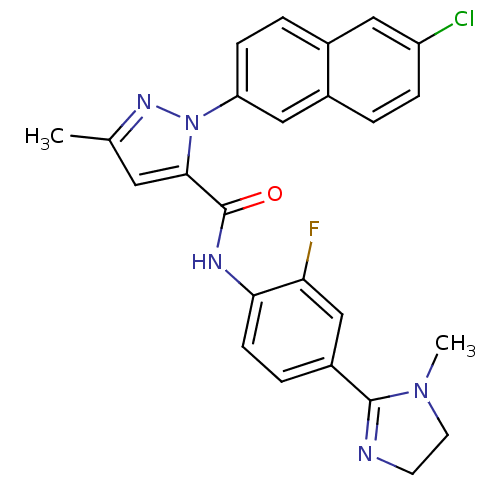
(2-(6-Chloro-naphthalen-2-yl)-5-methyl-2H-pyrazole-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(C)nn2-c2ccc3cc(Cl)ccc3c2)c(F)c1 |c:4| Show InChI InChI=1S/C25H21ClFN5O/c1-15-11-23(32(30-15)20-7-4-16-12-19(26)6-3-17(16)13-20)25(33)29-22-8-5-18(14-21(22)27)24-28-9-10-31(24)2/h3-8,11-14H,9-10H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for human Coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
4,4'-diapophytoene synthase
(Staphylococcus aureus) | BDBM50292846
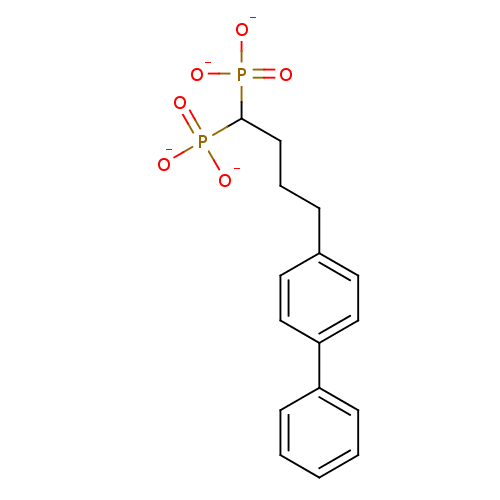
(4-(4-Biphenyl)butyldiphosphonic Acid Tetrapotassiu...)Show SMILES [O-]P([O-])(=O)C(CCCc1ccc(cc1)-c1ccccc1)P([O-])([O-])=O Show InChI InChI=1S/C16H20O6P2/c17-23(18,19)16(24(20,21)22)8-4-5-13-9-11-15(12-10-13)14-6-2-1-3-7-14/h1-3,6-7,9-12,16H,4-5,8H2,(H2,17,18,19)(H2,20,21,22)/p-4 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Inhibition of Staphylococcus aureus dehydrosqualene synthase expressed in Escherichia coli BL21 (DE3) cells by continuous spectrophotometric assay |
J Med Chem 52: 976-88 (2009)
Article DOI: 10.1021/jm801023u
BindingDB Entry DOI: 10.7270/Q2VM4C87 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50115933
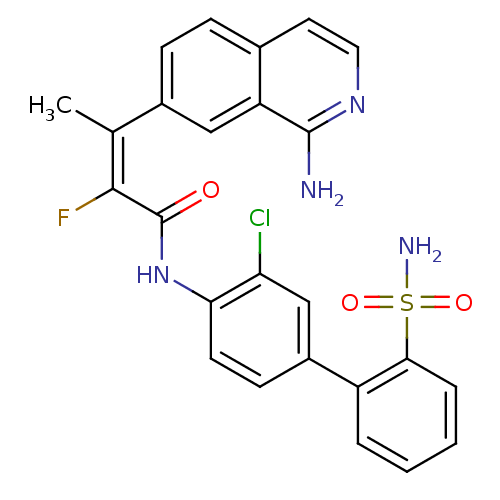
((E)-3-(1-Amino-isoquinolin-7-yl)-2-fluoro-but-2-en...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Cl)-c1ccccc1S(N)(=O)=O)c1ccc2ccnc(N)c2c1 Show InChI InChI=1S/C25H20ClFN4O3S/c1-14(16-7-6-15-10-11-30-24(28)19(15)12-16)23(27)25(32)31-21-9-8-17(13-20(21)26)18-4-2-3-5-22(18)35(29,33)34/h2-13H,1H3,(H2,28,30)(H,31,32)(H2,29,33,34)/b23-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Concentration required for inhibition of Coagulation factor X |
Bioorg Med Chem Lett 12: 2043-6 (2002)
BindingDB Entry DOI: 10.7270/Q2PZ584G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50443018
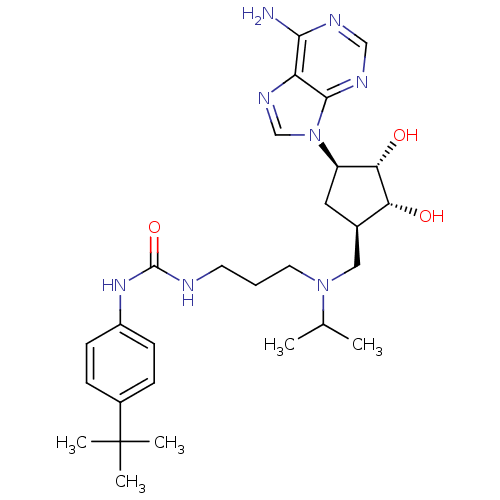
(CHEMBL3087503)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H42N8O3/c1-17(2)35(12-6-11-30-27(39)34-20-9-7-19(8-10-20)28(3,4)5)14-18-13-21(24(38)23(18)37)36-16-33-22-25(29)31-15-32-26(22)36/h7-10,15-18,21,23-24,37-38H,6,11-14H2,1-5H3,(H2,29,31,32)(H2,30,34,39)/t18-,21-,23-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50443018

(CHEMBL3087503)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)C[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H42N8O3/c1-17(2)35(12-6-11-30-27(39)34-20-9-7-19(8-10-20)28(3,4)5)14-18-13-21(24(38)23(18)37)36-16-33-22-25(29)31-15-32-26(22)36/h7-10,15-18,21,23-24,37-38H,6,11-14H2,1-5H3,(H2,29,31,32)(H2,30,34,39)/t18-,21-,23-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactor |
J Med Chem 56: 8972-83 (2013)
Article DOI: 10.1021/jm4007752
BindingDB Entry DOI: 10.7270/Q2D50PDQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140415
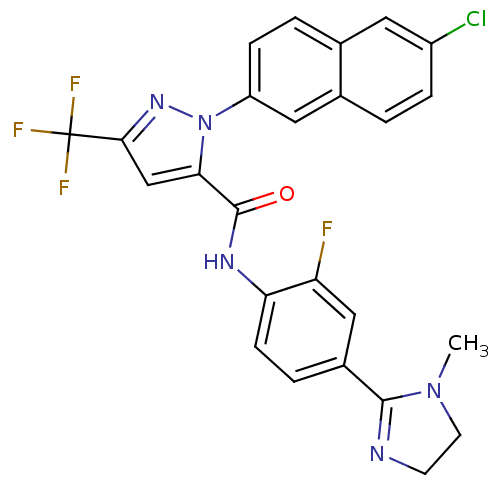
(2-(6-Chloro-naphthalen-2-yl)-5-trifluoromethyl-2H-...)Show SMILES CN1CCN=C1c1ccc(NC(=O)c2cc(nn2-c2ccc3cc(Cl)ccc3c2)C(F)(F)F)c(F)c1 |c:4| Show InChI InChI=1S/C25H18ClF4N5O/c1-34-9-8-31-23(34)16-4-7-20(19(27)12-16)32-24(36)21-13-22(25(28,29)30)33-35(21)18-6-3-14-10-17(26)5-2-15(14)11-18/h2-7,10-13H,8-9H2,1H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human coagulation factor X |
Bioorg Med Chem Lett 14: 1229-34 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.054
BindingDB Entry DOI: 10.7270/Q2QN6664 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50115936
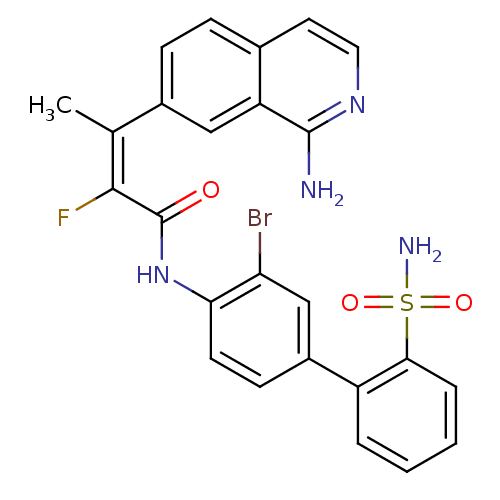
((E)-3-(1-Amino-isoquinolin-7-yl)-2-fluoro-but-2-en...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1Br)-c1ccccc1S(N)(=O)=O)c1ccc2ccnc(N)c2c1 Show InChI InChI=1S/C25H20BrFN4O3S/c1-14(16-7-6-15-10-11-30-24(28)19(15)12-16)23(27)25(32)31-21-9-8-17(13-20(21)26)18-4-2-3-5-22(18)35(29,33)34/h2-13H,1H3,(H2,28,30)(H,31,32)(H2,29,33,34)/b23-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor X |
Bioorg Med Chem Lett 12: 2043-6 (2002)
BindingDB Entry DOI: 10.7270/Q2PZ584G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50443019
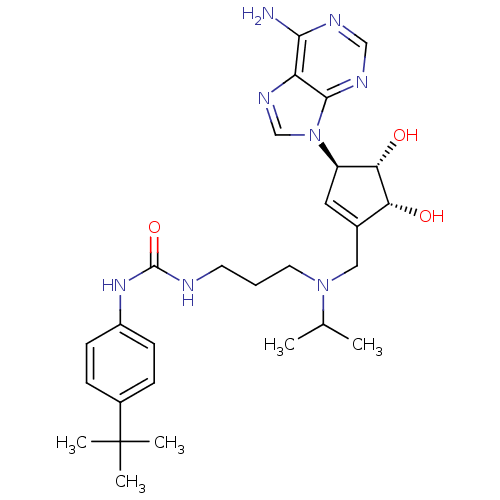
(CHEMBL3087502)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1=C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r,t:23| Show InChI InChI=1S/C28H40N8O3/c1-17(2)35(12-6-11-30-27(39)34-20-9-7-19(8-10-20)28(3,4)5)14-18-13-21(24(38)23(18)37)36-16-33-22-25(29)31-15-32-26(22)36/h7-10,13,15-17,21,23-24,37-38H,6,11-12,14H2,1-5H3,(H2,29,31,32)(H2,30,34,39)/t21-,23-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant human DOT1L using adenosine/deazaadenosine as substrate and SAM cofactor |
J Med Chem 56: 8972-83 (2013)
Article DOI: 10.1021/jm4007752
BindingDB Entry DOI: 10.7270/Q2D50PDQ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50443019

(CHEMBL3087502)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1=C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r,t:23| Show InChI InChI=1S/C28H40N8O3/c1-17(2)35(12-6-11-30-27(39)34-20-9-7-19(8-10-20)28(3,4)5)14-18-13-21(24(38)23(18)37)36-16-33-22-25(29)31-15-32-26(22)36/h7-10,13,15-17,21,23-24,37-38H,6,11-12,14H2,1-5H3,(H2,29,31,32)(H2,30,34,39)/t21-,23-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DOT1L (catalytic domain 1 to 472) using [3H]-SAM by scintillation containing |
Medchemcomm 4: 822-826 (2013)
Article DOI: 10.1039/c3md00021d
BindingDB Entry DOI: 10.7270/Q2R49TR1 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50249295
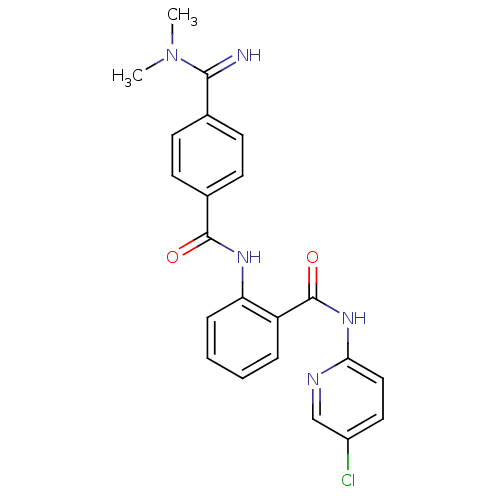
(CHEMBL471725 | N-(5-chloropyridin-2-yl)-2-(4-(N,N-...)Show SMILES CN(C)C(=N)c1ccc(cc1)C(=O)Nc1ccccc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C22H20ClN5O2/c1-28(2)20(24)14-7-9-15(10-8-14)21(29)26-18-6-4-3-5-17(18)22(30)27-19-12-11-16(23)13-25-19/h3-13,24H,1-2H3,(H,26,29)(H,25,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of Factor 10a (unknown origin) |
Bioorg Med Chem Lett 19: 2179-85 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.111
BindingDB Entry DOI: 10.7270/Q22Z15F5 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50134709
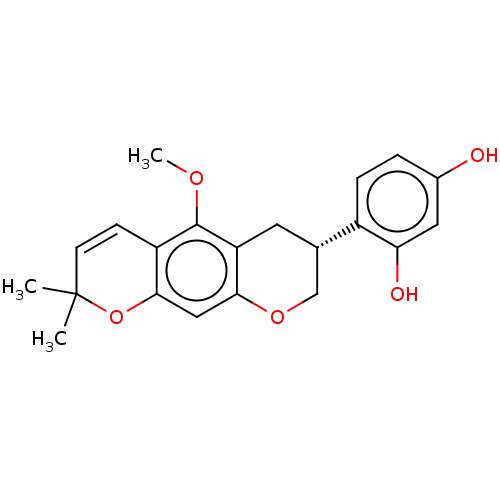
(CHEMBL3747057)Show SMILES COc1c2C[C@@H](COc2cc2OC(C)(C)C=Cc12)c1ccc(O)cc1O |r,c:16| Show InChI InChI=1S/C21H22O5/c1-21(2)7-6-15-19(26-21)10-18-16(20(15)24-3)8-12(11-25-18)14-5-4-13(22)9-17(14)23/h4-7,9-10,12,22-23H,8,11H2,1-3H3/t12-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Time dependent inhibition of monophenolase activity of mushroom tyrosinase using L-tyrosine as substrate measured every 30 secs by Morrison and Walsh... |
Bioorg Med Chem 24: 153-9 (2016)
Article DOI: 10.1016/j.bmc.2015.11.040
BindingDB Entry DOI: 10.7270/Q2RN39PT |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50409510
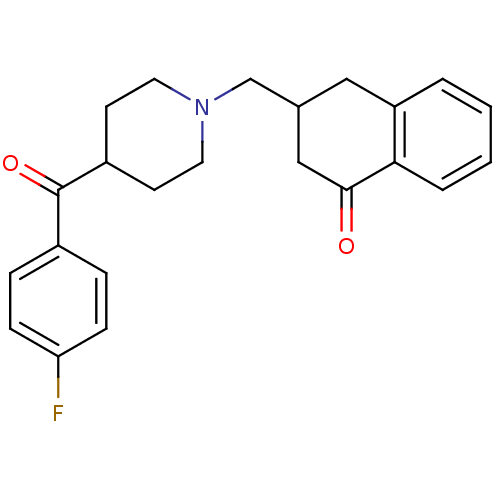
(CHEMBL308480)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CC2CC(=O)c3ccccc3C2)CC1 Show InChI InChI=1S/C23H24FNO2/c24-20-7-5-17(6-8-20)23(27)18-9-11-25(12-10-18)15-16-13-19-3-1-2-4-21(19)22(26)14-16/h1-8,16,18H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113964
BindingDB Entry DOI: 10.7270/Q2BP06TV |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50115935
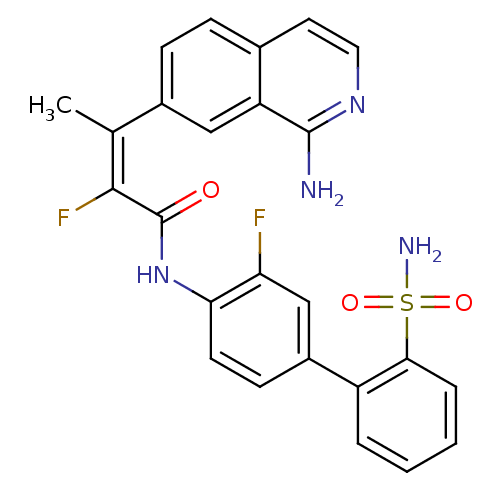
((E)-3-(1-Amino-isoquinolin-7-yl)-2-fluoro-but-2-en...)Show SMILES C\C(=C(/F)C(=O)Nc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)c1ccc2ccnc(N)c2c1 Show InChI InChI=1S/C25H20F2N4O3S/c1-14(16-7-6-15-10-11-30-24(28)19(15)12-16)23(27)25(32)31-21-9-8-17(13-20(21)26)18-4-2-3-5-22(18)35(29,33)34/h2-13H,1H3,(H2,28,30)(H,31,32)(H2,29,33,34)/b23-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor X |
Bioorg Med Chem Lett 12: 2043-6 (2002)
BindingDB Entry DOI: 10.7270/Q2PZ584G |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50163737
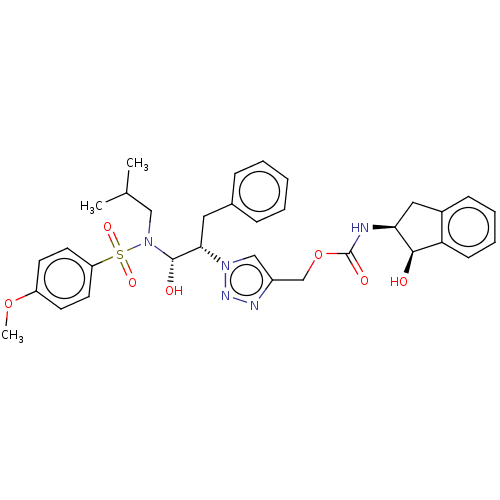
(CHEMBL3799941)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](O)[C@H](Cc1ccccc1)n1cc(COC(=O)N[C@H]2Cc3ccccc3[C@H]2O)nn1 |r| Show InChI InChI=1S/C33H39N5O7S/c1-22(2)19-38(46(42,43)27-15-13-26(44-3)14-16-27)32(40)30(17-23-9-5-4-6-10-23)37-20-25(35-36-37)21-45-33(41)34-29-18-24-11-7-8-12-28(24)31(29)39/h4-16,20,22,29-32,39-40H,17-19,21H2,1-3H3,(H,34,41)/t29-,30-,31+,32-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 62: 9375-9414 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00359
BindingDB Entry DOI: 10.7270/Q2BR8WFX |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110587

(4-Allyl-6-nitro-2-piperazin-1-yl-quinoline | CHEMB...)Show InChI InChI=1S/C16H18N4O2/c1-2-3-12-10-16(19-8-6-17-7-9-19)18-15-5-4-13(20(21)22)11-14(12)15/h2,4-5,10-11,17H,1,3,6-9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110579
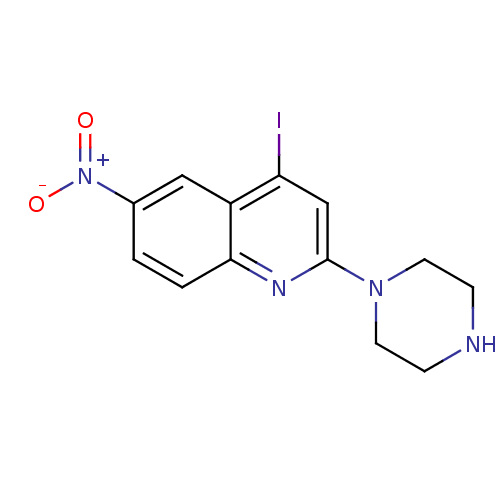
