

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18617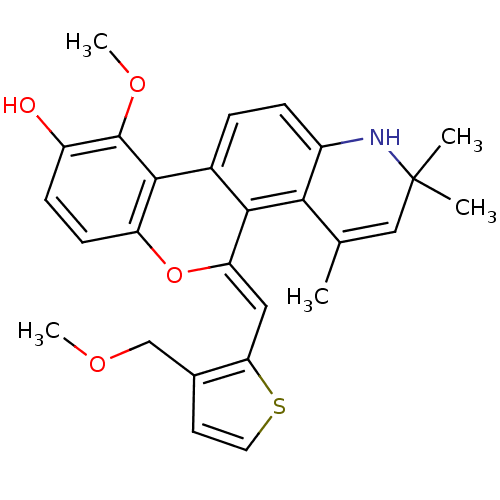 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -51.5 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36717 (1,25(OH)2D3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18622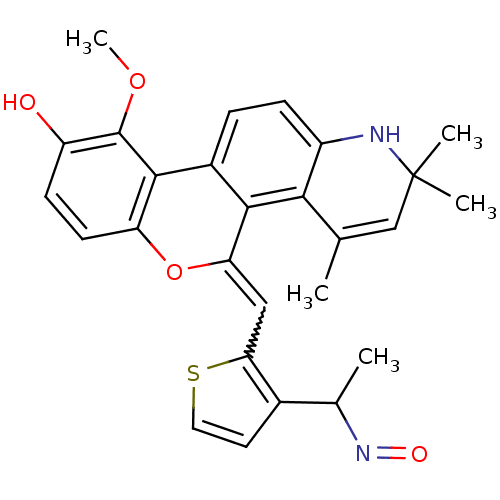 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -48.9 | 1.10 | n/a | 5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie Curated by ChEMBL | Assay Description Inhibition constant for human aromatase cytochrome P450 19A1 activity | Bioorg Med Chem Lett 8: 1041-4 (1999) BindingDB Entry DOI: 10.7270/Q2445KM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18616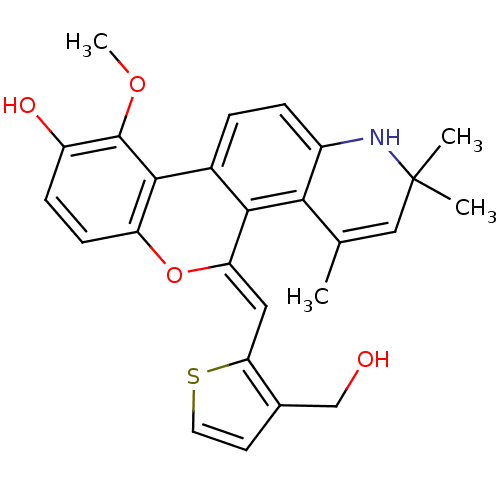 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -48.6 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18623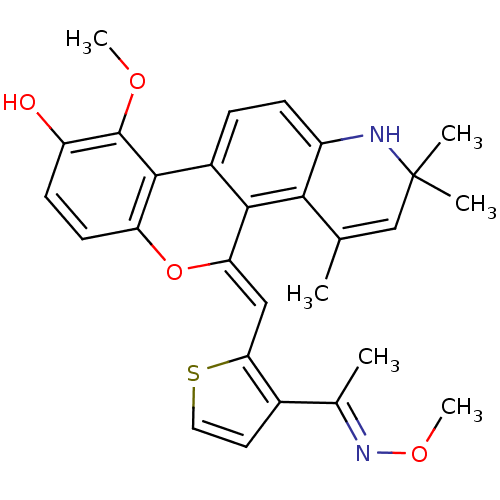 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -47.5 | 0.200 | n/a | 0.100 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18632 ((18Z)-18-{[3-(1-hydroxyethyl)thiophen-2-yl]methyli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | -47.3 | 1.60 | n/a | 1.30 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -47.0 | 0.400 | n/a | 0.300 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.10 | -46.0 | 1.40 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18626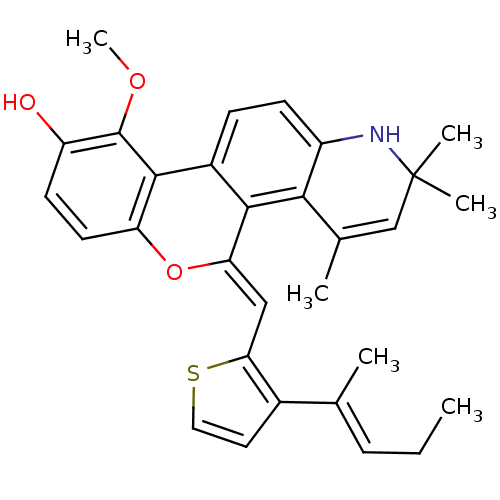 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(2E)-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | -44.3 | 6.10 | n/a | 0.5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50070097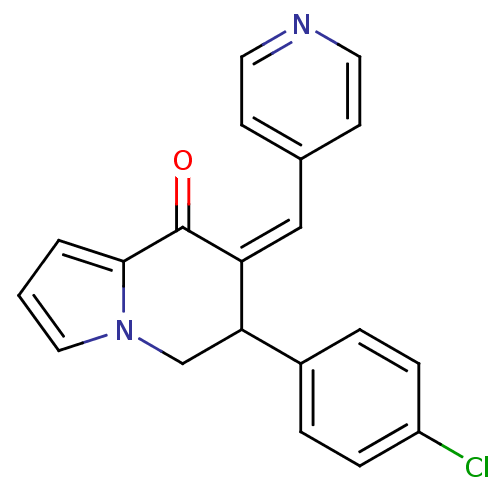 ((Z)-6-(4-chlorophenyl)-7-(pyridin-4-ylmethylene)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie Curated by ChEMBL | Assay Description Inhibition constant for human aromatase cytochrome P450 19A1 activity | Bioorg Med Chem Lett 8: 1041-4 (1999) BindingDB Entry DOI: 10.7270/Q2445KM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50070095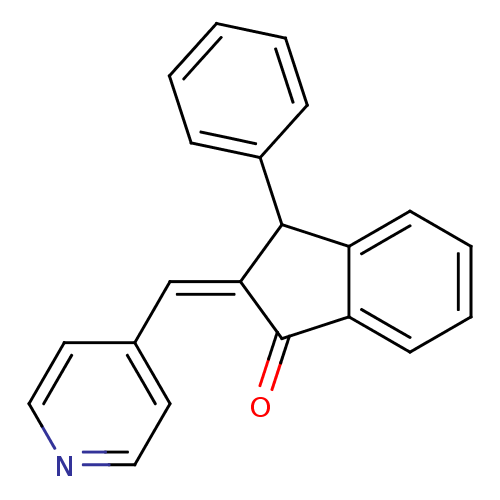 (3-Phenyl-2-[1-pyridin-4-yl-meth-(Z)-ylidene]-indan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie Curated by ChEMBL | Assay Description Inhibition constant for human aromatase cytochrome P450 19A1 activity | Bioorg Med Chem Lett 8: 1041-4 (1999) BindingDB Entry DOI: 10.7270/Q2445KM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18617 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18622 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18623 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18626 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(2E)-pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18623 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18626 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(2E)-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50215414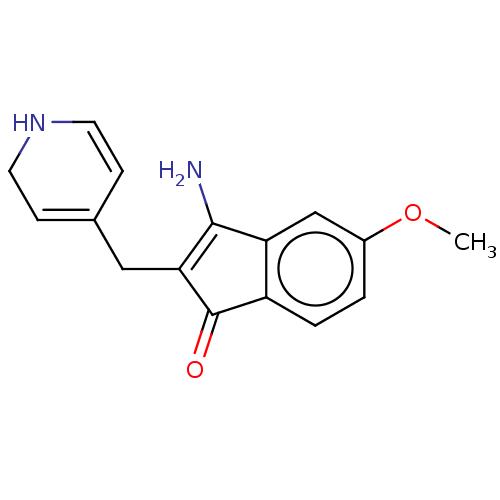 (CHEMBL1096024 | MR-20814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre d'Etudes et de Recherche sur le Médicament de Normandie Curated by ChEMBL | Assay Description Inhibition constant for human aromatase cytochrome P450 19A1 activity | Bioorg Med Chem Lett 8: 1041-4 (1999) BindingDB Entry DOI: 10.7270/Q2445KM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18623 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36722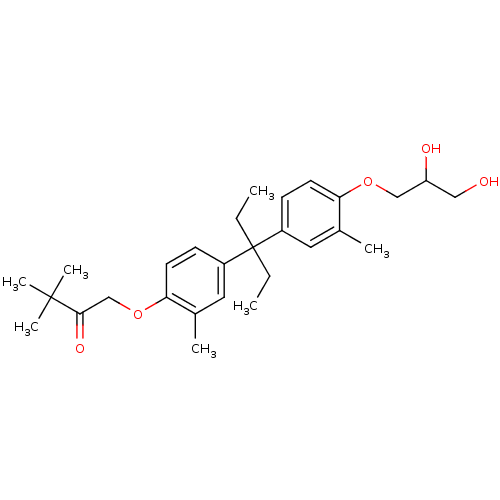 (LG190178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18617 