 Found 68 hits with Last Name = 'sopko' and Initial = 'mm'
Found 68 hits with Last Name = 'sopko' and Initial = 'mm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-1
(Homo sapiens (Human)) | BDBM50176493
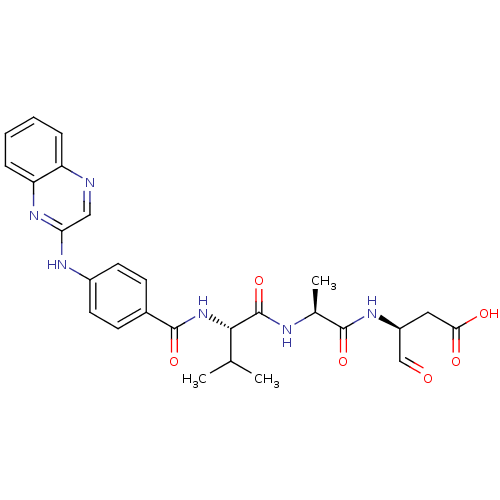
((S)-3-((S)-2-((S)-3-methyl-2-(4-(quinoxalin-2-ylam...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C27H30N6O6/c1-15(2)24(27(39)29-16(3)25(37)31-19(14-34)12-23(35)36)33-26(38)17-8-10-18(11-9-17)30-22-13-28-20-6-4-5-7-21(20)32-22/h4-11,13-16,19,24H,12H2,1-3H3,(H,29,39)(H,30,32)(H,31,37)(H,33,38)(H,35,36)/t16-,19-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176494
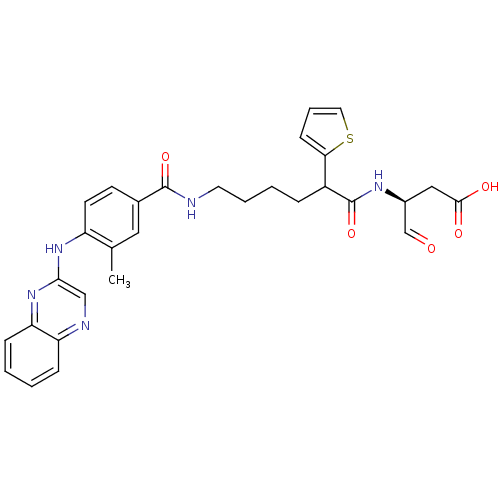
((3S)-3-(6-(3-methyl-4-(quinoxalin-2-ylamino)benzam...)Show SMILES Cc1cc(ccc1Nc1cnc2ccccc2n1)C(=O)NCCCCC(C(=O)N[C@@H](CC(O)=O)C=O)c1cccs1 Show InChI InChI=1S/C30H31N5O5S/c1-19-15-20(11-12-23(19)34-27-17-32-24-8-2-3-9-25(24)35-27)29(39)31-13-5-4-7-22(26-10-6-14-41-26)30(40)33-21(18-36)16-28(37)38/h2-3,6,8-12,14-15,17-18,21-22H,4-5,7,13,16H2,1H3,(H,31,39)(H,33,40)(H,34,35)(H,37,38)/t21-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176510
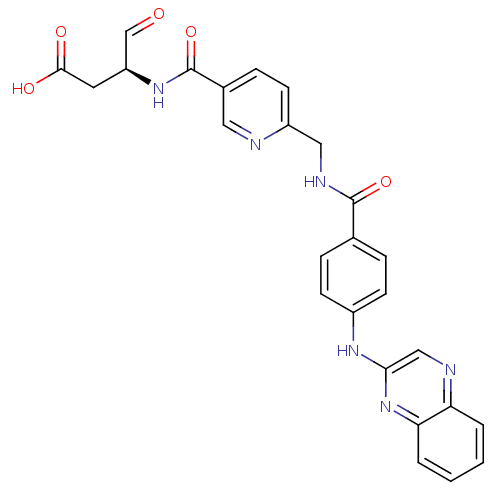
((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)c1ccc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)nc1)C=O |r| Show InChI InChI=1S/C26H22N6O5/c33-15-20(11-24(34)35)31-26(37)17-7-10-19(27-12-17)13-29-25(36)16-5-8-18(9-6-16)30-23-14-28-21-3-1-2-4-22(21)32-23/h1-10,12,14-15,20H,11,13H2,(H,29,36)(H,30,32)(H,31,37)(H,34,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-1
(Homo sapiens (Human)) | BDBM50176496
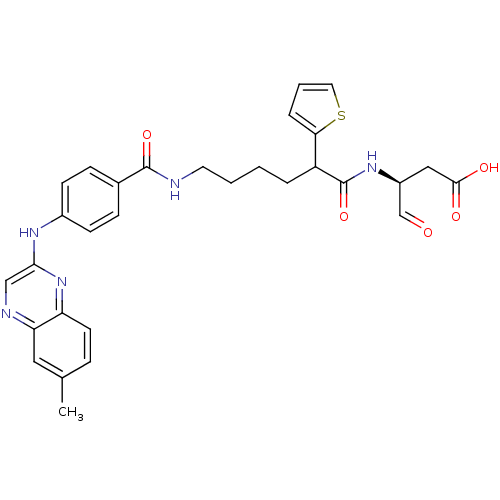
((3S)-3-(6-(4-(6-methylquinoxalin-2-ylamino)benzami...)Show SMILES Cc1ccc2nc(Nc3ccc(cc3)C(=O)NCCCCC(C(=O)N[C@@H](CC(O)=O)C=O)c3cccs3)cnc2c1 Show InChI InChI=1S/C30H31N5O5S/c1-19-7-12-24-25(15-19)32-17-27(35-24)33-21-10-8-20(9-11-21)29(39)31-13-3-2-5-23(26-6-4-14-41-26)30(40)34-22(18-36)16-28(37)38/h4,6-12,14-15,17-18,22-23H,2-3,5,13,16H2,1H3,(H,31,39)(H,33,35)(H,34,40)(H,37,38)/t22-,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176495
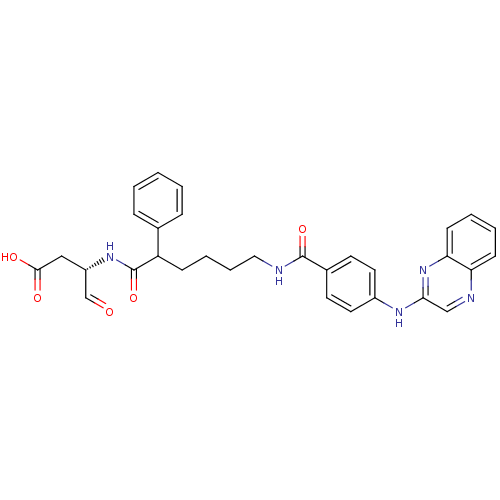
((3S)-4-oxo-3-(2-phenyl-6-(4-(quinoxalin-2-ylamino)...)Show SMILES OC(=O)C[C@H](NC(=O)C(CCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)c1ccccc1)C=O Show InChI InChI=1S/C31H31N5O5/c37-20-24(18-29(38)39)35-31(41)25(21-8-2-1-3-9-21)10-6-7-17-32-30(40)22-13-15-23(16-14-22)34-28-19-33-26-11-4-5-12-27(26)36-28/h1-5,8-9,11-16,19-20,24-25H,6-7,10,17-18H2,(H,32,40)(H,34,36)(H,35,41)(H,38,39)/t24-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176500
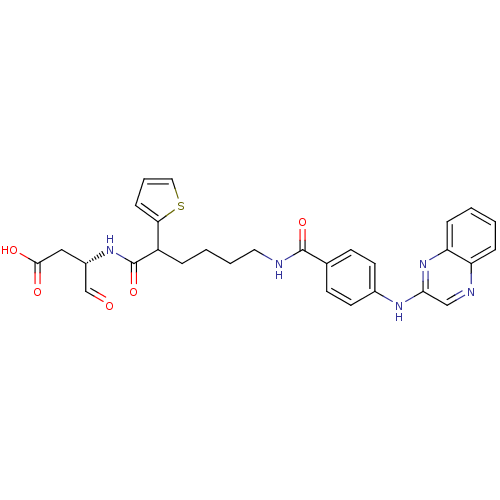
((3S)-4-oxo-3-(6-(4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)C(CCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)c1cccs1)C=O Show InChI InChI=1S/C29H29N5O5S/c35-18-21(16-27(36)37)33-29(39)22(25-9-5-15-40-25)6-3-4-14-30-28(38)19-10-12-20(13-11-19)32-26-17-31-23-7-1-2-8-24(23)34-26/h1-2,5,7-13,15,17-18,21-22H,3-4,6,14,16H2,(H,30,38)(H,32,34)(H,33,39)(H,36,37)/t21-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176519
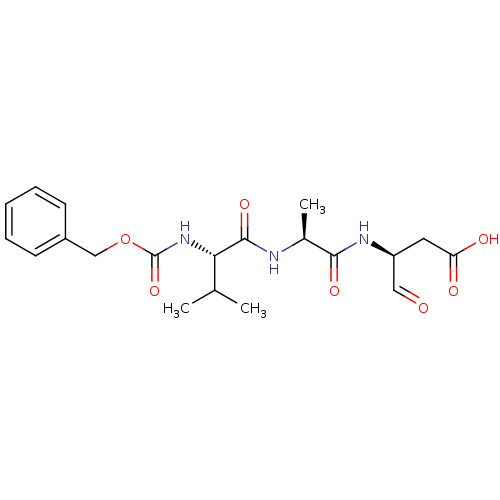
((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C20H27N3O7/c1-12(2)17(23-20(29)30-11-14-7-5-4-6-8-14)19(28)21-13(3)18(27)22-15(10-24)9-16(25)26/h4-8,10,12-13,15,17H,9,11H2,1-3H3,(H,21,28)(H,22,27)(H,23,29)(H,25,26)/t13-,15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176509
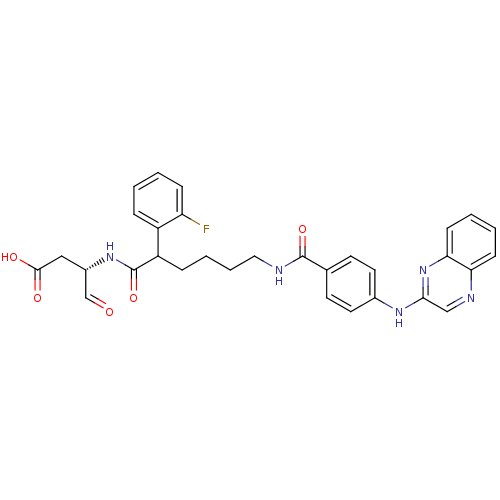
((3S)-3-(2-(2-fluorophenyl)-6-(4-(quinoxalin-2-ylam...)Show SMILES OC(=O)C[C@H](NC(=O)C(CCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)c1ccccc1F)C=O Show InChI InChI=1S/C31H30FN5O5/c32-25-9-2-1-7-23(25)24(31(42)36-22(19-38)17-29(39)40)8-5-6-16-33-30(41)20-12-14-21(15-13-20)35-28-18-34-26-10-3-4-11-27(26)37-28/h1-4,7,9-15,18-19,22,24H,5-6,8,16-17H2,(H,33,41)(H,35,37)(H,36,42)(H,39,40)/t22-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176505
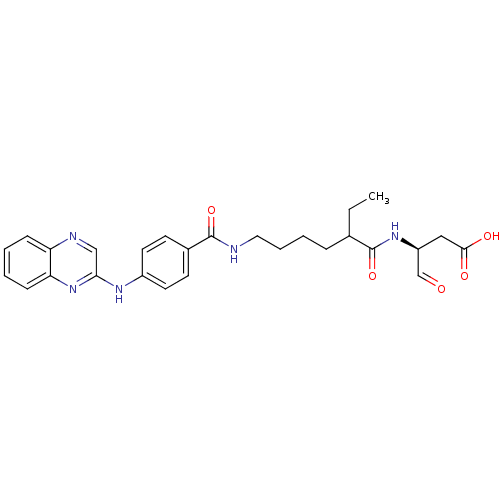
((3S)-3-(2-ethyl-6-(4-(quinoxalin-2-ylamino)benzami...)Show SMILES CCC(CCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C27H31N5O5/c1-2-18(27(37)31-21(17-33)15-25(34)35)7-5-6-14-28-26(36)19-10-12-20(13-11-19)30-24-16-29-22-8-3-4-9-23(22)32-24/h3-4,8-13,16-18,21H,2,5-7,14-15H2,1H3,(H,28,36)(H,30,32)(H,31,37)(H,34,35)/t18?,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176508
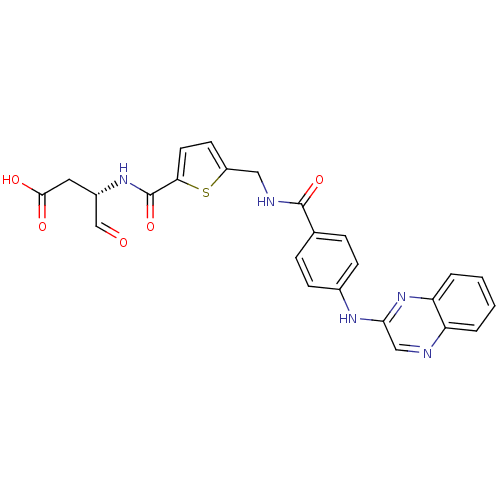
((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)c1ccc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)s1)C=O Show InChI InChI=1S/C25H21N5O5S/c31-14-17(11-23(32)33)29-25(35)21-10-9-18(36-21)12-27-24(34)15-5-7-16(8-6-15)28-22-13-26-19-3-1-2-4-20(19)30-22/h1-10,13-14,17H,11-12H2,(H,27,34)(H,28,30)(H,29,35)(H,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176502
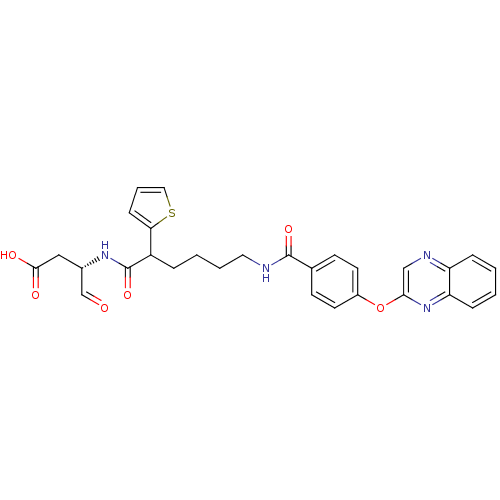
((3S)-4-oxo-3-(6-(4-(quinoxalin-2-yloxy)benzamido)-...)Show SMILES OC(=O)C[C@H](NC(=O)C(CCCCNC(=O)c1ccc(Oc2cnc3ccccc3n2)cc1)c1cccs1)C=O Show InChI InChI=1S/C29H28N4O6S/c34-18-20(16-27(35)36)32-29(38)22(25-9-5-15-40-25)6-3-4-14-30-28(37)19-10-12-21(13-11-19)39-26-17-31-23-7-1-2-8-24(23)33-26/h1-2,5,7-13,15,17-18,20,22H,3-4,6,14,16H2,(H,30,37)(H,32,38)(H,35,36)/t20-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176504
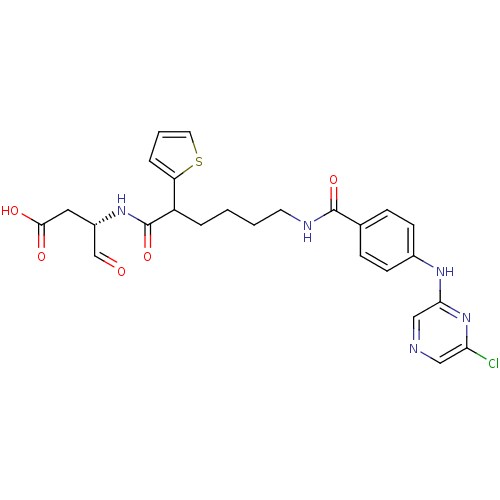
((3S)-3-(6-(4-(6-chloropyrazin-2-ylamino)benzamido)...)Show SMILES OC(=O)C[C@H](NC(=O)C(CCCCNC(=O)c1ccc(Nc2cncc(Cl)n2)cc1)c1cccs1)C=O Show InChI InChI=1S/C25H26ClN5O5S/c26-21-13-27-14-22(31-21)29-17-8-6-16(7-9-17)24(35)28-10-2-1-4-19(20-5-3-11-37-20)25(36)30-18(15-32)12-23(33)34/h3,5-9,11,13-15,18-19H,1-2,4,10,12H2,(H,28,35)(H,29,31)(H,30,36)(H,33,34)/t18-,19?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176497
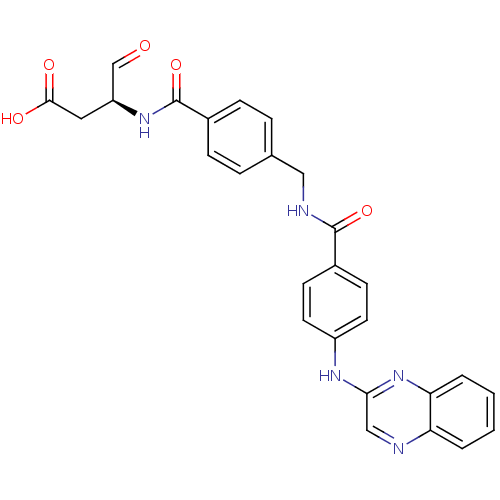
((S)-4-oxo-3-(4-((4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)c1ccc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)cc1)C=O Show InChI InChI=1S/C27H23N5O5/c33-16-21(13-25(34)35)31-27(37)19-7-5-17(6-8-19)14-29-26(36)18-9-11-20(12-10-18)30-24-15-28-22-3-1-2-4-23(22)32-24/h1-12,15-16,21H,13-14H2,(H,29,36)(H,30,32)(H,31,37)(H,34,35)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176513
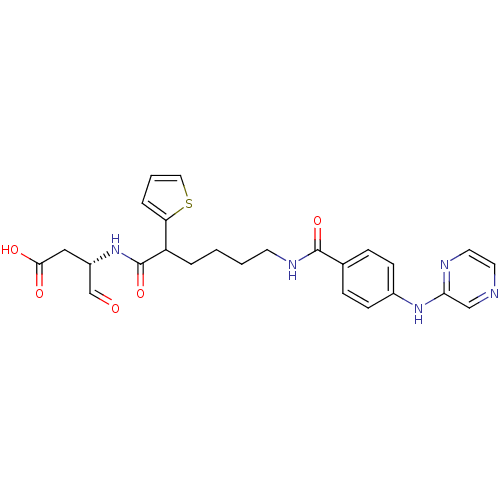
((3S)-4-oxo-3-(6-(4-(pyrazin-2-ylamino)benzamido)-2...)Show SMILES OC(=O)C[C@H](NC(=O)C(CCCCNC(=O)c1ccc(Nc2cnccn2)cc1)c1cccs1)C=O Show InChI InChI=1S/C25H27N5O5S/c31-16-19(14-23(32)33)30-25(35)20(21-5-3-13-36-21)4-1-2-10-28-24(34)17-6-8-18(9-7-17)29-22-15-26-11-12-27-22/h3,5-9,11-13,15-16,19-20H,1-2,4,10,14H2,(H,27,29)(H,28,34)(H,30,35)(H,32,33)/t19-,20?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176498
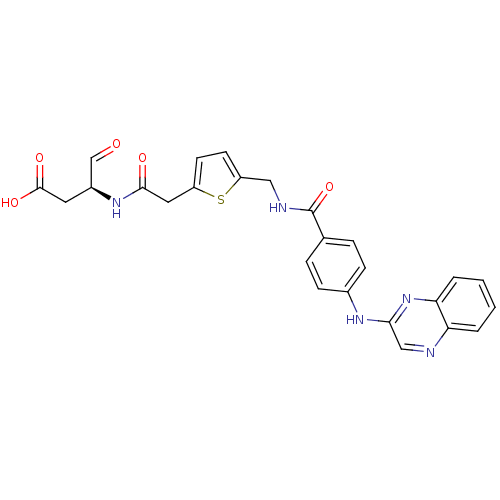
((S)-4-oxo-3-(2-(5-((4-(quinoxalin-2-ylamino)benzam...)Show SMILES OC(=O)C[C@H](NC(=O)Cc1ccc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)s1)C=O Show InChI InChI=1S/C26H23N5O5S/c32-15-18(11-25(34)35)30-24(33)12-19-9-10-20(37-19)13-28-26(36)16-5-7-17(8-6-16)29-23-14-27-21-3-1-2-4-22(21)31-23/h1-10,14-15,18H,11-13H2,(H,28,36)(H,29,31)(H,30,33)(H,34,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-1
(Homo sapiens (Human)) | BDBM50176512
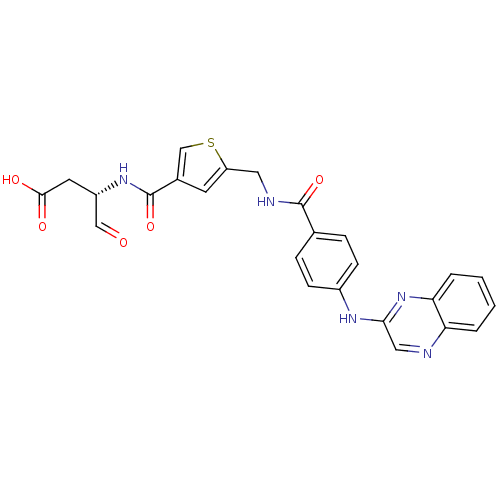
((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)c1csc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)c1)C=O Show InChI InChI=1S/C25H21N5O5S/c31-13-18(10-23(32)33)29-25(35)16-9-19(36-14-16)11-27-24(34)15-5-7-17(8-6-15)28-22-12-26-20-3-1-2-4-21(20)30-22/h1-9,12-14,18H,10-11H2,(H,27,34)(H,28,30)(H,29,35)(H,32,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176517
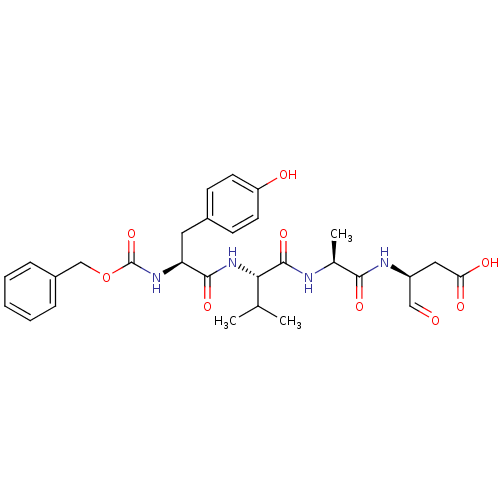
(CHEMBL159822 | Z-YVAD-CHO)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C29H36N4O9/c1-17(2)25(28(40)30-18(3)26(38)31-21(15-34)14-24(36)37)33-27(39)23(13-19-9-11-22(35)12-10-19)32-29(41)42-16-20-7-5-4-6-8-20/h4-12,15,17-18,21,23,25,35H,13-14,16H2,1-3H3,(H,30,40)(H,31,38)(H,32,41)(H,33,39)(H,36,37)/t18-,21-,23-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176515
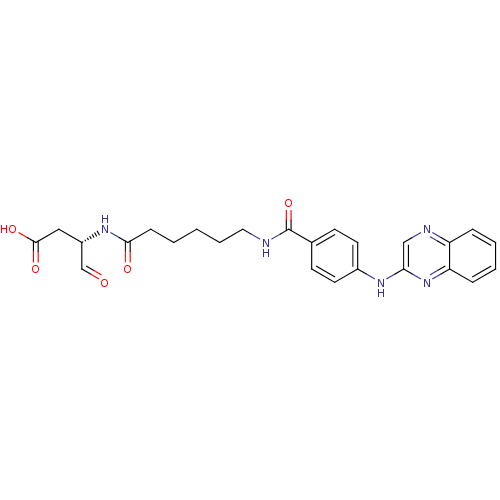
((S)-4-oxo-3-(6-(4-(quinoxalin-2-ylamino)benzamido)...)Show SMILES OC(=O)C[C@H](NC(=O)CCCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)C=O |r| Show InChI InChI=1S/C25H27N5O5/c31-16-19(14-24(33)34)29-23(32)8-2-1-5-13-26-25(35)17-9-11-18(12-10-17)28-22-15-27-20-6-3-4-7-21(20)30-22/h3-4,6-7,9-12,15-16,19H,1-2,5,8,13-14H2,(H,26,35)(H,28,30)(H,29,32)(H,33,34)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176511

((S)-4-oxo-3-(3-(4-(quinoxalin-2-ylamino)benzamido)...)Show SMILES OC(=O)C[C@H](NC(=O)c1cc(NC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)cs1)C=O Show InChI InChI=1S/C24H19N5O5S/c30-12-16(10-22(31)32)27-24(34)20-9-17(13-35-20)28-23(33)14-5-7-15(8-6-14)26-21-11-25-18-3-1-2-4-19(18)29-21/h1-9,11-13,16H,10H2,(H,26,29)(H,27,34)(H,28,33)(H,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176506
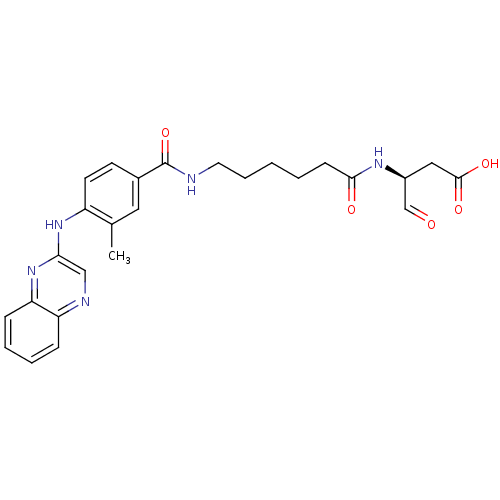
((S)-3-(6-(3-methyl-4-(quinoxalin-2-ylamino)benzami...)Show SMILES Cc1cc(ccc1Nc1cnc2ccccc2n1)C(=O)NCCCCCC(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C26H29N5O5/c1-17-13-18(10-11-20(17)30-23-15-28-21-7-4-5-8-22(21)31-23)26(36)27-12-6-2-3-9-24(33)29-19(16-32)14-25(34)35/h4-5,7-8,10-11,13,15-16,19H,2-3,6,9,12,14H2,1H3,(H,27,36)(H,29,33)(H,30,31)(H,34,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176507
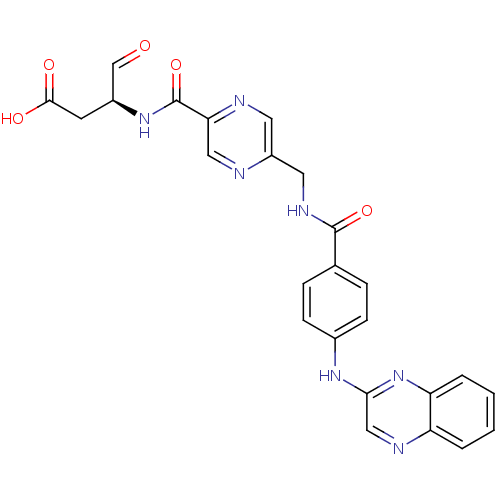
((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)c1cnc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)cn1)C=O Show InChI InChI=1S/C25H21N7O5/c33-14-17(9-23(34)35)31-25(37)21-12-26-18(10-27-21)11-29-24(36)15-5-7-16(8-6-15)30-22-13-28-19-3-1-2-4-20(19)32-22/h1-8,10,12-14,17H,9,11H2,(H,29,36)(H,30,32)(H,31,37)(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176503
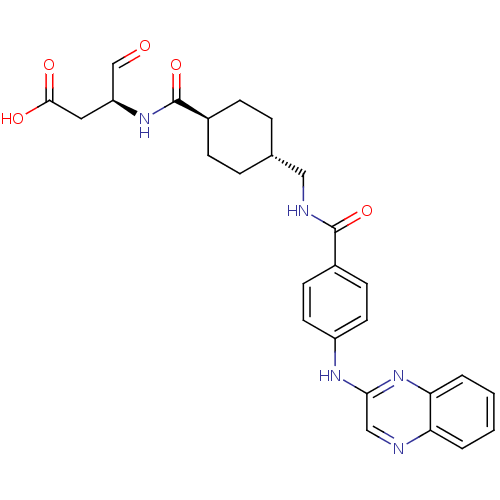
((S)-4-oxo-3-((1r,4S)-4-((4-(quinoxalin-2-ylamino)b...)Show SMILES OC(=O)C[C@H](NC(=O)[C@H]1CC[C@H](CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)CC1)C=O |wU:8.7,wD:4.4,11.11,(19.27,-37.83,;19.31,-36.28,;20.68,-35.56,;18.01,-35.47,;18.06,-33.93,;16.75,-33.12,;15.39,-33.84,;15.34,-35.38,;14.06,-33.08,;12.72,-33.85,;11.39,-33.09,;11.39,-31.56,;10.05,-30.79,;8.71,-31.88,;7.37,-31.11,;7.36,-29.57,;6.04,-31.89,;6.04,-33.43,;4.71,-34.21,;3.38,-33.43,;2.05,-34.21,;.71,-33.44,;.71,-31.89,;-.63,-31.13,;-1.96,-31.91,;-3.29,-31.14,;-4.62,-31.91,;-4.62,-33.46,;-3.29,-34.23,;-1.96,-33.45,;-.62,-34.22,;3.37,-31.9,;4.69,-31.13,;12.72,-30.77,;14.05,-31.54,;19.42,-33.21,;20.72,-34.02,)| Show InChI InChI=1S/C27H29N5O5/c33-16-21(13-25(34)35)31-27(37)19-7-5-17(6-8-19)14-29-26(36)18-9-11-20(12-10-18)30-24-15-28-22-3-1-2-4-23(22)32-24/h1-4,9-12,15-17,19,21H,5-8,13-14H2,(H,29,36)(H,30,32)(H,31,37)(H,34,35)/t17-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176514
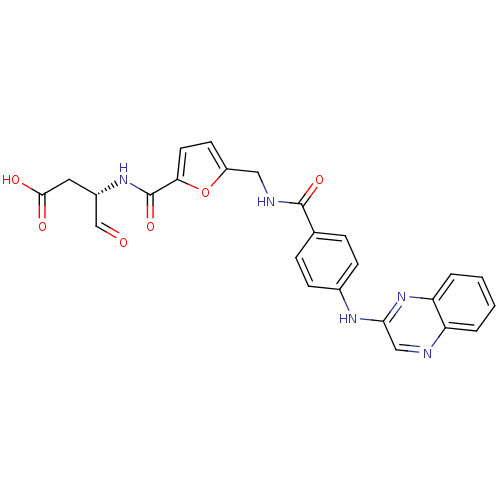
((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...)Show SMILES OC(=O)C[C@H](NC(=O)c1ccc(CNC(=O)c2ccc(Nc3cnc4ccccc4n3)cc2)o1)C=O Show InChI InChI=1S/C25H21N5O6/c31-14-17(11-23(32)33)29-25(35)21-10-9-18(36-21)12-27-24(34)15-5-7-16(8-6-15)28-22-13-26-19-3-1-2-4-20(19)30-22/h1-10,13-14,17H,11-12H2,(H,27,34)(H,28,30)(H,29,35)(H,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176516
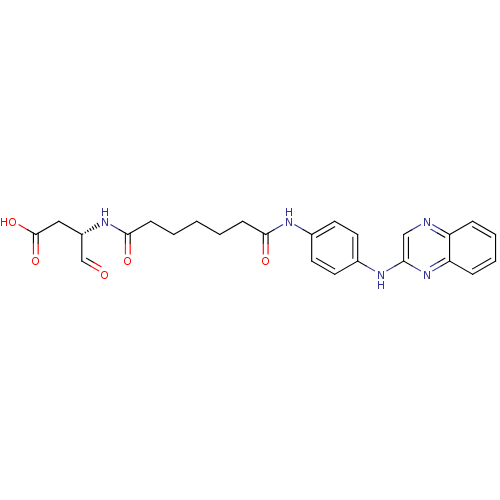
((S)-4-oxo-3-(7-oxo-7-(4-(quinoxalin-2-ylamino)phen...)Show SMILES OC(=O)C[C@H](NC(=O)CCCCCC(=O)Nc1ccc(Nc2cnc3ccccc3n2)cc1)C=O Show InChI InChI=1S/C25H27N5O5/c31-16-19(14-25(34)35)29-24(33)9-3-1-2-8-23(32)28-18-12-10-17(11-13-18)27-22-15-26-20-6-4-5-7-21(20)30-22/h4-7,10-13,15-16,19H,1-3,8-9,14H2,(H,27,30)(H,28,32)(H,29,33)(H,34,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176501
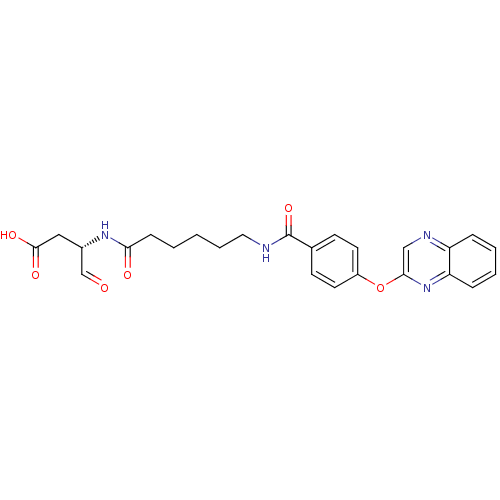
((S)-4-oxo-3-(6-(4-(quinoxalin-2-yloxy)benzamido)he...)Show SMILES OC(=O)C[C@H](NC(=O)CCCCCNC(=O)c1ccc(Oc2cnc3ccccc3n2)cc1)C=O Show InChI InChI=1S/C25H26N4O6/c30-16-18(14-24(32)33)28-22(31)8-2-1-5-13-26-25(34)17-9-11-19(12-10-17)35-23-15-27-20-6-3-4-7-21(20)29-23/h3-4,6-7,9-12,15-16,18H,1-2,5,8,13-14H2,(H,26,34)(H,28,31)(H,32,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176520
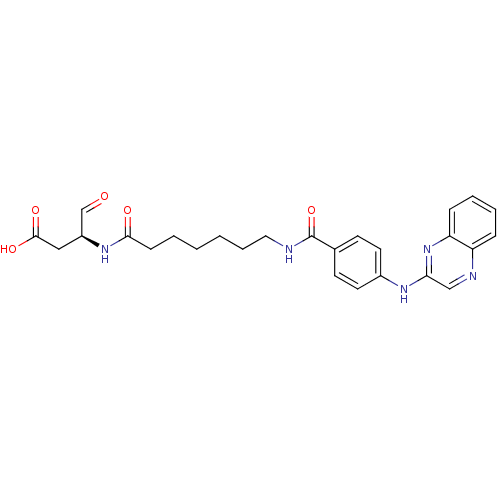
((S)-4-oxo-3-(7-(4-(quinoxalin-2-ylamino)benzamido)...)Show SMILES OC(=O)C[C@H](NC(=O)CCCCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)C=O Show InChI InChI=1S/C26H29N5O5/c32-17-20(15-25(34)35)30-24(33)9-3-1-2-6-14-27-26(36)18-10-12-19(13-11-18)29-23-16-28-21-7-4-5-8-22(21)31-23/h4-5,7-8,10-13,16-17,20H,1-3,6,9,14-15H2,(H,27,36)(H,29,31)(H,30,33)(H,34,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176499
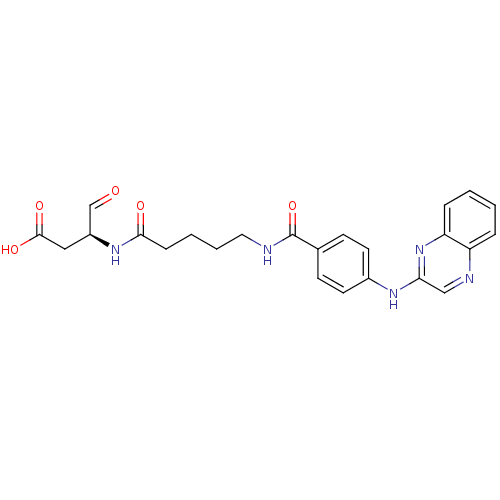
((S)-4-oxo-3-(5-(4-(quinoxalin-2-ylamino)benzamido)...)Show SMILES OC(=O)C[C@H](NC(=O)CCCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)C=O Show InChI InChI=1S/C24H25N5O5/c30-15-18(13-23(32)33)28-22(31)7-3-4-12-25-24(34)16-8-10-17(11-9-16)27-21-14-26-19-5-1-2-6-20(19)29-21/h1-2,5-6,8-11,14-15,18H,3-4,7,12-13H2,(H,25,34)(H,27,29)(H,28,31)(H,32,33)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176518
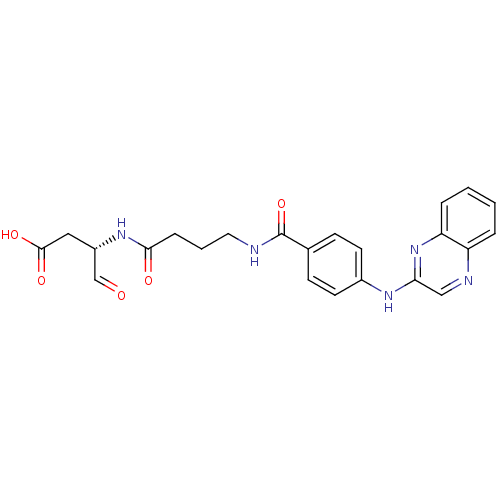
((S)-4-oxo-3-(4-(4-(quinoxalin-2-ylamino)benzamido)...)Show SMILES OC(=O)C[C@H](NC(=O)CCCNC(=O)c1ccc(Nc2cnc3ccccc3n2)cc1)C=O Show InChI InChI=1S/C23H23N5O5/c29-14-17(12-22(31)32)27-21(30)6-3-11-24-23(33)15-7-9-16(10-8-15)26-20-13-25-18-4-1-2-5-19(18)28-20/h1-2,4-5,7-10,13-14,17H,3,6,11-12H2,(H,24,33)(H,26,28)(H,27,30)(H,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50176492
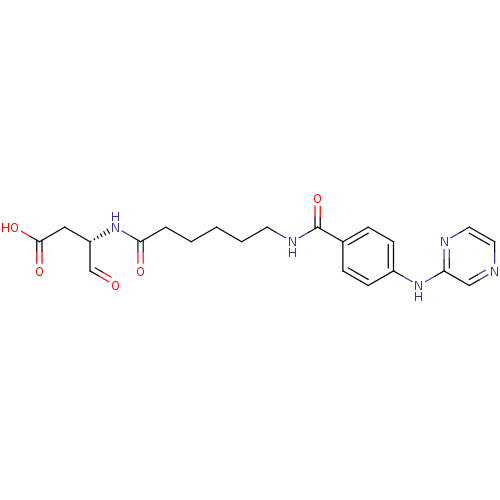
((S)-4-oxo-3-(6-(4-(pyrazin-2-ylamino)benzamido)hex...)Show SMILES OC(=O)C[C@H](NC(=O)CCCCCNC(=O)c1ccc(Nc2cnccn2)cc1)C=O Show InChI InChI=1S/C21H25N5O5/c27-14-17(12-20(29)30)26-19(28)4-2-1-3-9-24-21(31)15-5-7-16(8-6-15)25-18-13-22-10-11-23-18/h5-8,10-11,13-14,17H,1-4,9,12H2,(H,23,25)(H,24,31)(H,26,28)(H,29,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Caspase 1 |
Bioorg Med Chem Lett 16: 559-62 (2005)
Article DOI: 10.1016/j.bmcl.2005.10.048
BindingDB Entry DOI: 10.7270/Q2251HRD |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26333
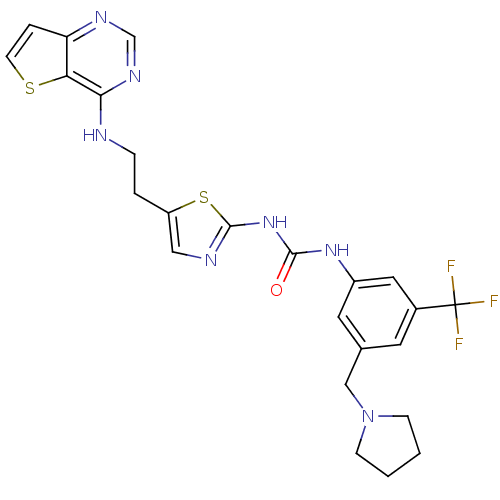
(1-[3-(pyrrolidin-1-ylmethyl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1cc(CN2CCCC2)cc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C24H24F3N7OS2/c25-24(26,27)16-9-15(13-34-6-1-2-7-34)10-17(11-16)32-22(35)33-23-29-12-18(37-23)3-5-28-21-20-19(4-8-36-20)30-14-31-21/h4,8-12,14H,1-3,5-7,13H2,(H,28,30,31)(H2,29,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26322
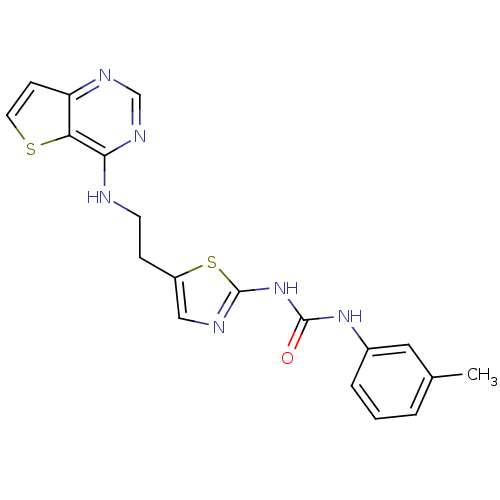
(1-(3-methylphenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Cc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H18N6OS2/c1-12-3-2-4-13(9-12)24-18(26)25-19-21-10-14(28-19)5-7-20-17-16-15(6-8-27-16)22-11-23-17/h2-4,6,8-11H,5,7H2,1H3,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26326
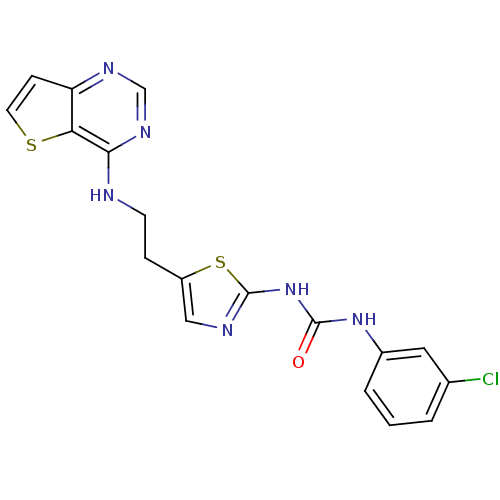
(1-(3-chlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Clc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15ClN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26330
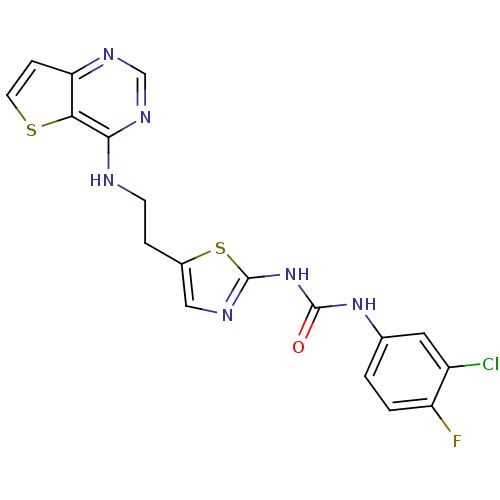
(1-(3-chloro-4-fluorophenyl)-3-[5-(2-{thieno[3,2-d]...)Show SMILES Fc1ccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)cc1Cl Show InChI InChI=1S/C18H14ClFN6OS2/c19-12-7-10(1-2-13(12)20)25-17(27)26-18-22-8-11(29-18)3-5-21-16-15-14(4-6-28-15)23-9-24-16/h1-2,4,6-9H,3,5H2,(H,21,23,24)(H2,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26329
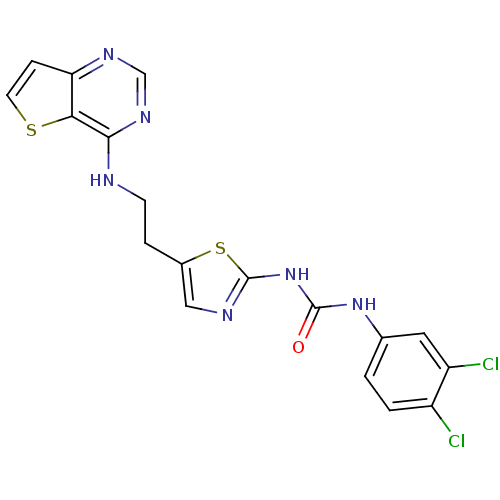
(1-(3,4-dichlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrim...)Show SMILES Clc1ccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)cc1Cl Show InChI InChI=1S/C18H14Cl2N6OS2/c19-12-2-1-10(7-13(12)20)25-17(27)26-18-22-8-11(29-18)3-5-21-16-15-14(4-6-28-15)23-9-24-16/h1-2,4,6-9H,3,5H2,(H,21,23,24)(H2,22,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26315
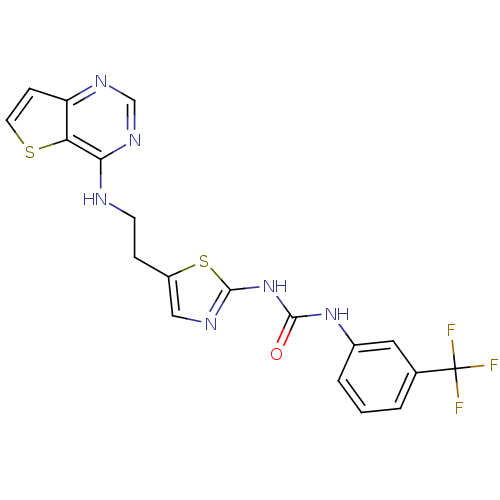
(3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino}ethyl)-1...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H15F3N6OS2/c20-19(21,22)11-2-1-3-12(8-11)27-17(29)28-18-24-9-13(31-18)4-6-23-16-15-14(5-7-30-15)25-10-26-16/h1-3,5,7-10H,4,6H2,(H,23,25,26)(H2,24,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26325
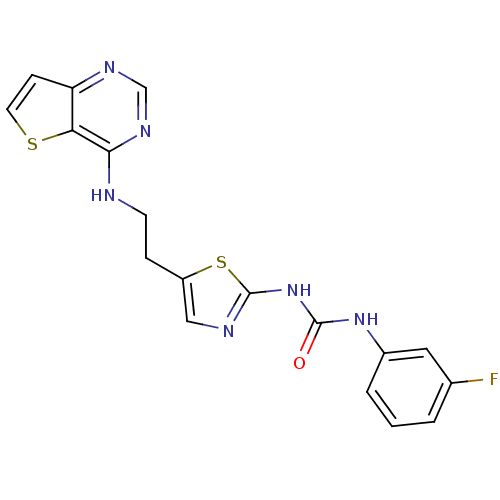
(1-(3-fluorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Fc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15FN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26331
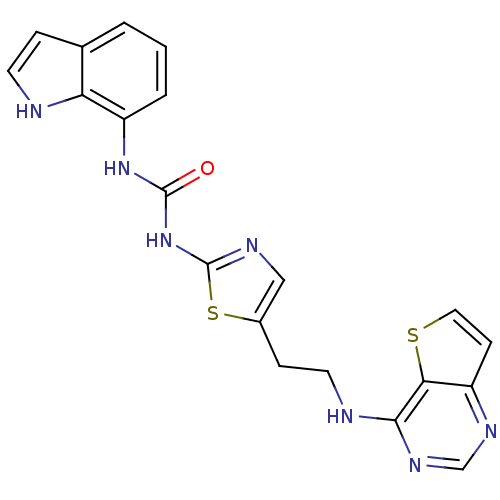
(1-1H-indol-7-yl-3-[5-(2-{thieno[3,2-d]pyrimidin-4-...)Show SMILES O=C(Nc1ncc(CCNc2ncnc3ccsc23)s1)Nc1cccc2cc[nH]c12 Show InChI InChI=1S/C20H17N7OS2/c28-19(26-14-3-1-2-12-4-7-21-16(12)14)27-20-23-10-13(30-20)5-8-22-18-17-15(6-9-29-17)24-11-25-18/h1-4,6-7,9-11,21H,5,8H2,(H,22,24,25)(H2,23,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26333

(1-[3-(pyrrolidin-1-ylmethyl)-5-(trifluoromethyl)ph...)Show SMILES FC(F)(F)c1cc(CN2CCCC2)cc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C24H24F3N7OS2/c25-24(26,27)16-9-15(13-34-6-1-2-7-34)10-17(11-16)32-22(35)33-23-29-12-18(37-23)3-5-28-21-20-19(4-8-36-20)30-14-31-21/h4,8-12,14H,1-3,5-7,13H2,(H,28,30,31)(H2,29,32,33,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26315

(3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino}ethyl)-1...)Show SMILES FC(F)(F)c1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H15F3N6OS2/c20-19(21,22)11-2-1-3-12(8-11)27-17(29)28-18-24-9-13(31-18)4-6-23-16-15-14(5-7-30-15)25-10-26-16/h1-3,5,7-10H,4,6H2,(H,23,25,26)(H2,24,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26325

(1-(3-fluorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Fc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15FN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26331

(1-1H-indol-7-yl-3-[5-(2-{thieno[3,2-d]pyrimidin-4-...)Show SMILES O=C(Nc1ncc(CCNc2ncnc3ccsc23)s1)Nc1cccc2cc[nH]c12 Show InChI InChI=1S/C20H17N7OS2/c28-19(26-14-3-1-2-12-4-7-21-16(12)14)27-20-23-10-13(30-20)5-8-22-18-17-15(6-9-29-17)24-11-25-18/h1-4,6-7,9-11,21H,5,8H2,(H,22,24,25)(H2,23,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26326

(1-(3-chlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Clc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C18H15ClN6OS2/c19-11-2-1-3-12(8-11)24-17(26)25-18-21-9-13(28-18)4-6-20-16-15-14(5-7-27-15)22-10-23-16/h1-3,5,7-10H,4,6H2,(H,20,22,23)(H2,21,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26327
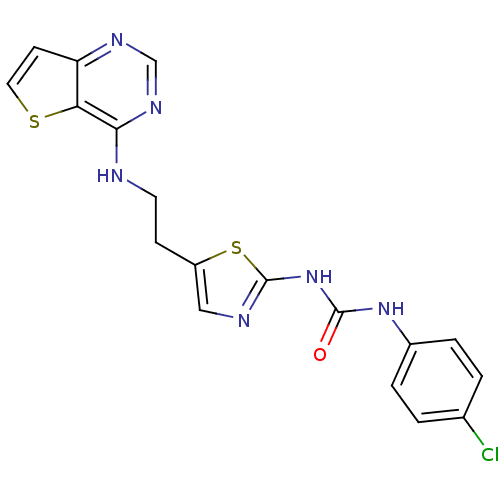
(1-(4-chlorophenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Clc1ccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)cc1 Show InChI InChI=1S/C18H15ClN6OS2/c19-11-1-3-12(4-2-11)24-17(26)25-18-21-9-13(28-18)5-7-20-16-15-14(6-8-27-15)22-10-23-16/h1-4,6,8-10H,5,7H2,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26324
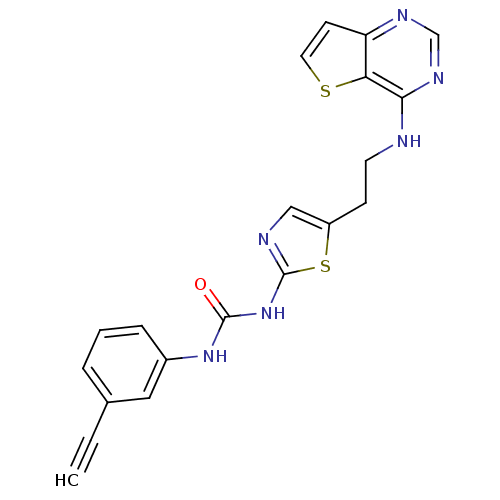
(1-(3-ethynylphenyl)-3-[5-(2-{thieno[3,2-d]pyrimidi...)Show SMILES O=C(Nc1ncc(CCNc2ncnc3ccsc23)s1)Nc1cccc(c1)C#C Show InChI InChI=1S/C20H16N6OS2/c1-2-13-4-3-5-14(10-13)25-19(27)26-20-22-11-15(29-20)6-8-21-18-17-16(7-9-28-17)23-12-24-18/h1,3-5,7,9-12H,6,8H2,(H,21,23,24)(H2,22,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26323
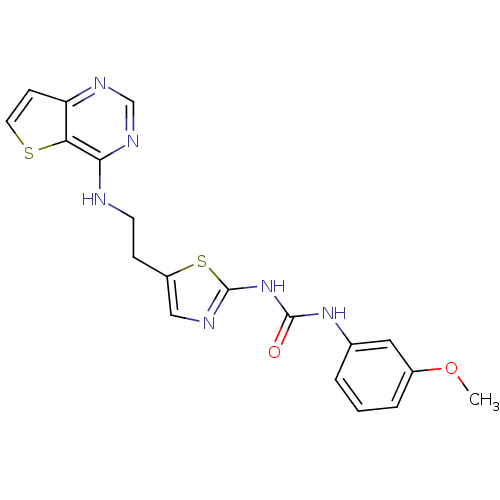
(1-(3-methoxyphenyl)-3-[5-(2-{thieno[3,2-d]pyrimidi...)Show SMILES COc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H18N6O2S2/c1-27-13-4-2-3-12(9-13)24-18(26)25-19-21-10-14(29-19)5-7-20-17-16-15(6-8-28-16)22-11-23-17/h2-4,6,8-11H,5,7H2,1H3,(H,20,22,23)(H2,21,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26330

(1-(3-chloro-4-fluorophenyl)-3-[5-(2-{thieno[3,2-d]...)Show SMILES Fc1ccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)cc1Cl Show InChI InChI=1S/C18H14ClFN6OS2/c19-12-7-10(1-2-13(12)20)25-17(27)26-18-22-8-11(29-18)3-5-21-16-15-14(4-6-28-15)23-9-24-16/h1-2,4,6-9H,3,5H2,(H,21,23,24)(H2,22,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26320
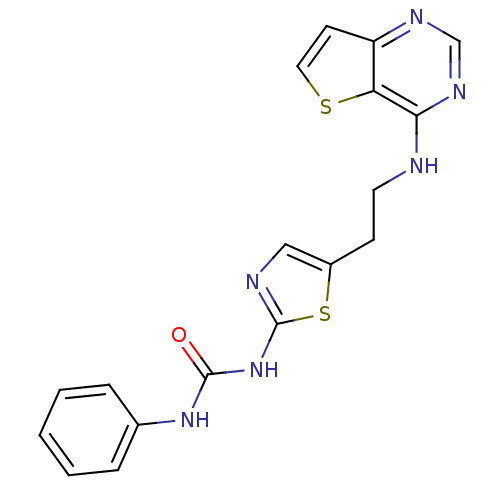
(1-phenyl-3-[5-(2-{thieno[3,2-d]pyrimidin-4-ylamino...)Show InChI InChI=1S/C18H16N6OS2/c25-17(23-12-4-2-1-3-5-12)24-18-20-10-13(27-18)6-8-19-16-15-14(7-9-26-15)21-11-22-16/h1-5,7,9-11H,6,8H2,(H,19,21,22)(H2,20,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM26334
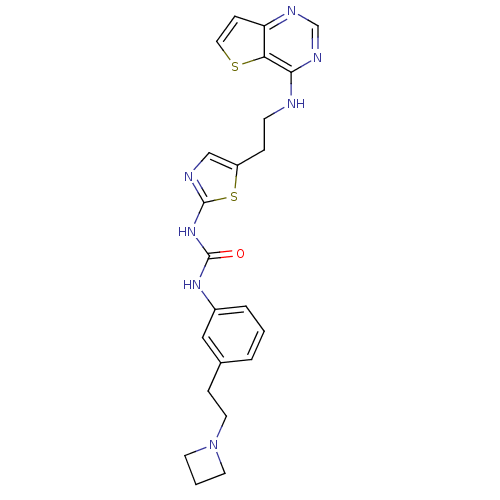
(1-{3-[2-(azetidin-1-yl)ethyl]phenyl}-3-[5-(2-{thie...)Show SMILES O=C(Nc1ncc(CCNc2ncnc3ccsc23)s1)Nc1cccc(CCN2CCC2)c1 Show InChI InChI=1S/C23H25N7OS2/c31-22(28-17-4-1-3-16(13-17)6-11-30-9-2-10-30)29-23-25-14-18(33-23)5-8-24-21-20-19(7-12-32-20)26-15-27-21/h1,3-4,7,12-15H,2,5-6,8-11H2,(H,24,26,27)(H2,25,28,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.2 | 21 |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26322

(1-(3-methylphenyl)-3-[5-(2-{thieno[3,2-d]pyrimidin...)Show SMILES Cc1cccc(NC(=O)Nc2ncc(CCNc3ncnc4ccsc34)s2)c1 Show InChI InChI=1S/C19H18N6OS2/c1-12-3-2-4-13(9-12)24-18(26)25-19-21-10-14(28-19)5-7-20-17-16-15(6-8-27-16)22-11-23-17/h2-4,6,8-11H,5,7H2,1H3,(H,20,22,23)(H2,21,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM26332
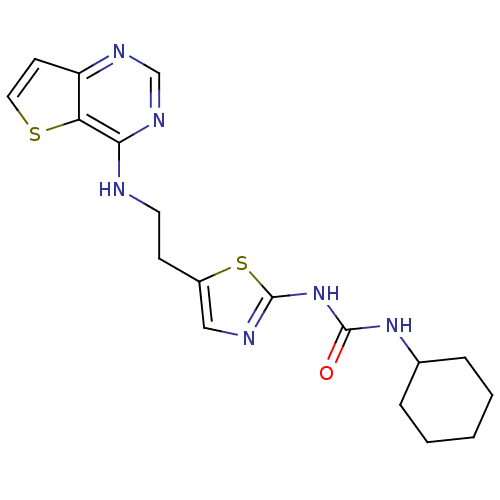
(1-cyclohexyl-3-[5-(2-{thieno[3,2-d]pyrimidin-4-yla...)Show InChI InChI=1S/C18H22N6OS2/c25-17(23-12-4-2-1-3-5-12)24-18-20-10-13(27-18)6-8-19-16-15-14(7-9-26-15)21-11-22-16/h7,9-12H,1-6,8H2,(H,19,21,22)(H2,20,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals
| Assay Description
Compounds were diluted into assay buffer containing Aurora kinase and FAM-PKAtide. The kinase reaction was initiated by adding ATP in assay buffer. T... |
Bioorg Med Chem Lett 18: 4880-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.073
BindingDB Entry DOI: 10.7270/Q279430N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data