(4-Iodo-6-nitro-2-piperazin-1-yl-quinoline | CHEMBL...)Show InChI InChI=1S/C13H13IN4O2/c14-11-8-13(17-5-3-15-4-6-17)16-12-2-1-9(18(19)20)7-10(11)12/h1-2,7-8,15H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110585

(4-(4-Bromo-6-nitro-quinolin-2-yl)-piperazine-1-car...)Show SMILES [O-][N+](=O)c1ccc2nc(cc(Br)c2c1)N1CCN(CC1)C=O Show InChI InChI=1S/C14H13BrN4O3/c15-12-8-14(18-5-3-17(9-20)4-6-18)16-13-2-1-10(19(21)22)7-11(12)13/h1-2,7-9H,3-6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]citalopram binding to the rat cortical Serotonin transporter |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110583
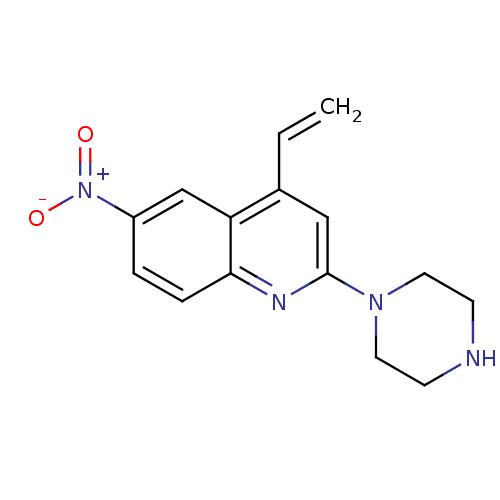
(6-Nitro-2-piperazin-1-yl-4-vinyl-quinoline | CHEMB...)Show InChI InChI=1S/C15H16N4O2/c1-2-11-9-15(18-7-5-16-6-8-18)17-14-4-3-12(19(20)21)10-13(11)14/h2-4,9-10,16H,1,5-8H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Inha University
Curated by ChEMBL
| Assay Description
Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT) |
Bioorg Med Chem Lett 12: 811-5 (2002)
BindingDB Entry DOI: 10.7270/Q2S46SHN |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50144092
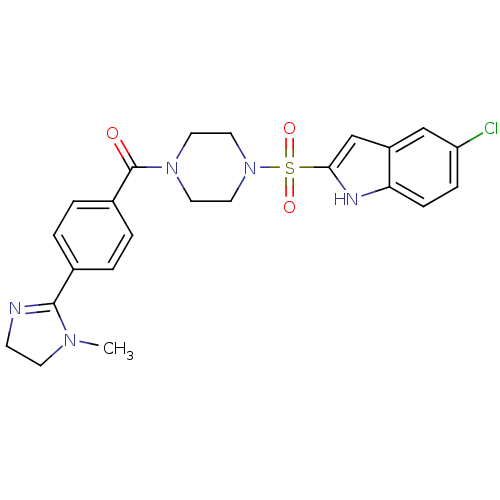
(CHEMBL294121 | [4-(5-Chloro-1H-indole-2-sulfonyl)-...)Show SMILES CN1CCN=C1c1ccc(cc1)C(=O)N1CCN(CC1)S(=O)(=O)c1cc2cc(Cl)ccc2[nH]1 |c:4| Show InChI InChI=1S/C23H24ClN5O3S/c1-27-9-8-25-22(27)16-2-4-17(5-3-16)23(30)28-10-12-29(13-11-28)33(31,32)21-15-18-14-19(24)6-7-20(18)26-21/h2-7,14-15,26H,8-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 14: 2073-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.049
BindingDB Entry DOI: 10.7270/Q2P84B98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data