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18617 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18622 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18616 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18626 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(2E)-pent...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18632 ((18Z)-18-{[3-(1-hydroxyethyl)thiophen-2-yl]methyli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18622 ((18Z)-18-({3-[(1E)-1-(hydroxyimino)ethyl]thiophen-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18616 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36721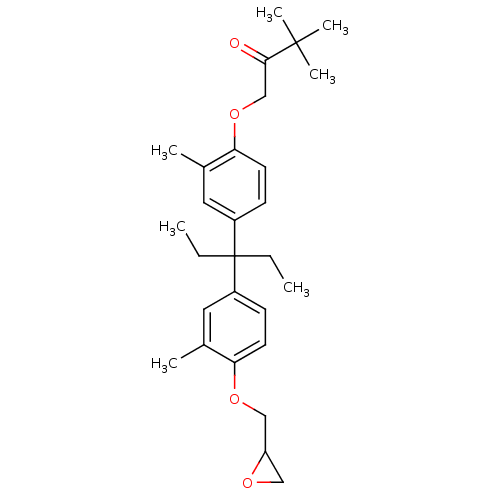 (LG190176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36720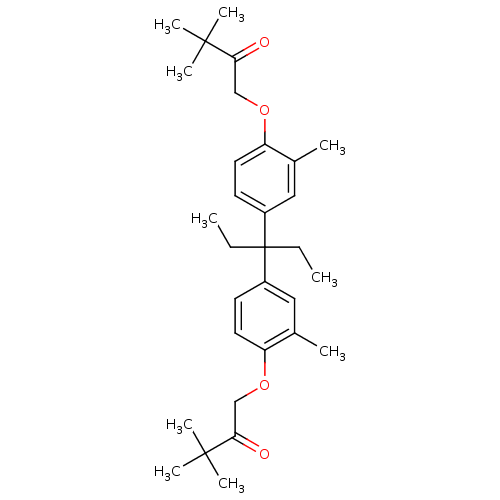 (LG190155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36719 (LG190119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin D3 receptor (Homo sapiens (Human)) | BDBM36718 (LG190090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals, Inc | Assay Description VDR in vitro ligand-binding assay using human VDR constructed into a yeast expression plasmid. | Chem Biol 6: 265-75 (1999) Article DOI: 10.1016/S1074-5521(99)80072-6 BindingDB Entry DOI: 10.7270/Q2NC5ZJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18616 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18632 ((18Z)-18-{[3-(1-hydroxyethyl)thiophen-2-yl]methyli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18632 ((18Z)-18-{[3-(1-hydroxyethyl)thiophen-2-yl]methyli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18629 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(1S)-2,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18621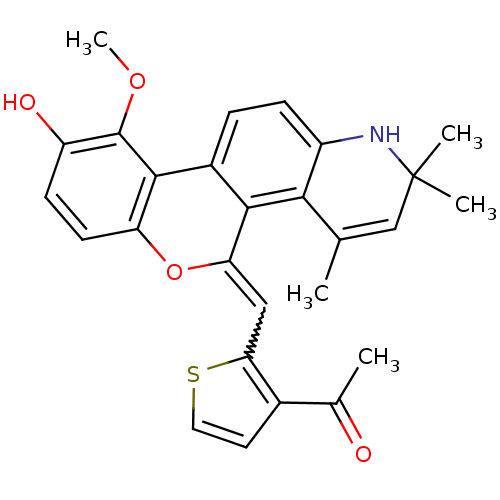 (1-(2-{[(18Z)-13-hydroxy-12-methoxy-3,5,5-trimethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | 0.600 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18624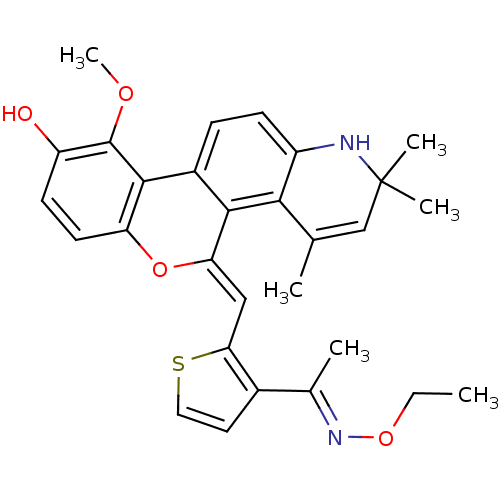 ((18Z)-18-({3-[(1E)-1-(ethoxyimino)ethyl]thiophen-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | 1.90 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18624 ((18Z)-18-({3-[(1E)-1-(ethoxyimino)ethyl]thiophen-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | 0.400 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18630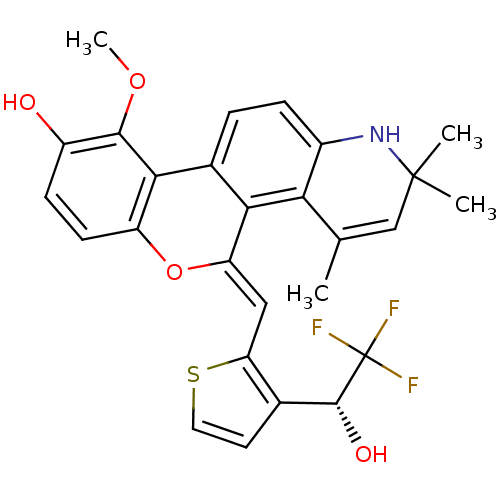 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(1R)-2,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | 1.40 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18620 (2-{[(18Z)-13-hydroxy-12-methoxy-3,5,5-trimethyl-17...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 0.200 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18619 ((18Z)-12-methoxy-3,5,5-trimethyl-18-[(3-{[(2,2,2-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | 0.5 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18633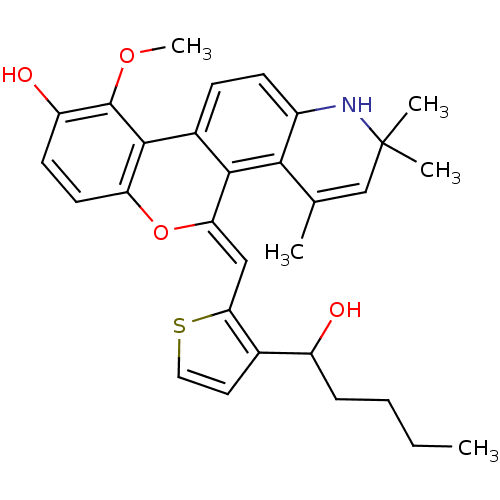 ((18Z)-18-{[3-(1-hydroxypentyl)thiophen-2-yl]methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | 0.600 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18631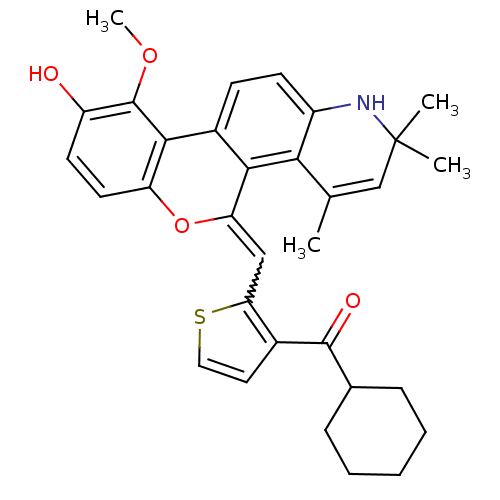 ((18Z)-18-[(3-cyclohexanecarbonylthiophen-2-yl)meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | 0.400 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18618 ((18Z)-18-({3-[(2-hydroxyethoxy)methyl]thiophen-2-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | 1.60 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |